Preemptive antiviral therapy with entecavir can reduce acute deterioration of hepatic function following transarterial chemoembolization
Article information
Abstract
Background/Aims
Hepatic damage during transarterial chemoembolization (TACE) is a critical complication in patients with hepatitis B virus (HBV)-related hepatocellular carcinoma (HCC). Apart from its role in preventing HBV reactivation, there is some evidence for the benefits of preemptive antiviral therapy in TACE. This study evaluated the effect of preemptive antiviral therapy on acute hepatic deterioration following TACE.
Methods
This retrospective observational study included a prospectively collected cohort of 108 patients with HBV-related HCC who underwent TACE between January 2007 and January 2013. Acute hepatic deterioration following TACE was evaluated. Treatment-related hepatic decompensation was defined as newly developed encephalopathy, ascites, variceal bleeding, elevation of the bilirubin level, prolongation of prothrombin time, or elevation of the Child-Pugh score by ≥2 within 2 weeks following TACE. Univariate and multivariate analyses were conducted to identify factors influencing treatment-related decompensation. Preemptive antiviral therapy involves directing prophylaxis only toward high-risk chronic hepatitis B patients in an attempt to prevent the progression of liver disease. We regarded at least 6 months as a significant duration of preemptive antiviral treatment before diagnosis of HCC.
Results
Of the 108 patients, 30 (27.8%) patients received preemptive antiviral therapy. Treatment-related decompensation was observed in 25 (23.1%) patients during the follow-up period. Treatment-related decompensation following TACE was observed more frequently in the nonpreemptive group than in the preemptive group (29.5% vs. 6.7%, P=0.008). In the multivariate analysis, higher serum total bilirubin (Hazard ratio [HR] =3.425, P=0.013), hypoalbuminemia (HR=3.990, P=0.015), and absence of antiviral therapy (HR=7.597, P=0.006) were significantly associated with treatment-related hepatic decompensation.
Conclusions
Our findings suggest that preemptive antiviral therapy significantly reduces the risk of acute hepatic deterioration. Preventing hepatic deterioration during TACE by applying such a preemptive approach may facilitate the continuation of anticancer therapy and thus improve long-term outcomes.
INTRODUCTION
Hepatocellular carcinoma (HCC) is the fifth most common cancer in the world and the most common primary liver cancer [1]. There are a variety of therapies for treatment of HCC; among them, transarterial chemoembolization (TACE) is one of the most commonly used treatment modalities [2]. TACE induces ischemic tumor necrosis by obstruction of hepatic artery blood flow and exerts an anticancer effect via chemotherapeutic agents such as adriamycin or cisplatin mixed with Lipiodol. In the American Association for the Study of Liver Diseases (AASLD) guidelines for the management of HCC, TACE is recommended for patients with intermediate-stage HCC according to the Barcelona Clinic Liver Cancer (BCLC) staging system, because TACE was found to improve survival compared with the best supportive care in patients with unresectable HCC [3-5]. Although TACE is the established standard of care only for intermediate-stage HCC, in recent years, TACE has been used widely even in treatment of advanced-stage HCC [6-9]. The most frequent side effects of TACE are fever, nausea, and abdominal pain, and these side effects are self-limiting in the majority of patients. However, acute deterioration of hepatic function following TACE is a potentially life-threatening complication that occasionally interferes with continuation of TACE in patients with HCC [10]. Although TACE has marked direct antitumor effects, it can also result in more complications than conservative management, because the ischemic damage caused by TACE can influence not only tumor tissue but non-tumorous liver tissue.
Antiviral therapy against hepatitis B virus (HBV) is known to reduce the incidence of hepatic decompensation and delay clinical progression in patients with advanced fibrosis or cirrhosis [11]. In particular, entecavir, one of the nucleot(s)ide analogues, is a potent inhibitor of HBV replication. It is known that preemptive antiviral therapy prevents the reactivation of HBV in patients undergoing TACE [12-14]. Apart from its role in preventing HBV reactivation, there are limited data on the benefits of preemptive antiviral therapy in deterioration of hepatic function related to TACE. This study aimed to evaluate the effect of preemptive antiviral therapy with entecavir on acute deterioration of hepatic function following TACE.
MATERIAL AND METHODS
Study population
This is a retrospective observational study of 108 patients in a prospectively collected cohort with HBV-related HCC who underwent TACE at the Catholic university of Korea Incheon St. Mary’s hospital between January 2007 and January 2013 (Fig. 1). Primary HCC was diagnosed in 561 patients in our institution. Patients who met the following criteria were entered into the study: age ≥ 18 years; hepatitis B surface antigen positive for at least 6 months; Child-Pugh Class A or B status; Barcelona Clinic Liver Cancer stage 0, A, or B HCC; treated with TACE; and antiviral therapy using entecavir 0.5 mg or no antiviral therapy. Patients were excluded for the following reasons: the presence of other liver diseases, including chronic hepatitis C, alcoholic liver disease, or autoimmune hepatitis (n=199); Child-Pugh class C status (n=43); BCLC stage C or D (n=137); other treatment for HCC including surgery, radiofrequency ablation, percutaneous ethanol injection, liver transplantation, or radiotherapy (n=50); mutation of HBV (n=3); or other antiviral therapy (n=21). Therefore, the study cohort consisted of 108 patients treated with TACE for HBV-related BCLC stage 0-B HCC, of whom 30 were given entecavir 0.5 mg prior to TACE. The diagnosis of HCC was done on the basis of the AASLD practice guidelines [3]. The initiation of antiviral therapy was generally based on the Korean Association for the Study of the Liver guidelines [15] and the Korean national insurance policy: HBV DNA≥2,000 IU/mL and serum aminotransferase level above upper limit normal. We regarded at least 6 months as a significant duration of preemptive antiviral treatment before diagnosis of HCC. This study was approved by the local ethical committee and Institutional Review Board of the Catholic University of Korea.
TACE procedure and follow-up
TACE was performed by injection of doxorubicin hydrochloride (50 mg/session) and iodized oil (Lipiodol Ultra Fluid; Guerbet, France). After the first TACE session, follow-up TACE sessions were performed every 2 months until CT scans suggested complete tumor remission and TACE showed no more hypervascular staining of the tumor. After TACE, liver and renal function tests, including albumin, total bilirubin, aspartate transaminase, alanine transaminase, prothrombin time-international normalized ratio, and creatinine levels were monitored at least every 3 days. CT scans were performed every 2 months to evaluate treatment response and progression of liver cirrhosis.
Assessment of outcomes
The primary endpoint was development of treatment-related deterioration of hepatic function after TACE for HBV-related HCC. Secondary endpoints included development of HBV reactivation, hepatitis, severe hepatitis, or ≥grade 3 common toxicity criteria of hepatic measures, including alanine aminotransferase, aspartate aminotransferase, and blood bilirubin. Acute deterioration of hepatic function was defined as newly developed encephalopathy, ascites, variceal bleeding, hepatorenal syndrome, a bilirubin level more than 2.5 times the upper normal limit, prolongation of prothrombin time by more than 3 seconds, or an elevation of the Child-Pugh score (≥2) within 2 weeks following TACE [10,16,17]. HBV reactivation was defined as a greater than 10-fold increase in serum HBV DNA from nadir during antiviral therapy. Hepatitis and severe hepatitis were defined as AST or ALT scores that were more than 2 times and 5 times the upper normal limits, respectively. Hepatic common toxicity criteria were based upon the common terminology criteria (CTC) for adverse events version 4.0 [18].
Statistical analyses
Categorical variables are described as frequencies and percentages. Continuous variables are presented as means ± standard deviations (SD) and medians with interquartile ranges for parametric and non-parametric variables, respectively. Baseline characteristics were analyzed using the χ2 test for categorical variables and Student’s t test for continuous variables. Cox proportional hazards models were used to assess the risk factors for treatment-related decompensation after TACE. The variables used for multivariate analysis were selected on the basis of statistical significance in the univariate analysis (P<0.10). Multivariate analysis was performed using a forward conditional stepwise procedure to exclude confounding variables. Kaplan-Meier curves and a log-rank test were used to estimate the cumulative incidence of treatment-related decompensation. A P-value less than 0.05 on a two-tailed test was regarded as statistically significant. Statistical analyses were carried out using the Statistical Package for the Social Sciences version 16.0 for Windows (SPSS, Inc., Chicago, IL, USA).
RESULTS
Characteristics of the study population
The clinical characteristics of the patients at the time of diagnosis of HCC are shown in Table 1. The study population was comprised of 108 patients who met the eligibility criteria, 30 of whom received antiviral therapy (preemptive group; entecavir 0.5mg) and 78 of whom did not receive antiviral therapy (non-preemptive group) prior to TACE. The mean age of the 108 patients was 57.6 years (SD: 9.6 years), and 84 (77.8%) were male. In baseline liver function, 84 patients (77.8%) were Child-Pugh class A and 24 patients (22.2%) were class B. Regarding tumor characteristics, 23 patients (21.3%) were BCLC stage 0, 45 patients (41.7%) were stage A, and 40 patients (37.0%) were stage B. There were no significant differences in age, gender, HBeAg positivity, HBV DNA levels, total bilirubin, ALT, creatinine, α-fetoprotein, total tumor size, Child-Pugh class, and BCLC stage of HCC between the two groups. However, patients in the preemptive group had significantly lower serum albumin levels (3.6 vs. 3.8 mg/dL, P=0.036) and platelet counts (102 vs. 136 ×103/mm3, P=0.008), and higher levels of PT INR (1.23 vs. 1.13, P=0.008).
Incidence of acute hepatic deterioration after TACE
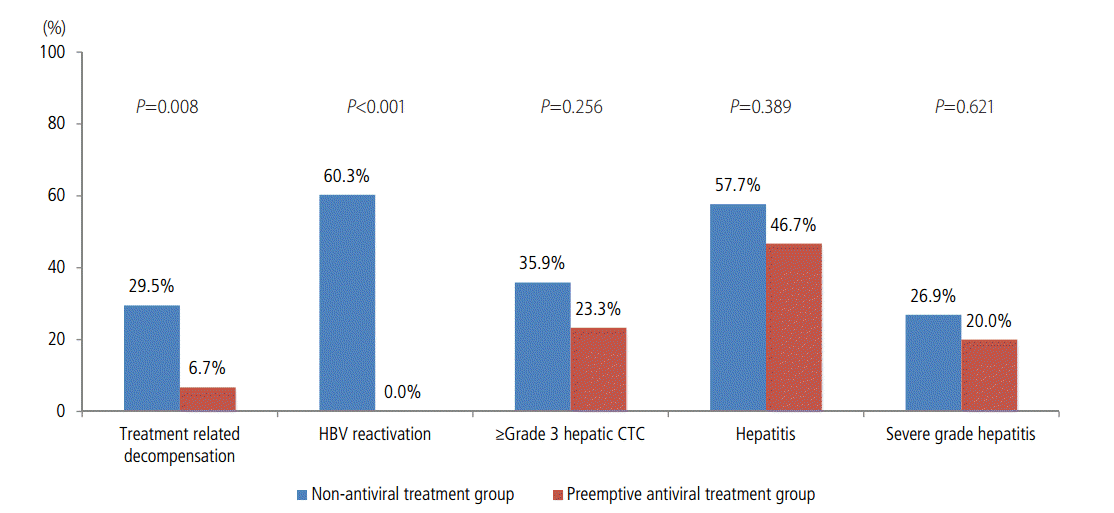
Among all 108 patients, 25 (23.1%) suffered from treatment-related decompensation. The rate of decompensation was significantly lower in the preemptive group than in the non-preemptive group (6.7% vs. 29.5%, P=0.008). HBV reactivation occurred more frequently in the non-preemptive group than in the preemptive group (60.3% vs. 0.0%, P<0.001). Hepatic CTC for adverse events grade 3 or 4, hepatitis, and severe-grade hepatitis following TACE developed in 35 (32.4%), 59 (54.6%), and 27 (25.0%) patients, respectively. However, there were no significant differences in the hepatic CTC for adverse events grade 3 or 4 (P=0.245), hepatitis (P=0.317), and severe-grade hepatitis (P=0.485) following TACE between the preemptive group and the non-preemptive group. Although the results revealed no significant statistical differences, the incidences of hepatic adverse events, hepatitis, and severe-grade hepatitis were lower in the preemptive group than in the non-preemptive group (Fig. 2).
Risk factors for treatment-related decompensation
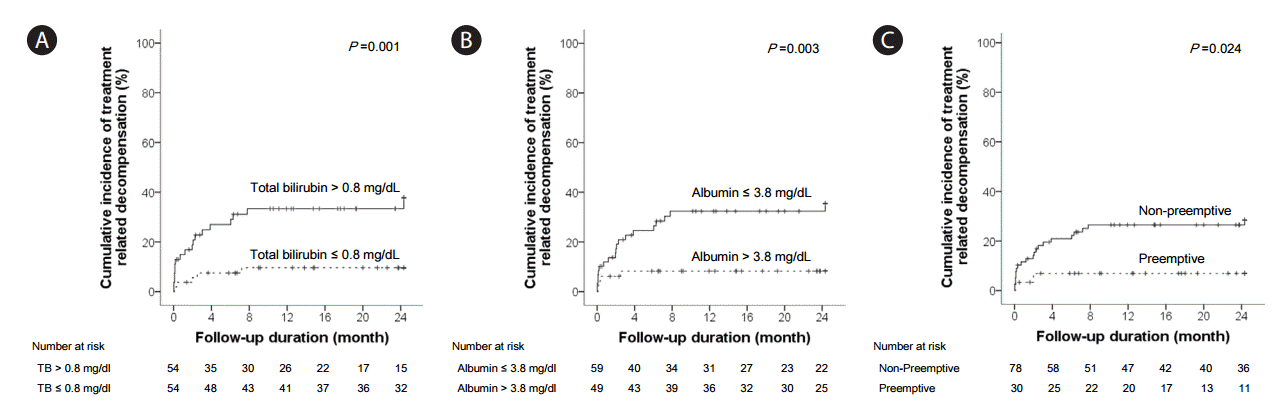
Treatment-related decompensation was observed in 25 patients (23.1%); 3 patients developed ascites, 1 developed hepatic encephalopathy, 7 demonstrated an increase in bilirubin levels, and 21 demonstrated an increase in Child-Pugh score. Univariate analysis was performed to screen the risk factors for treatment-related decompensation (Table 2). The predictive factors included in the univariate analysis were gender, age, HBeAg positivity, HBV DNA levels, Child-Pugh class, total bilirubin, ALT, albumin, creatinine, platelet counts, PT INR, alpha-fetoprotein (AFP) levels, total tumor size, BCLC stage of HCC, and preemptive antiviral therapy. Univariate analysis revealed that treatment-related decompensation was significantly associated with higher levels of total bilirubin (≥0.8 mg/dL, P=0.003), lower levels of albumin (≤3.8 g/dL, P=0.003), and non-antiviral therapy (P=0.040). In multivariate analysis, higher levels of total bilirubin (≥0.8 mg/dL; hazard ratio (HR), 3.425; 95% CI, 1.294-9.066; P=0.013), lower levels of albumin (≤3.8 g/dL; HR, 3.990; 95% CI, 1.307-12.182; P=0.015), and non-antiviral therapy (HR 7.597; 95% CI, 1.768-32.645; P=0.006) were the statistically significant factors predictive of treatment-related decompensation (Table 3). The cumulative incidence of treatment-related decompensation was significantly higher for patients with higher levels of total bilirubin (≥0.8 mg/dL, P=0.001) or lower levels of albumin (≤3.8 g/dL, P=0.001) (Fig. 3A, 3B). Patients who did not receive preemptive antiviral therapy had a higher risk of developing treatment-related decompensation than those who were given preemptive antiviral therapy (P=0.024) (Fig. 3C).
DISCUSSION
Although TACE is one of the most effective treatments for HCC, repeated TACE can affect remnant hepatic function and interfere with the ability to continue treatment in patients with HCC. Furthermore, patients with HBV-related HCC can be affected not only by repeated TACE but also by hepatitis B virus replication. This is because TACE is usually performed with administration of chemotherapeutic agents such as adriamycin or cisplatin. A prospective study showed that preemptive antiviral therapy in patients with HBV-related HCC can reduce the incidence of hepatitis due to HBV reactivation during TACE [12].
This study was conducted to evaluate the effect of preemptive antiviral therapy with entecavir on acute deterioration of hepatic function following TACE. One hundred eight patients with HBV-related HCC treated with TACE were enrolled in this study. A comparison was made between 38 patients who received preemptive antiviral therapy with entecavir (0.5mg/day) and 70 patients who did not in terms of development of acute hepatic deterioration following TACE. This study is distinct from earlier studies in that all patients in the preemptive group received entecavir only, while patients in other studies received various antiviral agents, such as lamivudine, adefovir, dipivoxil, and telbivudine [19,20].
In a retrospective study of 49 patients, Kuzuya et al. compared changes in remnant liver function after curative treatment for HCC according to whether patients received antiviral treatment or not. Remnant hepatic function was significantly better at the time of HCC recurrence in patients who received antiviral therapy [21]. In addition to treating HCC, nucleos(t)ide analogues can markedly inhibit acute deterioration of hepatic function after TACE. Lao et al. [19] found that HBV reactivation after TACE was significantly reduced in patients who received antiviral therapy, and the rate of deterioration of liver function was higher in the HBV reactivation group than in the non-reactivation group. Another study showed that antiviral therapy with lamivudine can improve hepatic function and produce better outcomes after liver resection [22]. In the present study, the cumulative incidence of treatment-related decompensation was found to be 23.1% in all patients. In our study population, the patients in the preemptive group started out with higher levels of serum HBV DNA and significantly worse markers of hepatic function, such as lower levels of albumin, lower platelet counts, and prolonged PT INR. Nevertheless, patients who received preemptive antiviral therapy showed a significantly lower incidence of TACE-related decompensation compared with the non-preemptive group (6.7% vs. 29.5%, P=0.008).
Other studies proposed higher AFP, larger tumor size, and increased ALT levels as factors that influenced the development of complications or deterioration after TACE [10,19,23,24]. However, in the present study, AFP, total tumor size, BCLC stage of HCC, and serum ALT levels had no significant effect on acute deterioration of liver function following TACE. Instead, higher serum total bilirubin and lower serum albumin levels significantly influenced treatment-related hepatic decompensation, as did not receiving preemptive antiviral therapy (Table 3).
We also analyzed the incidence of acute hepatic deterioration following TACE as well as the treatment-related decompensation. The incidence of acute hepatic deterioration, including hepatitis, severe hepatitis, and hepatic adverse events of grades 3 or 4 was greater in the non-preemptive group (57.7%, 26.9%, and 35.9%, respectively) than in the preemptive group (46.7%, 20.0%, and 23.3%), although the difference was not statistically significant (P=0.389, P=0.621, and P=0.256). The lack of statistical significance might be due to the small number of patients in the preemptive group rather than to a lack of effect of preemptive antiviral therapy.
The present study has several limitations. First, it was not a randomized controlled study. The study also lacked measures of long-term outcomes of HBV-related HCC treated with TACE. However, it is a post-hoc analysis from a database collected in a prospective manner. All patients received only TACE with a fixed dose of doxorubicin using the same technique in a single center, and patients in the preemptive group received entecavir only. The nature of this study population represents, therefore, a homogeneous and indicates that confounding factors behind management were significantly minimized. Reduction with antivirals in acute hepatic deterioration following TACE is likely to influence long-term clinical course. Future studies with larger numbers of patients and longer follow-up periods are necessary to further elucidate these findings.
In conclusion, our results demonstrated that preemptive antiviral therapy significantly reduces the risk of acute deterioration in hepatic function following TACE. Thus, preemptive antiviral therapy should be administered to patients undergoing TACE. Prevention of hepatic deterioration during TACE with such a preemptive approach may facilitate continuing anti-cancer therapy and thus potentially improve long-term outcomes.
Acknowledgements
The authors thank Dr. Won Sohn for his insightful and valuable comments during the preparation of this manuscript.
Notes
Funding support: This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2016R1D-1A1B03930457).
Conflicts of Interest: The authors have no conflicts to disclose.
Abbreviations
AASLD
American Association for the Study of Liver Diseases
ALT
alanine aminotransferase
AST
aspartate aminotransferase
AFP
alpha-fetoprotein
BCLC
Barcelona Clinic Liver Cancer
CTC
common terminology criteria
HBV
hepatitis B virus
HCC
hepatocellular carcinoma
HR
hazard ratio
SD
standard deviation
TACE
transarterial chemoembolization
HBeAg
hepatitis B envelope antigen