| Clin Mol Hepatol > Volume 23(2); 2017 > Article |
|
See the commentary-article "Can metronomic chemotherapy be an alternative to sorafenib in advanced hepatocellular carcinoma?" on page 123.
ABSTRACT
Background/Aims
Metronomic chemotherapy (MET) is frequently administered in comparatively low doses as a continuous chemotherapeutic agent. The aim of this study was to evaluate the feasibility and overall survival (OS) of MET compared to sorafenib for advanced hepatocellular carcinoma (HCC) patients with portal vein tumor thrombosis (PVTT).
Methods
A total of 54 patients with advanced HCC and PVTT who had undergone MET were analyzed between 2005 and 2013. A total of 53 patients who had undergone sorafenib therapy were analyzed as the control group. The primary endpoint of this study was OS.
Results
The median number of MET cycles was two (1-15). The OS values for the MET group and sorafenib group were 158 days (132-184) and 117 days (92-142), respectively (P=0.029). The Cox proportional-hazard model showed that a higher risk of death was correlated with higher serum alpha fetoprotein level (Ōēź400 mg/dL, hazard ratio [HR]=1.680, P=0.014) and Child-Pugh class B (HR=1.856, P=0.008).
Conclusions
MET was associated with more favorable outcomes in terms of overall survival than was sorafenib in patients with advanced HCC with PVTT, especially in patients with poor liver function. Therefore, MET can be considered as a treatment option in patients with advanced HCC with PVTT and poor liver function.
Hepatocellular carcinoma (HCC) is one of the most frequently diagnosed cancer and was the second leading cause of cancer-related death worldwide in 2012 [1]. In patients with very early or early stage HCC, there are currently some applicable curative treatment options, including liver transplantation, resection, or radiofrequency ablation. In patients with intermediate stage HCC, transarterial chemoembolization (TACE) is a treatment option; however, in patients with advanced stage HCC, sorafenib (Nexavar®, Bayer HealthCare, Leverkusen, Germany) is the only applicable treatment option in the Barcelona Clinic Liver Cancer (BCLC) staging classification [2-5]. In patients with advanced HCC with portal vein tumor thrombosis (PVTT) treated with supportive care, the overall survival (OS) was reported to range from two to four months [6], while that in patients with advanced HCC with PVTT treated with sorafenib ranged from six to eight months. However, in patients with Child-Pugh class B and advanced HCC with PVTT treated with sorafenib, the OS ranged from two to three months [7-9]. Because of these poor outcomes and lack of treatment options in advanced HCC with PVTT, additional treatment options are needed.
Metronomic chemotherapy (MET) is frequently administered in comparatively low doses that do not require an extended break [10]. MET is advantageous because it can inhibit tumor growth through anti-angiogenic mechanisms with less toxicity and fewer systemic side effects than maximum tolerated dose therapy. Several clinical trials have evaluated the efficacy and safety of metronomic chemotherapy in various human cancers, including breast cancer, ovarian cancer, lymphoma, renal cell carcinoma, and HCC [11].
Including our preclinical model and pilot study [12,13], some preclinical and clinical trials have evaluated MET in HCC [14]. In these studies, MET showed anti-angiogenic activity and a lower toxic effect compared with the conventional maximum tolerated dose therapy in HCC. Therefore, the aim of this study was to evaluate the feasibility and OS of MET administered via hepatic arterial infusion chemoport compared to sorafenib for patients with advanced HCC with PVTT in real clinical practice.
This was a retrospective cohort study conducted in Seoul St. MaryŌĆÖs Hospital, Seoul, Korea. We reviewed the medical records of patients who were treated with MET chemotherapy or sorafenib for advanced HCC with PVTT between June 2005 and August 2013. The diagnosis and stage of HCC were assessed according to the American Association for the Study of Liver Disease guidelines and the BCLC staging classification. Inclusion criteria were (1) age 18-80 years, (2) an Eastern Cooperative Oncology Group performance status of zero to two, (3) Child-Pugh Class A or B, (4) advanced HCC according to the BCLC staging classification (confirmed PVTT in the main [Vp4], first [Vp3], or second branch [Vp2] of the portal vein or extrahepatic metastasis), (5) acceptable blood cell counts (absolute neutrophil countŌēź1.0├Ś109/L and platelet countŌēź50├Ś109/L), and (6) vascular access to the lesion for implantation of a chemoport.
Exclusion criteria were (1) evidence of hepatic decompensation including uncontrolled ascites, gastrointestinal bleeding, or hepatic encephalopathy, (2) concurrent serious medical condition(s) such as underlying cardiac or renal disease, (3) other concurrent primary malignancy, (4) another chemotherapy such as hepatic arterial infusion chemotherapy (HAIC) or systemic chemotherapy, (5) adjuvant MET chemotherapy or sorafenib after curative treatment, or (6) absence of an intrahepatic lesion. The trial was conducted according to the Declaration of Helsinki, and the protocol was approved by the Institutional Ethics Committee of The Catholic University of Korea.
The total number of HCC patients in our institute between June 2005 and August 2013 was 2,381. A total of 303 patients of advanced HCC treated with MET chemotherapy or sorafenib were enrolled in this period. In these patients, 61 patients were MET group and 242 patients were sorafenib group. In MET group, seven patients were excluded because they did not have PVTT. In sorafenib group, 151 patients were excluded because they did not have PVTT. In remaining 91 patients, three patients were excluded by post-liver transplantation usage of sorafenib, 18 patients were excluded by combined with other treatment modality, one patient was excluded by adjuvant usage of sorafenib after curative surgery, and 16 patients were excluded because they did not follow up after their first prescriptions. Therefore, 54 patients with advanced HCC and PVTT who had undergone MET were assigned to the MET group, and 53 patients who had undergone sorafenib treatment were assigned to the sorafenib group (Fig. 1).
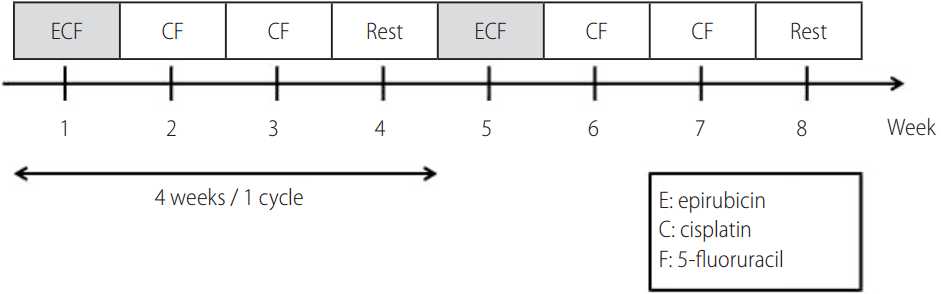
Chemotherapeutic agents were delivered via hepatic arterial infusion chemoport with an arterial infusion pump. Catheter insertion and placement were performed as previously described [15]. The metronomic chemotherapeutic agents were cisplatin and 5-fluorouracil (5-FU), which were infused weekly for three weeks with one week break between each cycle.
Single doses of cisplatin and 5-FU were 15 mg/m2 and 50 mg/m2, respectively. Cisplatin and 5-FU were infused via hepatic arterial infusion chemoport with an arterial pump for two hours. Along with the metronomic chemotherapy, epirubicin (30 mg/m2) was infused monthly via hepatic arterial infusion chemoport without the use of lipiodol or gelfoam embolization. This protocol was repeated every four weeks and continued until disease progression or unacceptable toxicity was evident or the patient refused to continue (Fig. 2) [12]. In instances including cisplatin infusion, intravenous hydration was performed to prevent nephrotoxicity, and an appropriate anti-emetic agent was administered to all patients. Dose adjustments were made depending on the toxicity observed in the preceding treatment cycle. The following cycle of treatment was reduced by 30% in cases of repeated grade two toxicity during the preceding cycle. Treatment was stopped in all grade three or grade four toxicity cases and was not reinitiated until symptom resolution. If a patient required a delay longer than four weeks for recovery, they were excluded from the study.
Principally, patients who were treated with sorafenib received standard doses of sorafenib (400 mg twice a day, orally). Dose adjustments of sorafenib were made depending on clinically significant toxicity (more severe than grade two based on the National Cancer Institute Common Terminology Criteria for Adverse Events Version 4.0) or the clinicianŌĆÖs determination of patient tolerance. During sorafenib treatment, the patients visited the outpatient clinic every three or four weeks for safety and tolerability assessments.
During the follow-up period, laboratory tests including alphafetoprotein (AFP), albumin, bilirubin, aspartate aminotransferase, alanine aminotransferase, and prothrombin time and a liver dynamic computed tomography (CT) scan (non-enhanced, arterial, portal, and delayed venous phases) or a liver dynamic magnetic resonance imaging (MRI) scan with hepatocyte specific contrast were performed to evaluate treatment response and reserved liver function every four to eight weeks after treatment. The treatment response was assessed using the modified Response Evaluation Criteria in Solid Tumors (mRECIST) [16]. An objective response was defined as complete response, and partial response and disease control included complete response, partial response, and stable disease.
The primary endpoints were OS and time to progression in each treatment group. The OS was calculated from the initiation of treatment to death or final follow-up visit. The time to progression was calculated from the initiation of treatment to radiologic progression. The secondary endpoints were objective response rate and disease control rate at eight weeks of treatment. The treatment-related toxicity assessment was based on the National Cancer Institute Common Terminology Criteria for Adverse Events Version 4.0.
Continuous data were expressed as median and range, and categorical data as percentage. Treatment response between groups was compared using the Chi-square or FisherŌĆÖs exact test. Other variables between different groups were compared using these tests or an independent t test, as appropriate. Cumulative overall survival was calculated using the Kaplan-Meier method, and the difference between groups was compared using the log-rank test. A Cox proportional-hazards model was used to identify independent clinical factors or groups that had effects on the overall survival rate. A P-value<0.05 was considered statistically significant (SPSS ver. 20.0, SPSS Inc., Chicago, IL, USA).
A total of 107 patients were analyzed into this study between June 2005 and August 2013. Among these patients, 54 with advanced HCC and PVTT who had undergone MET were assigned to the MET group, and 53 who had undergone sorafenib treatment were assigned to the sorafenib group. Table 1 shows the baseline characteristics of all patients enrolled in this study. The mean age was 56.2┬▒10.3 years; 89 patients were male, and 18 patients were female. The most common cause of underlying liver disease was hepatitis B virus infection (81.3%). Fifty-nine patients were Child-Pugh class A, and 48 patients were Child-Pugh class B. The HCC stage of all patients was BCLC C stage. The maximal tumor diameters of the MET group and sorafenib group were 12.5┬▒4.6 cm and 9.2┬▒5.1 cm, respectively (P=0.001). The median number of treatment cycles in the MET group was two (range 1-15 cycles), and the median treatment duration of the sorafenib group was 51 days (range, 11-725 days). The baseline characteristics between the two treatment groups were similar except for tumor number, maximal tumor diameter, and treatment history.
Table 2 shows the treatment response of each group at eight weeks of treatment. According to the mRECIST, complete response, partial response, stable disease, and progressive disease were noted in zero (0.0%), four (7.4%), 21 (38.9%), and 29 (53.7%) patients in the MET group and zero (0.0%), zero (0.0%), nine (22.0%), and 32 (78.0%) patients in the sorafenib group (P=0.026). The disease control rate was higher in the MET group than the sorafenib group (46.3% vs. 22.0%, P=0.014). However, the objective response rate showed no statistically significant difference between the two groups (7.4% vs. 0.0%, P=0.131).
The median OS of 107 patients during follow-up was 127 days (range, 12-2,666 days). In the MET group, 51 patients (94.4%) died, three patients (5.6%) were lost to follow-up, and only one patient (1.7%) survived. In the sorafenib group, 46 patients (86.8%) died, six patients (11.3%) were lost to follow-up, and no patient survived. The most common cause of death in both groups was tumor progression (31.4% and 58.7% in the MET group and sorafenib group, respectively). The second most common cause was hepatic failure, including hepatorenal syndrome (23.5 % and 23.9% in two groups, respectively), and other causes included variceal bleeding and infection (9.8% and 0.0%, and 5.9% and 2.2%, respectively). There was a statistically significant difference between the two groups (P=0.007) based on cause, and there were no deaths related to treatment.
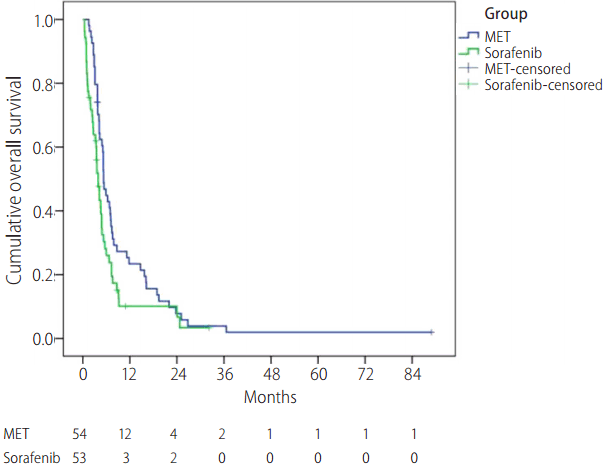
The median survival time in the MET and sorafenib groups was 158 days (95% confidence interval [CI], 132-184 days) and 117 days (95% CI, 92-142 days), respectively (Fig. 3). The overall survival rate showed significantly better results in the MET treatment group than in the sorafenib group (P=0.029). The time to progression in the two groups was 62 days (95% CI, 42-82 days) and 78 days (95% CI, 63-93 days), respectively; however, the difference was not statistically significant (P=0.472).
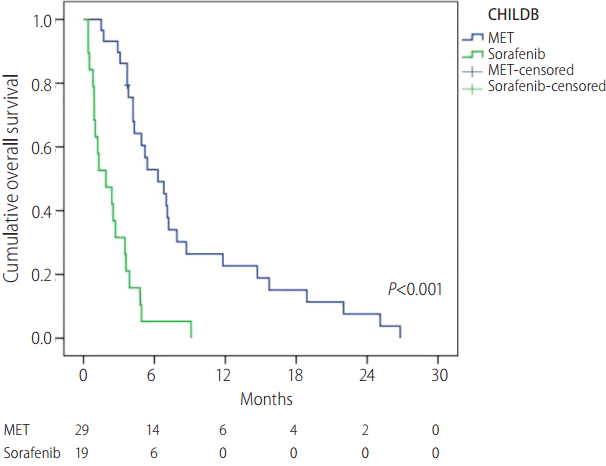
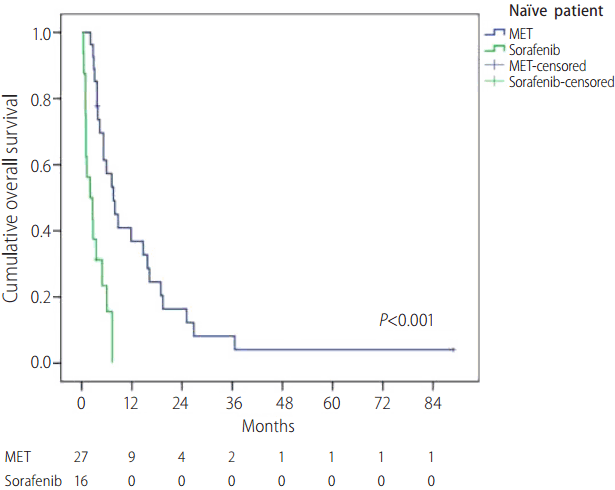
Univariate analysis was performed using the Cox proportional hazards model, which identified three prognostic factors for OS: Child-Pugh class, treatment modality, and serum AFP level. MET was an independent and more accurate prognostic factor associated with OS (hazard ratio [HR]=0.471, 95% CI, 0.296-0.749, P=0.001) than sorafenib, and Child-Pugh class B and higher serum AFP level (Ōēź400 mg/dL) were independent, less accurate, OS-associated prognostic factors (HR=1.856, 95% CI, 1.173-2.973, P=0.008, and HR=1.680, 95% CI, 1.112-2.538, P=0.014, respectively) (Table 3). In subgroup analysis in patients with Child-Pugh class B disease, there was no statistically significant difference in the baseline characteristics between MET and sorafenib group (Supplementary Table 1) and the median OS of the MET and sorafenib groups was 190 days and 58 days, respectively (29 and 19 patients, P<0.001) (Fig. 4). Otherwise, in subgroup analysis of patients with Child-Pugh class A disease, there was no statistically significant difference in the median OS of the MET and sorafenib groups. (157 days and 143 days, respectively, P=0.813). In subgroup analysis in na├»ve patients, there was no statistically significant difference in the baseline characteristics between MET and sorafenib group except for sex (Supplementary Table 2) and the median OS of the MET and sorafenib groups was 229 days and 61 days, respectively (27 and 16 patients, P<0.001) (Fig. 5).
Table 4 shows the treatment-related toxicity of both treatments. The majority of MET-related toxicity was hematological adverse events. Leukopenia developed in 48% of patients treated with MET. Thrombocytopenia, hyperbilirubinemia, and increased alanine aminotransferase level developed in 22%, 30%, and 17% of patients treated with MET, respectively. The majority of sorafenib-related toxicity was mucosal toxicity, including skin and gastrointestinal tract toxicity. Hand-foot syndrome and gastrointestinal tract toxicity developed in 28% and 40% of patients treated with sorafenib, respectively, and hyperbilirubinemia and alanine aminotransferase elevation developed in 36% and 17% of such patients. Patients who were experienced grade 3/4 toxicities in MET and sorafenib group, were 25 and 16, respectively and there was no statistically significant difference between two groups (P= 0.488).
Despite the emergence of new treatment modalities for HCC, the prognosis of advanced HCC with PVTT remains poor, especially in patients with poor liver function [6]. According to current guidelines, including the BCLC staging classification, sorafenib is the primary recommended treatment modality for advanced HCC with PVTT. However, due to its adverse effects, moderate efficacy, and high cost, the availability of sorafenib is limited [17]. Therefore, other alternative treatment modalities are needed. HAIC, TACE or surgery in select cases, external radiation, radioembolization, and combination therapies are alternative treatment options [6]. Additionally, MET could be applied as an alternative treatment modality [14].
The mechanisms of MET include inhibition of angiogenesis, stimulation of immunity, and direct inhibition of tumor cell proliferation. The main mechanism is inhibition of angiogenesis, which is related to direct destruction or inhibition of endothelial cell proliferation, up-regulation of endogenous anti-angiogenic factors like thrombospondin-1, down-regulation of endogenous angiogenic factors like hypoxia inducible factor 1╬▒, and reduction of endothelial progenitor cell mobilzation. Stimulation of immunity is also a mechanism of action and is related to a reduction in regulatory T-cells and stimulation of dendritic cell maturation [14].
To date, there are several clinical trials evaluating metronomic chemotherapy for HCC [14,15,18-21]. Among them, our institute previously reported a prospective trial for advanced HCC with PVTT treated with MET. The OS in that study was 5.6 months [6]. In the present study, the OS of MET and sorafenib was 158 days and 117 days, respectively. Our data showed relatively low OS with sorafenib compared with those in other studies [6-8], since 36% of the sorafenib group was Child-Pugh class B. We reported that MET might be a safe and useful palliative treatment in patients with advanced HCC with major PVTT [6]. To our knowledge, this study is the first comparison report of metronomic chemotherapy and sorafenib in patients with advanced HCC with PVTT in real clinical practice.
The time to progression of the sorafenib group in our data was shorter than that of the sorafenib group in the Asia-Pacific trial (2.6 months and 2.8 months, respectively) [2]. The macrovascular invasion of hepatocelluar carcinoma was the non-favorable risk factor of the time to pregression of sorafenib therapy [22]. In the Asia-Pacific trial, 36% of patients treated with sorafenib had macrovascular invasion, however in our study, all patients treated with sorafenib had macrovascular invasion [2]. Higher rate of macrovascular invasion in this study could explain the discrepancy of time to progression between the two groups.
Subgroup analysis in patients with Child-Pugh class B in this study found that MET was associated with greater OS compared to that of sorafenib treatment (190 days and 58 days, respectively). In patients with advanced HCC with PVTT and Child-Pugh class B liver function, their OS of sorafenib treatment was similar to those in previous studies [7-9]. Because of the low OS and higher incidence of cirrhosis-related complications, new therapeutic approaches that were appropriate for HCC patients with Child-Pugh class B were recommended. Although our study showed relatively lower OS and disease control rate in the sorafenib treatment group in Child-Pugh class B patients, this could be because of the inclusion of patients with HCC and PVTT [9]. In patients with Child-Pugh class B, the major cause of death in the sorafenib group was tumor progression (63% of the total 19 deaths); however, tumor progression was found in 22% of the total 27 deaths in the MET group. A possible explanation for this difference in OS in patients with Child-Pugh class B is that MET was tolerable in patients with poor liver function because it has low toxicity and controls tumor progression through its anti-angiogenic and anti-proliferative effects.
In this study, the schedule for MET and chemotherapeutic agent delivery via hepatic arterial infusion chemoport was the same as in our previous study [12]. The chemotherapeutic regimen of MET in the present study was modified based on previous reports that the efficacy of epirubicin, cisplatin, and 5-FU-based transcatheter arterial infusion chemotherapy was superior to that of adriamycin-based TACE [23, 24].
Because epirubicin was administrated every four weeks based on a maximum tolerated dose schedule, not a metronomic schedule like 5-FU and cisplatin, it could cause frequent hematologic adverse events, especially leucopenia. However, most cases of hematologic toxicity were manageable with conservative care and granulocyte colony-stimulating factor injection or transfusion.
Vu et al. [25] reported that doxorubicin and 5-FU-resistant hepatic cancer cells demonstrate stem-like properties. They found that the expression of AFP, CD56, CD117, Nanog, Sox-2, and Oct4 in hepatic cancer stem cells from primary HCC cells was much higher than that in primary HCC cells [25]. This could be a reason for the higher serum AFP level was an independent poor prognostic factor associated with OS in the multivariate analysis.
There were several limitations to our study. Firstly, this was a retrospectively designed study performed in a single center. Secondly, 8.4% of total patients were lost to follow up. Therefore, a prospectively designed randomized controlled trial for MET is needed. Thirdly, there were differences in tumor maximal diameter and previous treatments between the groups. However, these differences were not an independent prognostic factor in multivariate analysis; therefore, the difference in tumor maximal diameter did not significantly influence OS. Fourthly, since the study period includes about two years of pre-sorafenib era, there was selection bias in this study. We tried to partially overcome this limitation by the subgroup analysis in naïve patients. Lastly, all the tumor response evaluation was assessed using the mRECIST criteria. Because the mRECIST criteria were established in 2010, the results might be discordant in patients who were actually assessed with different methods in the pre-mRECIST era.
In previous study, we compared between MET and TACE in patient with Child-Pugh class B advanced HCC. We reported that MET showed better survival benefit than TACE in patients with Child-Pugh class B advanced HCC [26]. Additional studies are needed to compare the metronomic chemotherapy with other modalities such as HAIC.
In conclusion, although it was limited by the statistical design, our study showed that MET is an alternative treatment option for patients with advanced HCC and PVTT, especially in patients with poor liver function. Additional prospective studies are needed to compare the efficacy and safety of MET with other modalities, especially in advanced HCC with PVTT.
SUPPLEMENTAL MATERIAL
Supplementary materials are available at Clinical and Molecular Hepatology website (http://www.e-cmh.org)
Supplemental┬ĀTable┬Ā1.
Patient baseline characteristics of the Child-Pugh class B subgroup
Supplemental┬ĀTable┬Ā2.
Patient baseline characteristics of naïve patients
Figure┬Ā1.
Flow diagram of the study design. MET, metronomic chemotherapy; PVTT, portal vein tumor thrombosis.
Figure┬Ā4.
Overall survival rates according to treatment group in patients with Child-Pugh class B. MET, metronomic chemotherapy.
Figure┬Ā5.
Overall survival rates according to treatment group in naïve patients. MET, metronomic chemotherapy.
Table┬Ā1.
Patient baseline characteristics
Table┬Ā2.
Treatment responses
| MET (n=54) | Sorafenib* (n=41) | P-value | |
|---|---|---|---|
| Response | 0.026 | ||
| ŌĆāCR | 0 (0.0) | 0 (0.0) | |
| ŌĆāPR | 4 (7.4) | 0 (0.0) | |
| ŌĆāSD | 21 (38.9) | 9 (22.0) | |
| ŌĆāPD | 29 (53.7) | 32 (78.0) | |
| ORR | 0.131 | ||
| ŌĆāResponse | 4 (7.4) | 0 (0.0) | |
| ŌĆāNon-response | 50 (92.6) | 41 (100.0) | |
| DCR | 0.014 | ||
| ŌĆāControl | 25 (46.3) | 9 (22.0) | |
| ŌĆāProgression | 29 (53.7) | 32 (78.0) |
Values are presented as n (%).
MET, metronomic chemotherapy; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; ORR, objective response rate; DCR, disease control rate.
* 12 patients in the sorafenib group were not assessed for treatment response: six patients were followed up loss before eight weeks without imaging study for treatment response evaluation, five patients have expired before eight weeks without imaging study for treatment response evaluation, and one patient was not evaluated with imaging study for treatment response evaluation after sorafenib treatment.
Table┬Ā3.
Factors that influence overall survival according to univariate and multivariate analyses
Table┬Ā4.
Treatment-related toxicity
Abbreviations
5-FU
5-fluorouracil
AFP
alpha-fetoprotein
RECIST
modified Response Evaluation Criteria in Solid Tumors
BCLC
Barcelona Clinic Liver Cancer
HAIC
hepatic arterial infusion chemotherapy
HCC
hepatocellular carcinoma
HR
hazard ratio
MET
metronomic chemotherapy
OS
overall survival
PVTT
portal vein tumor thrombosis
TACE
transarterial chemoembolization
REFERENCES
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108.


2. Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009;10:25-34.


3. Llovet JM, Br├║ C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis 1999;19:329-338.



5. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378-390.


6. Woo HY, Heo J. New perspectives on the management of hepatocellular carcinoma with portal vein thrombosis. Clin Mol Hepatol 2015;21:115-121.



7. DA Fonseca LG, Barroso-Sousa R, Bento AD, Blanco BP, Valente GL, Pfiffer TE, et al. Safety and efficacy of sorafenib in patients with Child-Pugh B advanced hepatocellular carcinoma. Mol Clin Oncol 2015;3:793-796.



8. Lee SH, Song IH, Noh R, Kang HY, Kim SB, Ko SY, et al. Clinical outcomes of patients with advanced hepatocellular carcinoma treated with sorafenib: a retrospective study of routine clinical practice in multi-institutions. BMC Cancer 2015;15:236.




9. Kim JE, Ryoo BY, Ryu MH, Chang HM, Suh DJ, Lee HC, et al. Sorafenib for hepatocellular carcinoma according to Child-Pugh class of liver function. Cancer Chemother Pharmacol 2011;68:1285-1290.


10. Hanahan D, Bergers G, Bergsland E. Less is more, regularly: metronomic dosing of cytotoxic drugs can target tumor angiogenesis in mice. J Clin Invest 2000;105:1045-1047.



11. Pasquier E, Kavallaris M, Andr├® N. Metronomic chemotherapy: new rationale for new directions. Nat Rev Clin Oncol 2010;7:455-465.



12. Woo HY, Youn JM, Bae SH, Jang JW, Cha JH, Kim HL, et al. Efficacy and safety of metronomic chemotherapy for patients with advanced primary hepatocellular carcinoma with major portal vein tumor thrombosis. Korean J Hepatol 2012;18:32-40.



13. Park ST, Jang JW, Kim GD, Park JA, Hur W, Woo HY, et al. Beneficial effect of metronomic chemotherapy on tumor suppression and survival in a rat model of hepatocellular carcinoma with liver cirrhosis. Cancer Chemother Pharmacol 2010;65:1029-1037.


14. Torimura T, Iwamoto H, Nakamura T, Koga H, Ueno T, Kerbel RS, et al. Metronomic chemotherapy: possible clinical application in advanced hepatocellular carcinoma. Transl Oncol 2013;6:511-519.



15. Woo HY, Bae SH, Park JY, Han KH, Chun HJ, Choi BG, et al. A randomized comparative study of high-dose and low-dose hepatic arterial infusion chemotherapy for intractable, advanced hepatocellular carcinoma. Cancer Chemother Pharmacol 2010;65:373-382.


16. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 2010;30:52-60.



17. Song DS, Song MJ, Bae SH, Chung WJ, Jang JY, Kim YS, et al. A comparative study between sorafenib and hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma with portal vein tumor thrombosis. J Gastroenterol 2015;50:445-454.


18. Treiber G, Wex T, Malfertheiner P. Impact of different anticancer regimens on biomarkers of angiogenesis in patients with advanced hepatocellular cancer. J Cancer Res Clin Oncol 2009;135:271-281.


19. Shao YY, Lin ZZ, Hsu C, Lee KD, Hsiao CH, Lu YS, et al. Efficacy, safety, and potential biomarkers of thalidomide plus metronomic chemotherapy for advanced hepatocellular carcinoma. Oncology 2012;82:59-66.


20. Hsu CH, Shen YC, Lin ZZ, Chen PJ, Shao YY, Ding YH, et al. Phase II study of combining sorafenib with metronomic tegafur/uracil for advanced hepatocellular carcinoma. J Hepatol 2010;53:126-131.


21. Brandi G, de Rosa F, Agostini V, di Girolamo S, Andreone P, Bolondi L, et al. Metronomic capecitabine in advanced hepatocellular carcinoma patients: a phase II study. Oncologist 2013;18:1256-1257.



22. Sohn W, Paik YH, Cho JY, Lim HY, Ahn JM, Sinn DH, et al. Sorafenib therapy for hepatocellular carcinoma with extrahepatic spread: treatment outcome and prognostic factors. J Hepatol 2015;62:1112-1121.


23. Kim HY, Kim JD, Bae SH, Park JY, Han KH, Woo HY, et al. A comparative study of high-dose hepatic arterial infusion chemotherapy and transarterial chemoembolization using doxorubicin for intractable, advanced hepatocellular carcinoma. Korean J Hepatol 2010;16:355-361.



24. Jang JW, Park YM, Bae SH, Choi JY, Yoon SK, Chang UI, et al. Therapeutic efficacy of multimodal combination therapy using transcatheter arterial infusion of epirubicin and cisplatin, systemic infusion of 5-fluorouracil, and additional percutaneous ethanol injection for unresectable hepatocellular carcinoma. Cancer Chemother Pharmacol 2004;54:415-420.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Supplement1
Supplement1 Print
Print




