| Clin Mol Hepatol > Volume 22(4); 2016 > Article |
|
ABSTRACT
Background/Aims
This study aimed to evaluate the efficacy and safety of emergency variceal ligation for the prevention of rebleeding in cirrhotic patients who are found on initial endoscopy to have blood clots in the stomach but no actively bleeding esophageal and gastric varices or stigmata.
Methods
This study included 28 cirrhotic patients who underwent emergency prophylactic EVL and 41 who underwent an elective intervention between January 2009 and June 2014. Clinical outcomes were analyzed, including the rebleeding, 6-week mortality, and rebleeding-free survival rates.
Results
The rebleeding rate was higher in the emergency than in the elective group (28.6% vs. 7.3%, P=0.041). Multivariate analysis showed that emergency prophylactic EVL (odds ratio [OR] = 7.4, 95% confidence interval [CI]=1.634.8, P=0.012) and Child-Pugh score C (OR=10.6, 95% CI=1.4-80.8, P=0.022) were associated with rebleeding. In the emergency group, the gastric varices were associated with rebleeding (OR=12.0, 95% CI=1.7-83.5, P=0.012).
Cirrhosis-associated gastroesophageal variceal bleeding is a major complication of portal hypertension [1]. Gastroesophageal varices are present in approximately half of patients with cirrhosis; these varices have been estimated to develop in about 8% of cirrhotic patients without varices per year [2]. The yearly rate of first gastroesophageal variceal bleeding in patients with cirrhosis is 12% [3], and survivors of an episode of active bleeding have a 60% risk of recurrent bleeding within 1 year [4]. The risk of rebleeding is greatest immediately after cessation of active bleeding, then declines, reaching close to baseline at 6 week [5]. The 6-week mortality rate following each episode of variceal bleeding is approximately 10-20% [6].
The diagnostic criteria of acute variceal bleeding include: (1) endoscopic detection of active bleeding from a varix; (2) the finding of a sign of recent variceal bleeding, including a white nipple; and (3) the presence of varices and recent hemorrhagic sign without any other source [7]. Emergency endoscopic intervention at the initial diagnostic endoscopy is considered as gold standard for the management of acute variceal bleeding [8]. Endoscopic variceal ligation (EVL) is the preferred therapy when esophageal varices are the cause of bleeding, whereas endoscopic variceal obturation (EVO) with tissue adhesive is recommended for acute bleeding from type 2 gastroesophageal varices and isolated gastric varices [9]. However, the optimal treatment has not been determined in these situations diagnosed as acute variceal bleeding when esophageal and/or gastric varices have no sign of bleeding and the recent hemorrhagic sign is present.
Patients who survive after an episode of variceal bleeding should be started on treatment to prevent rebleeding as soon as possible, after a period of at least 24 hours without evidence of bleeding [2]. Emergency prophylactic EVL is a reasonable method for reducing the rebleeding rate after an episode of acute variceal bleeding, defined as the presence of varices without any other source of bleeding when blood clots present in the stomach. Elective endoscopic intervention after emergency endoscopy requires an additional endoscopy, increasing medical costs to patients. If emergency prophylactic EVL does not have a negative impact on patient safety and the quality of medical care, it may be useful in optimizing medical resources. This study, therefore compared outcomes of emergency prophylactic EVL with elective intervention during second-look endoscopy and aimed to suggest an optimal timing of preventive intervention.
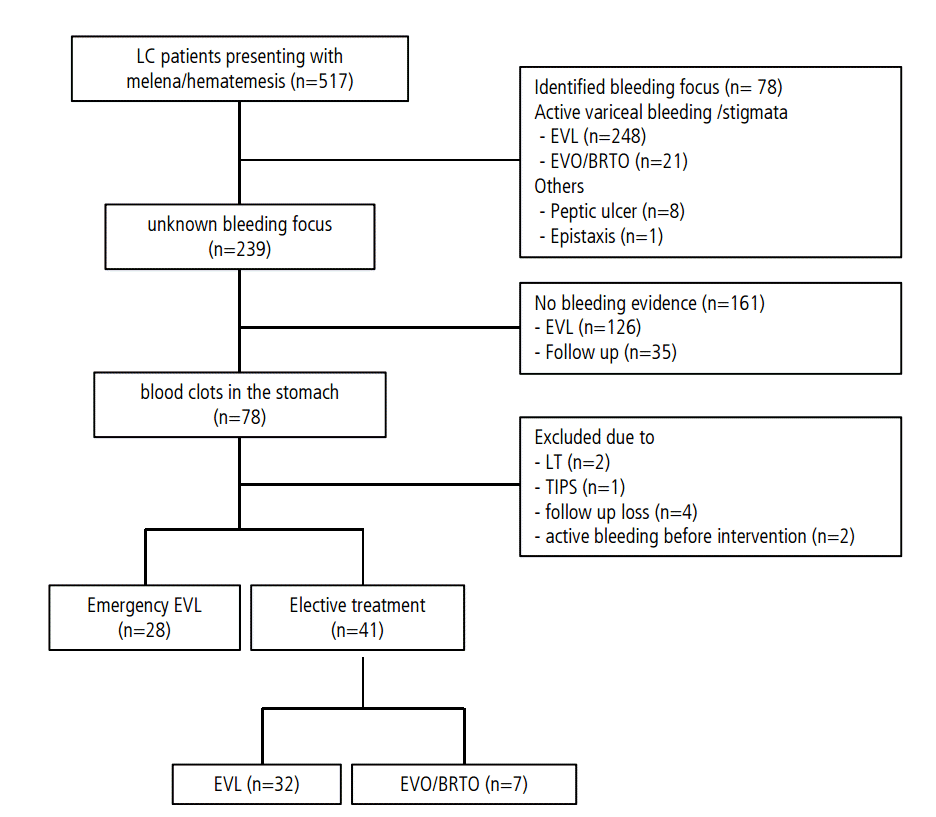
Between January 2009 and June 2014, 517 cirrhotic patients presenting with melena or hematemesis visited Pusan National University Yangsan Hospital. Patients were excluded if they (1) had active variceal bleeding or stigmata indicating recent bleeding, (2) had undergone liver transplantation or placement of a transjugular intrahepatic portosystemic shunt within 6 week of variceal bleeding, (3) had active variceal bleeding before elective prophylactic intervention, (4) had non variceal bleeding, or (5) were lost to follow up within 6 week after the intervention. Among the patients eligible for inclusion, 28 underwent emergency prophylactic EVL, and 41 underwent elective intervention, including EVL, EVO or balloon-occluded retrograde transvenous obliteration (BRTO) (Fig. 1). All procedures were performed by three endoscopists, each with >5 years of experience in therapeutic endoscopy, and the treatment strategy for each patient was at the discretion of the endoscopist. The medical records of all 69 patients were retrospectively reviewed, and the outcomes were compared between the two groups. The study was approved by the ethics committee of Pusan National University Yangsan Hospital and was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all patients.
All patients with bleeding from gastroesophageal varices received vasoactive drugs (terlipressin/somatostatin) and intravenous antibiotics (ceftriaxone) when variceal bleeding was suspected. Blood was transfused to maintain hemoglobin concentrations at 7-8 g/dL. All patients underwent upper endoscopy within 12 hours of presentation to identify the source of bleeding, unless the patient had severe hemodynamic instability, or refused consent. Propranolol was administered to prevent recurrent bleeding if patients had no contraindications to this agent. After full examination of the esophagus, stomach and duodenum, endoscopists confirmed the absence of active bleeding and stigmata.
Emergency prophylactic EVL to prevent a variceal rebleeding was performed from distal gastroesophageal varices to proximal 5 cm. Ligation bands were usually applied in a circumferential pattern spiraling gradually up the esophagus. Elective intervention was undertaken either after 1 or 2 days from the emergency endoscopy as second-look endoscopy. If an examination revealed the stigmata of recent bleeding, patients received EVL for esophageal varices or EVO for gastroesophageal varices. If no stigmata were found, prophylactic EVL was performed, except in one patient who underwent BRTO. In the absence of treatment guidelines, the choice of treatment strategy was at the discretion of endoscopists.
Secondary prophylaxis, consisting of a combination of treatment with a non-selective beta-blocker and band ligation, is usually started 6 days after successful hemostasis [6]. The risk of rebleeding declines to near baseline values by 6 week [5]. Therefore, the outcomes of study included rebleeding rates within 6 weeks. Secondary endpoint included 6-weeks overall survival (OS) and rebleeding-free survival. The endoscopic stage of esophageal varices was based on the general rules for recording endoscopic findings established by the Japanese Research Society for Portal Hypertension [10].
Patient demographic and clinical characteristics were compared using the Student’s t test or Mann–Whitney U test for continuous variables and the χ2 test or Fisher’s exact test for categorical variables. Variables with a P value <0.05 on univariate analysis were included in a backward stepwise multiple logistic regression model to identify factors independently associated with rebleeding and 6-week mortality. The null hypotheses of no difference was rejected if P<0.05. All analyses were performed using SPSS version 12.0 (SPSS, Inc., Chicago, IL, USA).
The baseline demographic characteristics of the patients are shown in Table 1. The mean age of patients in the emergency and elective groups were 60.0±10.2 years and 60.2±12.2 years, respectively. There were no significant differences between groups in sex, etiology of liver disease, grade of esophageal varices, Child-Turcotte-Pugh (CTP) classification, model for end-stage liver disease (MELD) score, presence of gastric varices or hepatocellular carcinoma (HCC), portal vein thrombosis, laboratory findings. A follow-up period ranged from days 3 to 5 years.
In the elective group, the stigmata was found during second-look endoscopy in 15 patients, including nine with esophageal and six with gastric varices. EVL or EVO was successful in treating the stigmata. One patient without stigmata underwent BRTO because of large gastric varices and a previous history of gastric variceal bleeding.
Rebleeding events occurred in 11 patients (15.9%, 11/69), with the rate being significantly higher in the emergency than in the elective group (28.6% [8/28] vs. 7.3% [3/41], P=0.041). Four patients in the emergency group experienced gastric variceal bleeding. In addition, three patients in this group experienced esophagael variceal bleeding and one experienced post-EVL site ulcer bleeding. In the elective group, two patients experienced esophageal variceal bleeding and one had a post-EVL site ulcer bleeding.
Multivariate analysis showed that emergency prophylactic EVL to treat acute variceal bleeding (odds ratios [OR] 7.4; 95% confidence interval [CI], 1.6-34.8; P=0.012) and advanced liver cirrhosis (CTP grade C; OR 10.6; 95% CI, 1.4-80.8; P=0.022) were associated with an increased risk of rebleeding (Table 2). In the emergency group, multivariate analysis found that the presence of gastric varices was an independent risk factor for rebleeding (OR 12.0; 95% CI, 1.7-83.5; P=0.012).
Five patients (17.9%) in the emergency group and three (7.3%) in the elective group died within 6 weeks after the intervention. In the emergency group, three patients died of gastric variceal bleeding, one died of the hepatorenal syndorne, and one died of the progression of HCC. In the elective group, two patients died of the hepatorenal syndrome, and one died of HCC rupture. The 6-week OS rate was similar in the emergency and elective groups (82.1% vs. 92.7%, P=0.169) (Fig. 2A), as well as being similar in these two groups with (70.0% vs. 93.3%, P=0.104) and without (88.9% vs. 92.3%, P=0.739) gastric varices. The 1-year survival rates also showed no significant difference between two groups (75.0% vs. 86.9%, P=0.129). Multivariate analysis showed that the rebleeding (OR 23.1; 95% CI, 2.4-239.3; P=0.008) and the high serum creatinine concentration (>1.5 µmoL/L; OR 27.1; 95% CI, 2.6-284.2; P=0.006) were significant risk factors for 6-week mortality (Table 3).
The 6-week rebleeding-free survival rates were similar in the emergency and elective groups (67.9% vs. 85.4%, P=0.079) (Fig. 2A). In patients with gastric varices, however, the 6-week rebleeding-free survival rate was significantly lower in the emergency than in the elective group (40.0% vs. 93.3%, P=0.002). However, these rates were similar in patients without gastric varices (83.3% vs. 80.8%, P=0.733) (Fig. 2B).
This study shows that; In initial endoscopy, if the blood clots are present in the stomach without active bleeding or stigmata, the rebleeding rate in patients receiving emergency EVL was significantly higher than in patinets who receiving elective intervention during second-look endoscopy. The total rebleeding rate was lower than that of previous studies, in which rebleeding rates ranged from 30% to 37% [11,12]. Our study excluded patients with active variceal bleeding or recent bleeding stigmata. Thus, included patients had less severe liver disease than other studies, which may explain the lower rebleeding rate of our study.
Emergency EVL was identified as a risk factor for rebleeding when compared with elective intervention. In patients who underwent emergency EVL, the presence of gastric varices was an independent risk factors for rebleeding. The percentage of patients with gastric varices was similar in the emergency and elective groups. In elective group, six patients underwent elective EVO for gastric varices due to the presence of stigmata during second-look endoscopy and one underwent BRTO due to a large gastric varix. In emergency group, blood clots interfered the endoscopic view during the emergency endoscopic exam, which might lead to missing the stigmata of gastric varices. In addition, endoscopists tends to perform EVL after searching the stigmata for short time in stressful situation like emergency setting. This can also result in increase of the chance to miss the stigmata of the esophageal varices (Fig. 3). Our study also found that advanced liver cirrhosis (CTP grade C) was associated with the rebleeding within 6 week. Previous reports indicated that rebleeding was associated with CTP classification and other factors related to the portal hypertension including gastric varices, high bilirubin and creatinine serum level, low albumin serum level, infection [13-17]. The severity of liver cirrhosis like CTP score C are mostly associated with the portal hypertension. Concerning about the higher proportion of patients with HCC and/or PVT (33 patients among 69 patients), the presence of HCC and/or PVT showed no association with rebleeding. This result is correlated with previous study to evaluate independent factors associated with recurrent bleeding in cirrhotic patients [18].
The most important concern regarding bleeding episodes is 6-week mortality. In this study, 6-week OS rates were similar in the emergency and elective groups. Rebleeding was found to be significantly associated with death within 6 week, therefore, 6-week mortality rate was suggested as the primary endpoint in treatment of patients with acute variceal bleeding [15,19,20]. In addition, CTP classification, MELD score, shock, renal failure, infection, HCC, active bleeding during endoscopy, portal vein thrombosis and HVPG >20 mmHg have been known as significant predictors for death [21-26]. Multivariate analysis that included these factors found that not only the rebleeding but also the high creatinine level were a risk factor for 6-week mortality. Renal failure is a severe complication in cirrhotic patients and has been associated with poor patient prognosis [27,28]. Our finding, that CTP classification and MELD score were not risk factors for death, may have been due to our inclusion of patients with less severe liver disease than other studies.
We also found that the 6-week rebleeding-free survival rates were not significantly different in the emergency and elective groups. However, the 6-week rebleeding-free survival rate was significantly lower in the emergency than in the elective group in subgroup with gastric varices. Seven patients with gastric varices experienced rebleeding episodes, with four (57.1%) having gastric variceal bleeding, and three of them (75.0%) dying of hypovolemic shock. Although gastric varices have a lower risk of bleeding than esophageal varices, gastric varices are associated with more severe blood loss and a higher mortality rate than esophageal varices [29-31]. Elective intervention during second-look endoscopy therefore should be considered as a treatment strategy when clots interfere with the accurate assessment of gastric varices. The 6-week rebleeding-free survival was similar in subgroups without gastric varices. All patients in this subgroup achieved successful bleeding control [6]. These results suggest that, in the absence of gastric varices, esophageal varices may be the cause of bleeding in patients with blood clots in the stomach. Considering two patients who experienced active esophageal variceal bleeding before elective intervention, prophylactic EVL during emergency endoscopy can reduce rebleeding before elective intervention. In addition, the intervention without additional endoscopy can optimize medical resource. Although peptic ulcer bleeding may occur under retained blood, gastric ulcers are usually located at the angular incisura, followed by the lesser curvature and antrum which endoscopic visualization is less impaired by blood clot in the stomach. Therefore, emergency EVL can be considered as a treatment option when clots are present in the stomach without gastric varices.
The present study is limited by its retrospective design, its small number of patients, and its inclusion of patients from a single institution. The retrospective design did not enable full evaluation of possible confounding factors such as the HVPG, which is an expensive and invasive procedure although it is significantly prognostic of cirrhosis. Small sample size may have influenced the interpretation of the differences in rebleeding and mortality rates. Second, the choice of emergency EVL or elective treatment after emergency study was at the discretion of endoscopists. This can cause selection bias, furthermore, it can be speculated that patients with poor condition tend to receive the emergency EVL. However, there are no significant difference in the baseline characteristics of patients including hemoglobin and CTP classification. Endoscopists usually performed emergency EVL when the next day was a weekend or holiday. Therefore, the number of patients who received the elective treatment after emergency endoscopy was higher than that of the emergency EVL group. Ideally, a randomized control study is needed to overcome these limitations.
Despite these limitations, this study demonstrated that emergency EVL can increase the risk of rebleeding compared with elective intervention when blood clots in the stomach without active esophageal and gastric variceal bleeding or stigmata is present. Therefore, elective interventions should be considered as a preventive measure. Emergency EVL can be a treatment option to prevent the rebleeding if the emergency endoscopy reveals no gastric varix.
Figure 2.
(A) Results of Kaplan-Meier analyses of 6-week overall survival and rebleeding-free survival curves. (B) Results of Kaplan-Meier analyses of 6-week rebleeding-free survival in patients with or without gastric varices (GV).
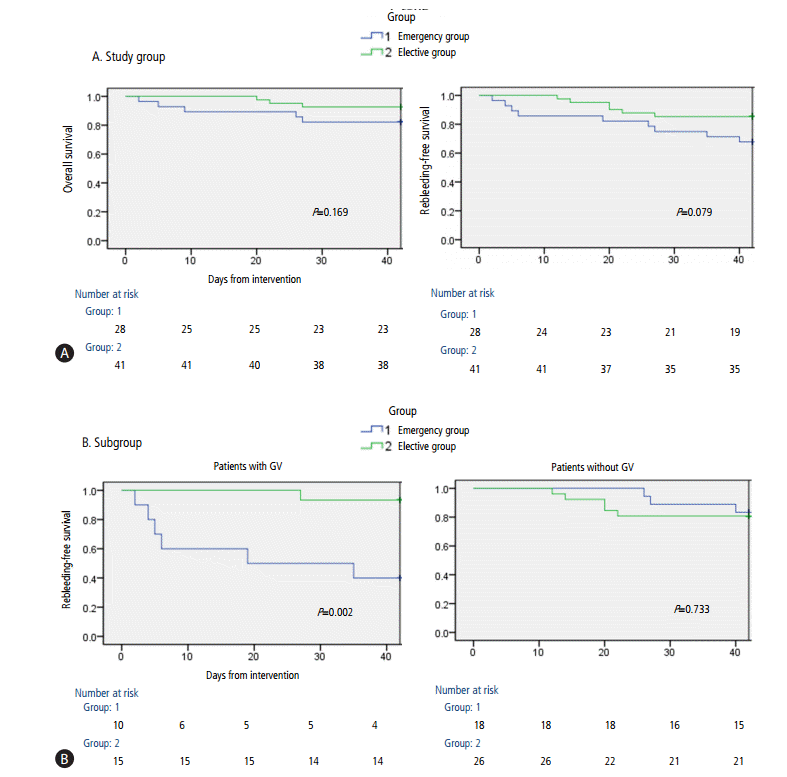
Figure 3.
Case 1. (A) Blood clots covered the GV mucosa. (B) Second-look endoscopy showed stigmata (arrow) of GV. Case 2. (C) Emergency endoscopy showed no evidence of a bleeding focus. Emergency EVL was performed. (D) Active GV bleeding (arrow) was present 5 days later. GV, gastric varices; EVL, endoscopic variceal ligation.

Table 1.
Baseline characteristics of the study subjects
| Emergency group (n=28) | Elective group(n=41) | P-value | |
|---|---|---|---|
| Age, year, mean (SD) | 60.0 (49.8-70.2) | 60.2 (48.0-72.4) | 0.935 |
| Male sex, n (%) | 22 (78.6) | 33 (80.5) | 0.846 |
| Etiology of cirrhosis, n (%) | 0.143 | ||
| Alcohol | 7 (25.0) | 14 (34.1) | |
| Hepatitis B | 15 (53.6) | 23 (56.1) | |
| Hepatitis C | 5 (17.9) | 1 (2.4) | |
| Others* | 1 (3.6) | 3 (7.3) | |
| EV grade, n (%) | 0.321 | ||
| F1 | 1 (3.6) | 6 (14.6) | |
| F2 | 13 (46.4) | 16 (39.0) | |
| F3 | 14 (50.0) | 19 (46.3) | |
| CTP classification, n (%) | 0.327 | ||
| A | 15 (53.6) | 16 (39.0) | |
| B | 8 (28.6) | 19 (46.3) | |
| C | 5 (17.9) | 6 (14.6) | |
| MELD score, mean (SD) | 13.3 (8.3-18.3) | 13.9 (5.7-22.1) | 0.758 |
| GV presence, n (%) | 10 (35.7) | 15 (36.6) | 0.941 |
| HCC presence, n (%) | 13 (46.4) | 20 (48.8) | 0.848 |
| PVT presence, n (%) | 15 (53.6) | 28 (70.0) | 0.167 |
| Hemoglobin (g/dL)±SD | 8.5±1.1 | 8.1±1.8 | 0.246 |
| Platelet count (×103/μL)±SD | 106.57±88.14 | 94.90±51.44 | 0.961 |
| Albumin (g/dL)±SD | 3.2±0.5 | 3.0±0.5 | 0.278 |
| Prothrombin time (INR)±SD | 1.4±0.2 | 1.8±1.9 | 0.191 |
| Creatinine (μmoL/L)±SD | 1.3±0.9 | 0.9±0.4 | 0.053 |
| Total bilirubin (mg/dL)±SD | 1.5±1.0 | 2.3±2.2 | 0.409 |
| Hx of variceal bleeding, n (%) | 13 (46.4) | 20 (48.8) | 0.848 |
Table 2.
Results of univariate and multivariate analyses of factors associated with rebleeding
OR, odds ratio; CI, confidence interval; EVL, endoscopic variceal ligation; EV, esophageal varices; CTP, child-turcotte-pugh; GV, gastric varices; HCC, hepatocellular carcinoma; PVT, portal vein thrombosis; MELD, model for end-stage liver disease; Cr, creatinine; Bil, bilirubin; PT, prothrombiin time; Hx, history.
Table 3.
Results of univariate and multivariate analyses of factors associated with 6-week mortality
OR, odds ratio; CI, confidence interval; EVL, endoscopic variceal ligation; EV, esophageal varices, CTP, child-turcotte-pugh; GV, gastric varices; HCC, hepatocellular carcinoma; PVT, portal vein thrombosis; MELD, model for end-stage liver disease; Cr, creatinine; Bil, bilirubin; PT, prothrombiin time; Hx, history.
REFERENCES
1. Garcia-Tsao G, Bosch J. Management of varices and variceal hemorrhage in cirrhosis. N Engl J Med 2010;362:823-832.


2. Garcia-Tsao G, Sanyal AJ, Grace ND, Carey W; Practice Guidelines Committee of AASLD; Practice Parameters Committee of the ACG. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology 2007;46:922-938.


3. D’Amico G, Pagliaro L, Bosch J. Pharmacological treatment of portal hypertension: an evidence-based approach. Semin Liver Dis 1999;19:475-505.


6. de Franchis R; Baveno V Faculty. Revising consensus in portal hypertension: report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol 2010;53:762-768.


7. de Franchis R, Pascal JP, Ancona E, Burroughs AK, Henderson M, Fleig W, et al. Definitions, methodology and therapeutic strategies in portal hypertension. A Consensus Development Workshop, Baveno, Lake Maggiore, Italy, April 5 and 6, 1990. J Hepatol 1992;15:256-261.


8. Triantos C, Kalafateli M. Endoscopic treatment of esophageal varices in patients with liver cirrhosis. World J Gastroenterol 2014;20:13015-13026.



9. Kahloon A, Chalasani N, DeWitt J, Liangpunsakul S, Vinayek R, Vuppalanchi R, et al. Endoscopic therapy with 2-octyl-cyanoacrylate for the treatment of gastric varices. Dig Dis Sci 2014;59:2178-2183.


10. Beppu K, Inokuchi K, Koyanagi N, Nakayama S, Sakata H, Kitano S, et al. Prediction of variceal hemorrhage by esophageal endoscopy. Gastrointest Endosc 1981;27:213-218.


11. Altamirano J, Zapata L, Agustin S, Muntaner L, González-Angulo A, Ortiz AL, et al. Predicting 6-week mortality after acute variceal bleeding: role of Classification and Regression Tree analysis. Ann Hepatol 2009;8:308-315.


12. Lee JY, Lee JH, Kim SJ, Choi DR, Kim KH, Kim YB, et al. Comparison of predictive factors related to the mortality and rebleeding caused by variceal bleeding: child-pugh score, MELD score, and rockall score. Korean J Hepatol 2002;8:458-464.
13. D’Amico G, Pagliaro L, Bosch J. The treatment of portal hypertension: a meta-analytic review. Hepatology 1995;22:332-354.


14. Goulis J, Armonis A, Patch D, Sabin C, Greenslade L, Burroughs AK. Bacterial infection is independently associated with failure to control bleeding in cirrhotic patients with gastrointestinal hemorrhage. Hepatology 1998;27:1207-1212.


15. D’Amico G, Luca A. Natural history. Clinical-haemodynamic correlations. Prediction of the risk of bleeding. Baillieres Clin Gastroenterol 1997;11:243-256.


16. Wang MT, Liu T, Ma XQ, He J. Prognostic factors associated with rebleeding in cirrhotic inpatients complicated with esophageal variceal bleeding. Chin Med J (Engl) 2011;124:1493-1497.

17. Zhao JR, Wang GC, Hu JH, Zhang CQ. Risk factors for early rebleeding and mortality in acute variceal hemorrhage. World J Gastroenterol 2014;20:17941-17948.



18. Lee SW, Lee TY, Chang CS. Independent factors associated with recurrent bleeding in cirrhotic patients with esophageal variceal hemorrhage. Dig Dis Sci 2009;54:1128-1134.


19. Sempere L, Palazón JM, Sánchez-Payá J, Pascual S, de Madaria E, Poveda MJ, et al. Assessing the short- and long-term prognosis of patients with cirrhosis and acute variceal bleeding. Rev Esp Enferm Dig 2009;101:236-248.


20. de Franchis R; Baveno VI Faculty. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol 2015;63:743-752.


21. Abraldes JG, Villanueva C, Bañares R, Aracil C, Catalina MV, Garci A-Pagán JC, et al. Hepatic venous pressure gradient and prognosis in patients with acute variceal bleeding treated with pharmacologic and endoscopic therapy. J Hepatol 2008;48:229-236.


22. Augustin S, Altamirano J, González A, Dot J, Abu-Suboh M, Armengol JR, et al. Effectiveness of combined pharmacologic and ligation therapy in high-risk patients with acute esophageal variceal bleeding. Am J Gastroenterol 2011;106:1787-1795.


23. Augustin S, Muntaner L, Altamirano JT, González A, Saperas E, Dot J, et al. Predicting early mortality after acute variceal hemorrhage based on classification and regression tree analysis. Clin Gastroenterol Hepatol 2009;7:1347-1354.


24. D’Amico G, De Franchis R; Cooperative Study Group. Upper digestive bleeding in cirrhosis. Post-therapeutic outcome and prognostic indicators. Hepatology 2003;38:599-612.


25. Moitinho E, Escorsell A, Bandi JC, Salmerón JM, García-Pagán JC, Rodés J, et al. Prognostic value of early measurements of portal pressure in acute variceal bleeding. Gastroenterology 1999;117:626-631.


26. Moitinho E, Planas R, Bañares R, Albillos A, Ruiz-del-Arbol L, Gálvez C, et al. Multicenter randomized controlled trial comparing different schedules of somatostatin in the treatment of acute variceal bleeding. J Hepatol 2001;35:712-718.


27. Moreau R, Lebrec D. Acute renal failure in patients with cirrhosis: perspectives in the age of MELD. Hepatology 2003;37:233-243.


28. Schepke M, Appenrodt B, Heller J, Zielinski J, Sauerbruch T. Prognostic factors for patients with cirrhosis and kidney dysfunction in the era of MELD: results of a prospective study. Liver Int 2006;26:834-839.


29. Sarin SK, Lahoti D, Saxena SP, Murthy NS, Makwana UK. Prevalence, classification and natural history of gastric varices: a long-term follow-up study in 568 portal hypertension patients. Hepatology 1992;16:1343-1349.


- TOOLS



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print




