New perspectives of biomarkers for the management of chronic hepatitis B
Article information
Abstract
With recent advances in molecular and genomic investigations, the impact of hepatitis B viral and host factors on the progression of chronic HBV infection has been explored. For viral factors, hepatitis B viral load is a strong predictor for liver disease progression. Hepatitis B viral kinetics appear to be important for successful anti-viral therapy. Serum HBsAg level serves as a complementary marker to viral load for the prediction of HBV-related adverse outcomes in patients with low viral load. In those with low viral load, high serum HBsAg level is associated with higher risks of cirrhosis and HCC. Hepatitis B core-related antigen (HBcrAg) induces host immune responses, and the reduction of the HBcrAg level as well as the increment of total anti-HBc level are significantly associated with favorable outcomes. HBV genotypes (genotype C/D) and mutants (basal core promoter and deletion mutation in pre-S genes) are well known viral genetic markers to predict disease progression. For host factors, serum inflammatory biomarkers have been developed to evaluate the HBV-associated hepatic necroinflammation and fibrosis. Host single nucleotide polymorphism on sodium taurocholate cotransporting polypeptide (NTCP, an HBV entry receptor) may be associated with a decreased risk for cirrhosis and HCC. In conclusion, patients with chronic hepatitis B should be evaluated with relevant viral and host markers to identify those who are at a higher risk of liver disease progression and then receive timely antiviral therapy.
INTRODUCTION
Despite the availability of effective hepatitis B virus (HBV) vaccine for more than three decades, HBV is still an important public health problem and the leading cause of chronic hepatitis, cirrhosis, and hepatocellular carcinoma (HCC) worldwide [1,2]. Although most of chronic hepatitis B (CHB) patients have favorable clinical outcomes, HBV eventually leads to develop cirrhosis, liver failure, or HCC in a significant proportion of patients [3]. Through the development of effective anti-HBV treatment, the prevention of disease progression and reduction of HCC risk in patients with chronic HBV infection have been achieved [4]. However, the optimal timing and the strategy of antiviral therapy toward HBV cure are still daunting challenges ahead. With recent advances in molecular investigations, several biomarkers associated with the natural history of chronic HBV infection and the efficacy of antiviral therapy have been identified [5-7]. Integration of these biomarkers may improve our understanding about the natural history of CHB and the response to antiviral therapy. In this article, a new perspective of biomarkers for the management of CHB will be reviewed and discussed.
HBV BIOMARKERS ASSOCIATED WITH PROGRESSION OF CHB
The natural history of HBV carriers who are infected early in life can be divided into 4 dynamic phases based on the virus-host interaction [1,6]. During the immune tolerance phase, extensive liver replication of HBV results in high HBV DNA levels (usually higher than 108 IU/mL) and hepatitis B e antigen (HBeAg) is present. Hepatitis activities are low with normal or mildly elevated serum alanine aminotransferase (ALT) levels in this phase. After 2 to 3 decades of persistent infection, the infection enters the immune clearance phase. HBV carriers have bouts of acute hepatitis flare accompany with the elevation of ALT level and the decline of serum HBV DNA level. The majority of carriers seroconvert from HBeAg to anti-HBe in this phase. However, following a series of hepatitis flare, the liver starts to have cirrhotic changes. The incidence of cirrhosis has been shown to be 2% per year, depending on the severity and extent of hepatic inflammation [8,9]. After HBeAg seroconversion, patients are usually in the residual or low replication phase, with low HBV DNA level (less than 2,000 IU/mL) and normal ALT, and even Hepatitis B surface antigen (HBsAg) seroclearance. Unfortunately, a small but significant proportion of patients continue to have moderate level of HBV DNA and active liver disease designated reactivation phase [10]. The frequency and severity of hepatitis flares during the immune clearance and/or reactivation phase predicts progression of liver disease. In general, early HBeAg seroconversion with subsequent HBsAg loss or seroconversion typically confers a favorable outcome, whereas late or absent HBeAg seroconversion after multiple hepatitis flares may accelerate the progression of chronic hepatitis to cirrhosis, and therefore, has a poor clinical outcome.
In real world practice, the clinical courses of CHB vary greatly among individuals. Recent cohort studies on Asian populations even showed that HCC still occurred in patients with spontaneous HBsAg clearance [11,12]. Therefore, accurate determination of the clinical phases of a given HBV patient is clinically mandatory. Currently, several biomarkers affecting clinical outcomes of CHB have been identified. By integration of these biomarkers, the nature history of CHB could be revisited (Fig. 1) [6].
HEPATITIS B VIRAL LOAD
Based on a population-based cohort of untreated CHB Taiwanese patients, the risk evaluation of viral load elevation and associated liver disease/cancer-hepatitis B virus (REVEAL-HBV) study first demonstrated that the cumulative incidence of HCC is correlated with serum hepatitis B viral load in a dose dependent manner [13,14]. The relative risk of HCC began to increase from HBV DNA level of 2,000 IU/mL to HBV DNA levels of 200,000 IU/mL. Several cross sectional and longitudinal cohort studies in Taiwan, Hong Kong, and China also confirmed the impact of HBV DNA level on HCC development [15-20]. In addition, several clinical trials revealed that a lower baseline HBV DNA level was predictive of HBeAg loss or seroconversion as well as viral suppression in patients receiving anti-viral therapy [5]. These lines of evidence indicate that measurements of HBV viral load may define which CHB patients are at an increased risk of liver disease progression.
QUANTITATIVE HBsAg LEVEL
HBsAg is a well established biomarker of HBV infection and is qualitatively used for the diagnosis of HBV infection in our daily practice. In the natural course of CHB, HBV DNA is only from mature infectious particles and its level reflects viral replication. Accordingly, a decline of HBV DNA means the reduction of HBV replication. In addition, HBsAg can also be derived from defective subviral particles. Thus, serum HBsAg level not only reflects the cccDNA transcription or mRNA translation, but also host immune control over HBV infection [21,22]. The combination of low serum HBsAg level and serum HBV DNA level can predict inactive HBV carrier status and HBsAg loss after HBeAg seroconversion [23,24]. Our previous study showed that serum HBsAg level was better than serum HBV DNA level for the prediction of spontaneous HBsAg loss in HBeAg-negative carriers with a low viral load (<2,000 IU/mL). Moreover, serum HBsAg level <10 IU/mL was the strongest predictor of HBsAg loss in patients with a low viral load [25].
The association between serum HBsAg level and HCC has been elucidated in both community-based and hospital-based cohort studies. In the community-based cohort study (REVEAL-HBV study), serum HBsAg and HBV DNA levels were independent predictors of HCC development in HBV carriers. Serum HBsAg level was complementary to HBV DNA level in stratifying HCC risks, especially in patients with HBV DNA level < 200,000 IU/mL [26]. In our hospital-based ERADICATE-B study (Elucidation of Risk Factors for Disease Control or Advancement in Taiwanese Hepatitis B Carriers), elevated HBV DNA and HBsAg levels were positively associated with HCC development in a dose-response manner in patients with HBV DNA levels >2,000 IU/mL [27]. Although the incidence of HCC is significantly associated with baseline serum HBV-DNA level, inactive or low risk HBV carriers (serum HBV-DNA levels <2,000 IU/mL) still have significantly higher hazard ratios for HCC compared with individuals without HBV infection [28]. Therefore, it is important to identify factors predictive of HCC other than HBV DNA level in these inactive or low risk HBV carriers. In the ERADICATE-B study, among 1,068 HBeAg-negative patients with HBV DNA levels <2000 IU/mL, the HCC risk significantly increased in those with HBsAg level ≥ 1000 IU/mL compared with those with HBsAg level <1,000 IU/mL. Multivariate analysis revealed that HBsAg level ≧1,000 IU/mL was an independent risk factor for HCC development [27]. Data from REVEAL-HBV study and ERADICATE-B study all showed that serum HBsAg and HBV DNA levels were complementary markers in predicting disease progression, including hepatitis flares, HBeAg-negative hepatitis, liver cirrhosis, and HCC [27,29,30].
Despite using immunoprophylaxis to interrupt mother-to-infant HBV transmission in clinical practice, mothers positive for HBeAg with a high viral load are the major causes of immunoprophylactic failure [31]. Furthermore, quantitative maternal HBsAg predicts infection in infants as well as maternal viral load does. Estimated rates of infection at maternal HBsAg levels of 4, 4.5, and 5 log10 IU/mL were 2.4%, 8.6%, and 26.4% [32]. Thus, antiviral therapy may be considered in pregnant women with high HBsAg level as well as high viral load to interrupt mother-to-infant transmission [33].
HEPATITIS B CORE-RELATED ANTIGEN LEVEL
Hepatitis B virus core-related antigen (HBcrAg) is comprised of hepatitis B core antigen (HBcAg), HBeAg and a precore protein (p22cr) coded with the precore/core region [34]. HBcAg is an inner nucleocapsid surrounding the viral DNA. HBeAg is a circulating peptide derived from the core gene, then modified and secreted from liver cells. Both HBcAg and HBeAg, sharing an identical 149 amino acid sequence, are the target of cytotoxic T-cell. Thus, HBcrAg can induce host cellular immune response. During follow-up of HBeAg-positive patients, the reduction in the HBcrAg levels is significantly associated with spontaneous HBeAg seroconversion [35]. Previous reports indicated that serum HBcrAg concentration is closely correlated with serum HBV DNA as well as intrahepatic cccDNA level [36-38]. Therefore, HBcrAg may help determine the phase of HBV infection. In a large European cohort mainly infected with HBV genotypes A or D, the median serum HBcrAg levels were high in the immune tolerance and immune clearance phases (>8 log U/mL, respectively), lower in HBeAg-negative chronic hepatitis (4.82 log U/mL) but very low in HBeAg-negative inactive carriers (only 2.00 log U/mL) [39]. Furthermore, recent studies revealed that serum HBcrAg level was associated with development of HCC in both nucleos(t)ide analogue-treated and untreated patients [40-42]. In a large cohort study, HBcrAg >2.9 log U/mL (HR, 5.05; 95% CI, 2.40-10.63) was associated with the incidence of HCC. Of particular note, time-dependent ROC analysis showed that HBcrAg was superior to HBV DNA in predictive power for HCC development throughout the follow-up period [42].
TOTAL ANTI-HBc LEVEL
Pathogenesis of HBV infection related to the interactions between host immune responses and HBV-encoded antigens. Immunoglobulin antibody to HBcAg is the first antibody to appear after infection, in the sequence of immunoglobulin M anti-HBc (IgM anti-HBc) followed by Immunoglobulin G anti-HBc (IgG anti-HBc). A novel assay for the quantification of total anti-HBc antibodies has been developed [43]. In the natural history of chronic HBV infection, total anti-HBc levels in the immune clearance and reactivation phases are significantly higher than those in the immune tolerance and inactive carrier phases. Although patients at the immune-tolerant phase have very high levels of HBV DNA, the total anti-HBc levels are similar to those of inactive carriers [44]. In addition, the baseline total anti-HBc levels could predict HBeAg seroconversion in HBeAg-positive CHB patients receiving antiviral therapy. Patients with higher baseline total anti-HBc levels exhibited a higher rate of HBeAg seroconversion [45,46]. Accordingly, total anti-HBc level is suggested to correlate with the host immune responses against HBV and maybe complementary to current quantitative viral markers, including HBsAg and HBV DNA levels.
HBV GENOTYPE
According to the divergence in the entire HBV genomic sequences, at least 10 HBV genotypes (A to J) have been defined with specific geographical distributions [47,48]. For example, Genotype A and D are prevalent in Africa, Europe, India and America. Genotypes B and C are most common in Asia-Pacific region. Genotype E is restricted to West Africa. Genotype F is found in Central and South America. Several studies suggested that HBV genotype can influence the long-term outcomes of HBV infection. HBV genotype C and D patients, compared to genotype A and B patients, have late or absent HBeAg seroconversion [49-52]. Patients with genotype C HBV infection have a higher risk of cirrhosis and HCC than those with genotype B infection [47,48,53,54]. Of interest, several reports have shown that HBV genotype B was associated with HCC development in young non-cirrhotic patients. Whereas genotype C was associated with HCC development in old cirrhotic patients [51,53,55]. The possible influence of other genotypes on the natural history of HBV infection remains limited. Early studies reported that HCC was more frequent in patients with HBV genotype D and F infection than those with genotype A infection [56,57]. Recent cohort study from Gambia, where genotype E prevail, revealed that the annual incidence of HBeAg and HBsAg seroclearance were similar to other genotypes (7.4 and 1.0 %, respectively). However, the incidence rate of HCC in treatment-naive male chronic HBV carriers in Gambia was higher than Europe but lower than in East Asia [58].
HBV MUTANTS
Because HBV lacks the ability of proofreading for the spontaneous error of viral replication through reverse transcription, HBV mutant strains occur during the natural course of infection as well as with anti-viral therapy. The common mutations in core promoter and deletion mutation in pre-S/S genes have been reported to be associated with the progression of liver disease, including cirrhosis and HCC. Previous studies revealed that dual mutations in basal core promoter (BCP) A1762T/G1764A were strongly associated with the risk of HCC development [17,18,59-62]. BCP A1762T/G1764A mutants also served as a quantitative viral marker for cirrhosis development. In our ERADICATE-B study, quantitative analysis using pyrosequencing revealed that risk of cirrhosis was higher in patients with BCP A1762T/G1764A mutants ≥45% compared to <45% [63].
Deletion mutations in the pre-S gene frequently occur in chronic HBV infection. The deletion over pre-S gene may cause accumulation of large surface protein in the endoplasmic reticulum (ER), resulting in ER stress and hepatocarcinogenesis [64,65]. Previous studies revealed that the presence of pre-S deletion mutations was an independent risk factor associated with the development of cirrhosis and HCC over time [66-68]. Meta-analysis studies also confirmed that pre-S deletion mutations are associated with HCC risk [62,69].
HOST INFLAMMATORY BIOMARKERS
In the course of chronic hepatitis B, necroinflammation along with architectural changes of the liver parenchyma often leads to hepatic fibrogenesis. The severity of hepatic fibrosis is one of the important prognostic factors in CHB [70]. With the limitations of liver biopsy, several non-invasive biomarkers, such as aspartate aminotransferase to platelet ratio index (APRI), FIB-4 index, FibroTest, Forns test, hepascore, have been developed to assess hepatic necroinflammation and fibrosis [71]. Among these biochemical indices, both APRI and FIB-4 index can be measured by using parameters from routine laboratory tests. In our recent study, the correlation between APRI, FIB-4 and histologic severity of 631 CHB patients was assessed. Areas under receiver operating characteristic curves of APRI and FIB-4 demonstrated moderate discriminatory power for the diagnosis of significant fibrosis and cirrhosis. Of particular note, our study showed that the diagnostic ability of FIB-4 was better than APRI. By applying FIB-4 index, significant fibrosis could be accurately excluded in 69.2% and presence of cirrhosis could be diagnosed in 84.4% of patients. Furthermore, 62% of patients could be identified correctly without performing invasive liver biopsy [72]. Recently, through comprehensive metabolomic profiling method, serum metabolite biomarkers were established for CHB stage stratification. A novel metabolite biomarker, PIPS index, based on phosphatidylinositol and phosphatidylserine, has high discriminatory power for the detection of early inflammation and fibrosis in CHB patients [73]. Taken together, serum inflammatory biomarker may serve as useful noninvasive methods to evaluate the severity of hepatic necroinflammation and fibrosis in CHB patients.
HOST GENETIC MARKERS
Not only hepatitis B viral factors associated with HCC are identified, host genetic variations also affect the long-term outcome of CHB. Although research on the natural history of CHB has made remarkable progress, host genetic factors associated with disease progression of CHB have not well addressed yet [74]. In a large cohort study, including patients with CHB, a single nucleotide polymorphism (SNP) (rs12979860) in the intronic region of interferon-λ4 is a strong predictor of hepatic fibrosis progression [75].
Through genome-wide association studies, two SNPs, rs3077 near HLA-DPA1 region and rs9277535 near HLA-DPB1 region, have been shown to be associated with HBV persistence after acute HBV infection [76]. Our case-control study demonstrated that HBeAg-negative patients with rs9277535 non-GG genotype had a higher likelihood of spontaneous HBsAg seroclearance (Odds ratio: 1.83, 95% confidence interval: 1.04-3.21, P =0.034) [77].
Sodium taurocholate cotransporting polypeptide (NTCP) has been identified as either the receptor or part of the receptor complex for HBV infection [78]. NTCP is encoded by the SLC10A1 gene, locates on chromosome 14. Recently, the association between SNP, the p.Ser267Phe (S267F) variant (rs2296651), on NTCP and HBV related cirrhosis and HCC has been determined. A genetic association study in a large cohort of patients with CHB in Taiwan demonstrated that S267F was significantly associated with decreased risk for cirrhosis and HCC. In addition, patients with undetectable HBV DNA and the GA or AA genotypes of S267F variant had a 25-fold decreased risk of developing HCC than patients with high viremia who carried the GG genotype[79]. Therefore, the S267F variant may serve as a genetic marker to identify CHB patients with very low risk of developing HCC.
Epigenetic change, the DNA methylation, reflects an interaction between genetic background and cellular microenvironment. DNA methylation might also contribute to the downregulation of gene expression, which may link to liver disease progression [80]. Recently, through hepatic genome-wide methylation profiling, hypermethylation of HOXA2 and HDAC4 along with hypomethylation of PPP1R18 were identified and significantly linked to severe fibrosis in patients with CHB. cytosine-guanine dinucleotide methylation within HOXA2, PPP1R18 and HDAC4 genes might serve as genetic markers for progression of CHB [81].
CONCLUSIONS
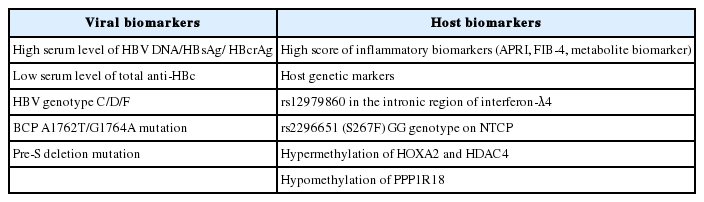
Over the past decade, through meticulous investigations on the molecular epidemiology of HBV, several hepatitis B viral biomarkers, including HBV viral load, quantitative HBsAg, HBcrAg, anti-HBc levels and HBV genotype/mutants have been shown to affect the long-term outcomes of patients with chronic HBV infection. In addition, the role of host factors in HBV-related liver disease progression, such as liver inflammation status, genetic variation and epigenetically modified genes have also been increasingly recognized. On the basis of these emerging data (Table 1), patients with CHB should be evaluated and monitored with relevant viral and host biomarkers to identify those who have a higher risk of liver disease progression and receive timely effective antiviral therapy.
Notes
Funding support: This work was supported by grants from the National Taiwan University Hospital, the Ministry of Health and Welfare, and the Ministry of Science and Technology, Executive Yuan, Taiwan.
Conflicts of Interest: The authors have no conflicts to disclose.
Abbreviations
ALT
alanine aminotransferase
APRI
aspartate aminotransferase to platelet ratio index
BCP
basal core promoter
CHB
chronic hepatitis B
ERADICATE-B
Elucidation of risk factors for disease control or advancement in Taiwanese hepatitis B carriers
HBcAg
hepatitis B core antigen
HBcrAg
Hepatitis B virus core-related antigen
HBeAg
hepatitis B e antigen
HBsAg
Hepatitis B surface antigen
HBV
Hepatitis B virus
HCC
hepatocellular carcinoma
NTCP
Sodium taurocholate cotransporting polypeptide
REVEAL-HBV
Risk evaluation of viral load elevation and associated liver disease/cancer-hepatitis B virus
SNP
single nucleotide polymorphism