Immunosuppression status of liver transplant recipients with hepatitis C affects biopsy-proven acute rejection
Article information
Abstract
Background/Aims
The relationship between patient survival and biopsy-proven acute rejection (BPAR) in liver transplant recipients with hepatitis C remains unclear. The aims of this study were to compare the characteristics of patients with and without BPAR and to identify risk factors for BPAR.
Methods
We retrospectively reviewed the records of 169 HCV-RNA-positive patients who underwent LT at three centers.
Results
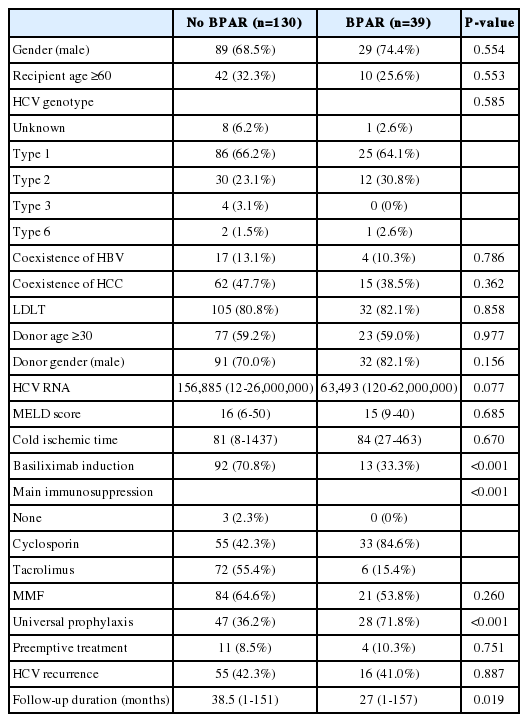
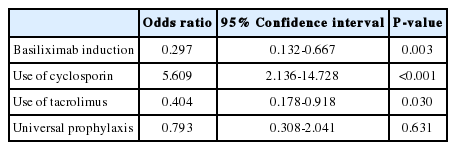
BPAR occurred in 39 (23.1%) of the HCV-RNA-positive recipients after LT. The 1-, 3-, and 5-year survival rates were 92.1%, 90.3%, and 88.5%, respectively, in patients without BPAR, and 75.7%, 63.4%, and 58.9% in patients with BPAR (P<0.001). Multivariate analyses showed that BPAR was associated with the non-use of basiliximab and tacrolimus and the use of cyclosporin in LT recipients with HCV RNA-positive.
Conclusion
The results of the present study suggest that the immunosuppression status of HCV-RNA-positive LT recipients should be carefully determined in order to prevent BPAR and to improve patient survival.
INTRODUCTION
Hepatitis C virus (HCV) infection is the most common underlying disease among patients requiring liver transplantation (LT) in Western countries. In Korea, 1-2% of the population is infected with HCV, and 15-20% of these infected individuals have chronic liver disease [1,2].
Although LT offers the optimal treatment for HCV-related end-stage liver disease and hepatocellular carcinoma (HCC), graft reinfection with HCV is not acute, but rather immediate and universal, in all patients who are hepatitis C virus ribonucleic acid (HCV RNA)-positive at transplantation [3]. Recurrent HCV infection after LT is associated with reduced graft and patient survival [4]. Factors related to the recipient, donor, and transplant are associated with a high risk of severe and progressive HCV disease. Donor liver quality, older donor age, prolonged warm and cold ischemia times, donation after cardiac death, and a high donor risk index have been found to be associated with lower graft survival and higher rates of advanced fibrosis [5,6].
Allograft rejection refers to graft damage arising as a consequence of an immunological reaction to foreign antigens on the graft. The incidence of clinically significant rejection is 10-40% in most series, and usually occurs within the first month after LT, when liver injury results in elevated levels of bilirubin, transaminases, and alkaline phosphatase [7]. The diagnosis is confirmed by characteristic histological findings of portal inflammation, bile duct injury, and venous inflammation often associated with centrilobular necroinflammation involving the hepatic venules and surrounding hepatocytes [8].
The relationship between biopsy-proven acute rejection (BPAR) and immunosuppression regimen in HCV LT recipients remains unclear. Herein, we compared patient characteristics and survival data between patients with and without BPAR, and identified risk factors for BPAR in HCV LT recipients.
PATIENTS AND METHODS
Patients
This study involved three LT centers in Korea: Samsung Medical Center, Asan Medical Center, and Seoul National University Hospital. Because this study was a retrospective, we were not able to obtain patient consent for use of clinical records. However, each center’s institutional review board (IRB) approved our protocols (Samsung Medical Center IRB no. 2014-07-031, Asan Medical Center IRB no. S2015-1341-0003, and Seoul National University Hospital IRB no. 1407-139-597). We retrospectively evaluated patients undergoing their first LT between 1994 and 2012 at Samsung Medical Center (n=42), Seoul National University Hospital (n=42), and Asan Medical Center (n=108). Data from all 192 consecutive HCV RNA positive cases during this period were reviewed following the same questionnaire for each study center. HCV RNA level was measured with a real-time PCR-based assay using COBAS® TaqMan® HCV Test Version 2.0 (Roche Molecular Systems, Pleasanton, California) with a lower limit of quantification of 12 IU/mL. However, the immunosuppression protocols, treatments for organ rejection, and treatments for recurrent HCV infections were not standardized across centers. The laboratory-based model for end-stage liver disease (MELD) score was calculated at the time of transplantation. The information and/or records of all patients were de-identified prior to analysis. We selected 169 patients after excluding hospital mortality cases (n=23).
Evaluated variables
The following variables were obtained from the medical record review in response to the survey: patient age, gender, HCV genotype, MELD score, co-occurrence with HCC, co-infection with hepatitis B virus (HBV) or human immunodeficiency virus, antiviral treatments received after LT, type of calcineurin inhibitor received, use of mycophenolate mofetil (MMF), steroid withdrawal, BPAR, HCV recurrence, and other outcomes. Additionally, donor age and ischemic time were added as variables. Unversal prophylaxis was defined as antiviral therapy that was routinely performed in all recipients with HCV after liver transplantation. Preemptive treatment was defined as antiviral therapy that was started when the serum HCV viral load increased. Finally, we recorded information on patient survival and calculated the time to death. However, we did not incorporate any other incomplete variables the analysis that may have been associated with patient survival, such as IL-28 gene polymorphisms, histological findings, biliary complications, or infectious episodes. Diagnosis of acute rejection was based on internationally accepted histologic criteria (Banff guidelines) using liver biopsy [9]. HCV recurrence was diagnosed based on histology, biochemistry, and/or the detection of HCV RNA in serum.
Statistical analyses
Continuous data are reported as the median and range and compared using the Mann-Whitney U test. Categorical variables are reported as numeric proportions. Comparisons between groups for categorical data were performed using the Fisher’s exact test. Patient survival rates were evaluated using the Kaplan-Meier method and compared using the log-rank test. Clinical variables found to have significance on univariate analysis were entered into a binary logistic regression analysis to determine which factors independently predicted BPAR. Statistical significance was set at a P-value less than 0.05. Statistical analyses were performed with SPSS for Windows version 21.0 (IBM, Armonk, NY, USA).
RESULTS
BPAR occurred in 39 (23.1%) HCV RNA-positive recipients after LT. The proportion of basiliximab induction and tacrolimus usage in patients with BPAR was lower than in patients without BPAR (33.3% vs. 70.8% for basiliximab induction and 15.4% vs. 55.4% for the use of tacrolimus). However, the proportion of universal prophylaxis in patients with BPAR was higher than in patients without BPAR (71.2% vs. 36.2%; Table 1). There were no statistically significant differences in the gender, recipient age, HCV genotype, coexistence of HBV or HCC, living donor LT, donor age, donor gender, HCV RNA status, MELD score, cold ischemic time, MMF usage, preemptive treatment or HCV recurrence between the two groups.
Most patients with BPAR were treated with an increased immunosuppression dosage (n=9) or a change in calcineurin inhibitor (n=23). Four patients were treated with steroid pulse therapy. However, patient survival rates were not different according to the treatments for BPAR.
The 1-, 3-, and 5-year patient survival rates were 92.1%, 90.3%, and 88.5%, respectively, in patients without BPAR and 75.7%, 63.4%, and 58.9% in patients with BPAR (Fig. 1). The patient survival curve for patients with BPAR was lower than that for patients without BPAR (P<0.001). Multivariate analyses showed that BPAR was associated with a lack of basiliximab induction and tacrolimus, and the use of cyclosporin among HCV RNA-positive LT recipients (Table 2). HCV RNA levels and universal prophylaxis were not associated with BPAR.
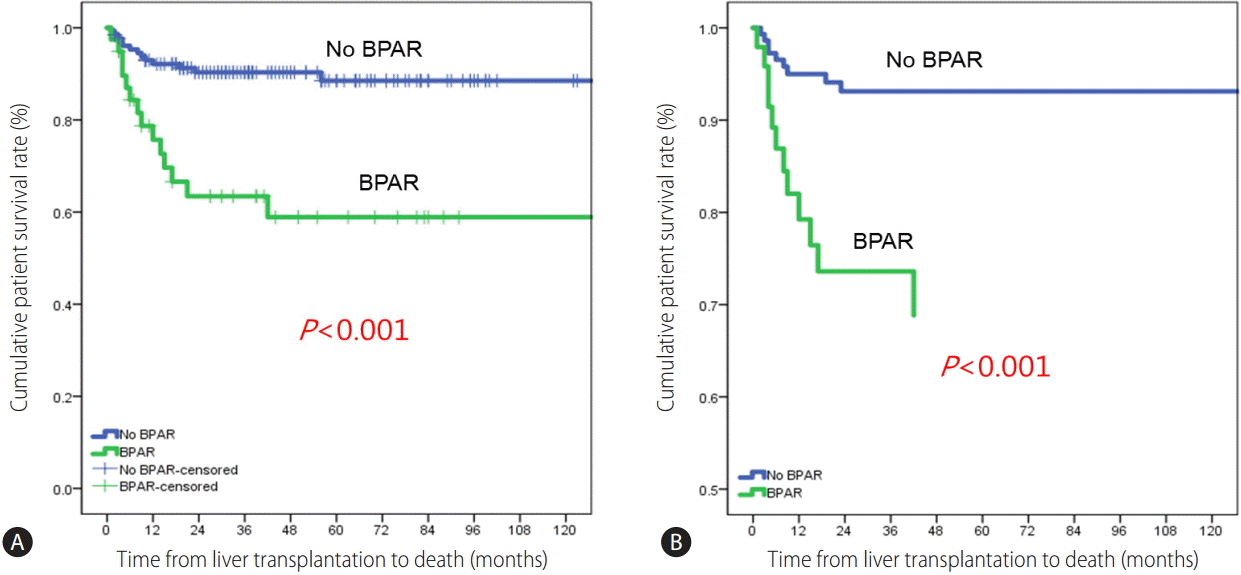
(A) Survival rates in patients with and without BPAR. (B) Adjusted survival curves using covariates including cyclosporin, tacrolimus, universal prophylaxis, and HCV RNA.
DISCUSSION
Immunosuppression for HCV patients requires a fine balance between suppressing immune response and maintaining an optimal host viral response [10]. Immunosuppression remains an area of uncertainty, due to the lack of good evidence on the factors that may improve or worsen outcomes [11]. In this study, the survival rate of HCV-positive recipients with BPAR was lower than that of HCV-positive recipients without BPAR, after LT. BPAR in HCV-positive recipients was closely associated with basiliximab induction and using tacrolimus; these immunosuppressants prevented BPAR in HCV-positive LT recipients. Acute rejection in conjunction with cyclosporin treatment is one of the most critical factors influencing patient survival.
There are adverse effects of early or repetitive episodes of acute cullular rejection [12]. Treated acute rejection is strongly associated with post-transplant HCV disease severity and the risk of cirrhosis [13]. Importantly, acute rejection is not associated with HCV disease severity or progression, but, rather, disease severity and progression are associated with the receipt of anti-rejection treatment [13]. For this reason, treatment with boluses of corticosteroids is not recommended for mild rejection; increased maintenance immunosuppression is the treatment of choice. Indeed, the overreaching goal in terms of immunosuppression in HCV-infected recipients is to provide sufficient immunosuppression to prevent moderate to severe rejection while simultaneously avoiding excess immunosuppression [13].
The immunosuppressive potency of oral tacrolimus is greater than that of cyclosporin and its bioavailability is excellent. Thus, it has become the main calcineurin inhibitor used in most immunosuppressive protocols, often in combination with corticosteroids [14]. Following the introduction of tacrolimus-based immunosuppression, the rate of acute rejection fell progressively to 20% and the rate of chronic rejection to 5%, and most cases of acute rejection are now controlled either by an increase in tacrolimus or by boluses of steroids with a reduced total immunosuppressive load and consequent reduction in infectious complications [15].
Although cyclosporin has weak antiviral activity against HCV replication in vitro [16], the effect of calcineurin inhibitors on HCV progression is highly controversial based on conflicting data regarding the relative risk of tacrolimus compared with cyclosporin. Most prospective studies suggest that there is no difference between cyclosporin- and tacrolimus-based regimens in terms of liver histology, acute rejection, graft survival, or mortality [17,18]. However, recent analysis of the Scientific Registry of Transplant Recipients also demonstrated that tacrolimus is associated with reduced mortality and graft cirrhosis in HCV patients [19]. Our study also suggests that careful avoidance of acute rejection in the post-transplant period through adequate use of tacrolimus is a preferable strategy, because cyclosporin is associated with a greater incidence of acute rejection.
The present study showed that universal prophylaxis was associated with BPAR in univariate analysis. However, multivariate analysis did not reveal any relationship between universal prophylaxis and BPAR. This result could be due to the possibility that only one of the centers in the study had a policy of universal prophylaxis cyclosporin use in HCV patients after liver transplantation.
Anti-interleukin-2 receptor antibodies are now widely used in LT as induction agents targeted at reducing the incidence of acute cellular rejection to protect renal function in the immediate post-operative period [20]. A recent study reported that induction with anti-interleukin-2 receptor antibodies has not been associated with worse or better HCV disease-specific outcomes [13]. However, our study revealed that anti-interleukin-2 receptor antibodies prevent biopsy-proven acute rejection, which contributes to the long-term survival of HCV-positive LT recipients.
There are several limitations inherent in the type of study design that we employed, including variability in documentation, differences in selection criteria and data collection, and missing data. The present study was a retrospective and nonrandomized case series. To minimize variability, we sent a standardized collection form containing 56 questions to each participating transplant center. The answers were either multiple-choice or involved providing a name or a specific value. However, the quality of the pretransplant interviews from which the baseline data were derived and the quality of the post-transplant follow-up data across the three centers were variable. Furthermore, subjects had varying follow-up durations. We did not have data on the onset of BPAR, the response to BPAR treatments, or the date of graft failure. Additionally, the present study did not include data on fibrosis upon biopsy, which is another important limitation. To address these limitations, a well-designed prospective study is needed.
In conclusion, our study showed that patients with BPAR have lower survival rates compared with patients without BPAR. Basiliximab induction and cyclosporin usage were associated with survival of HCV-positive recipients after LT. The present study suggests that the selection of immunosuppression in HCV RNA-positive recipients should be carefully reviewed to prevent BPAR and improve patient survival.
Notes
Authors’ contributions: Jong Man Kim: acquired, analyzed, and interpreted the data and wrote the manuscript.
Gi-Won Song and Kwang-Woong Lee: designed the study, acquired and interpreted the data, and helped write the manuscript.
Bo-Hyun Jung and Hae Won Lee: acquired and analyzed the data.
Nam-Joon Yi, ChoonHyuck David Kwon, Shin Hwang, Kyung-Suk Suh, Jae-Won Joh, Suk-Koo Lee, and Sung-Gyu Lee: interpreted the data
Acknowledgements
The authors have no affiliation with nor financial involvement in any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in this manuscript. The production of this manuscript included writing assistance.
Notes
Conflicts of Interest: The authors have no conflicts to disclose.
Abbreviations
BPAR
biopsy-proven acute rejection
HCC
hepatocellular carcinoma
HCV
hepatitis C virus
LT
liver transplantation
MELD
model for end-stage liver disease