Current status and strategies for the control of viral hepatitis A in Korea
Article information
Abstract
Hepatitis A virus is one of the most frequent causes of foodborne infection, which is closely associated with sanitary conditions and hygienic practices. The clinical spectrum of acute hepatitis A is wide, ranging from mild case without any noticeable symptoms to severe case with acute liver failure leading to mortality. The severity and outcome are highly correlated with age at infection. In developing countries, most people are infected in early childhood without significant symptom. Ironically, in area where sanitary condition has improved rapidly, adults who do not have immunity for viral hepatitis A (VH-A) in early childhood is accumulating. Adults without immunity are exposed to risks of symptomatic disease and large outbreaks in society. In Korea, where hygiene has improved rapidly, acute hepatitis A is a significant health burden that needs to be managed with nationwide health policy. The incidence of symptomatic VH-A has increased since 2000 and peaked in 2009. Korea has designated hepatitis A as a group 1 nationally notifiable infectious disease in 2001. Since 2001, mandatory surveillance system has been established to detect every single case of acute hepatitis A. Universal, nationwide vaccination program for newborns was introduced in 2015. In this review, we will present the current epidemiologic status of viral hepatitis A, and evaluate the effectiveness of the current nationwide strategies for the control of viral hepatitis A in Korea. Furthermore, we presented some action proposals that can help eliminate viral hepatitis A, which is a significant health burden in Korea.
INTRODUCTION
Hepatitis A virus (HAV) was first characterized in 1973 following the detection of the virus in stools of patients who were infected with HAV [1]. HAV is an acid-stable microorganism that can survive in acidic environments with a pH of 3.0, which means that it can also survive in the gastric acid of the human body [2]. HAV can survive in dried feces for 4 weeks, and in seawater or live oysters for 5 days. This virus is also heat stable, and can survive exposure to heat at 60°C for 60 min. HAV can be inactivated with heat at 85°C for 1 min [3]. The primary transmission route of this microorganism is the fecal–oral route. HAV can be transmitted through ingestion of contaminated food or water. This virus causes either asymptomatic or symptomatic acute hepatitis. When HAV is ingested, it traverses the small intestinal mucosa to the portal vein, reaches the hepatocytes, and exclusively replicates in the liver. This virus is then, released into the bile canaliculi and is excreted through the feces of a patient.
Korea is a developing country, with sanitary conditions that have been improved rapidly over the last 30 years. As a result, the seroprevalence of HAV in children and young adults dropped to less than 30%, which was noted in a 2008–2013 report. The major risk group includes children and adolescents with no history of infection and thus no immunity to hepatitis [4]. Adult patients have high symptom rates, and the high disease susceptibility in young adults led to the outbreak of acute viral hepatitis A (VH-A) in 2009.
CURRENT STATUS OF VH-A IN KOREA
Analysis of trends in the incidence of acute VH-A
The number of annual reported cases for symptomatic VH-A has rapidly increased in 2000s. Three- and five-fold increases in the number of cases were recorded in 2008 and 2009, respectively, compared to the number reported in 2007, and a peak of 15,231 cases was recorded in 2009. After the outbreak in 2009, the number of cases gradually decreased until 2013, when the number came down to less than 1000 per year. However, the number of reported cases gradually increased again from 2013 up to 4679 in 2016, showing an increase of 260% from the number in 2015 (Fig. 1) [5]. This re-increase is attributed to the increment of susceptible subjects, about 10 years passed after herd immunity by epidemic around late 2000s. VH-A outbreaks in Incheon and Chonnam areas in 2015 represent good examples suggesting the possibility of a major epidemic of VH-A occurring again.
Analysis of trends in the seroprevalence rates of HAV
As a result of the improved sanitary condition in Korea over the last 30 years, the rate of asymptomatic infection at early ages has decreased. Therefore, the graph presenting the seropositive rates in 2011–2013 by age groups shifted to the right for approximately 20 years compared with that in 1979–1981 (Fig. 2). Even after the outbreak in 2009, low positive rates was still observed in adolescents and young adults in 2011–2015, which may be one of the reasons for the recent rebound in the number of acute VH-A cases.
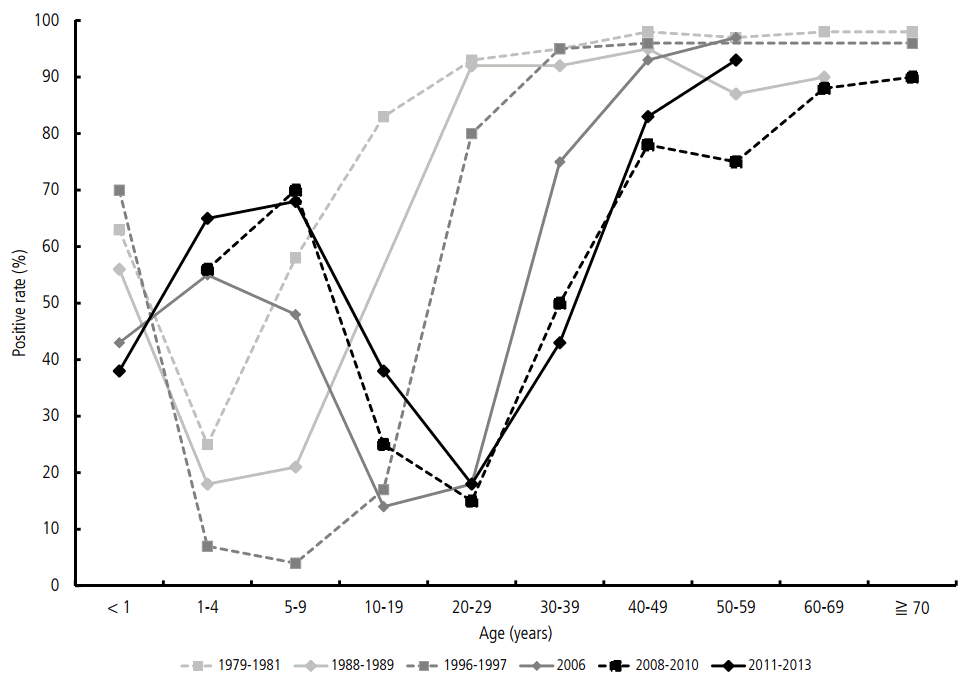
The trends of seropositive rates by ages and years in Korea for the last 30 years. The seropositivity graph has shifted horizontally to the left for 20 years in age. The age of people who do not have anti- HAV antibody is getting older because of an improved sanitation in Korea for the last 30 years.
Regional distribution of VH-A incidence
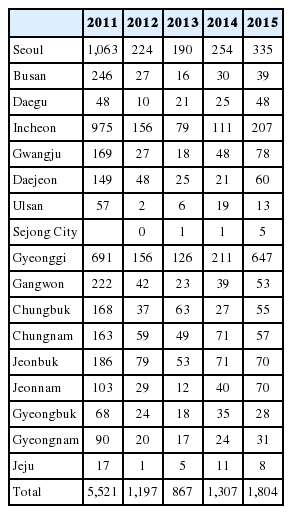
According to the report of Korea Centers for Disease Control and Prevention (KCDC), individuals living in mid-western regions, including Seoul and Incheon, were consistently at high risk for HAV infection from 2011 to 2015 (Table 1) [6]. In data from 2005–2009, the age-specific anti-HAV seroprevalence rates were different between the districts. Particularly, the seroprevalence rates of persons in their 40s were 80% in Seoul and 88.9–96.8% in other areas [7]. Therefore, 40–49-year-old people do not share the same risk of HAV infection with regard to their habitat.
Difference in the seroprevalence rates of HAV by age
The seropositive rate of the age group less than 10 years old is reported to be 50–70% after the epidemic in 2008–2009. However, recent data indicate that the seropositive rate in the 15–29 age group is still low (Table 2). Especially, the rate in the 20–29 age group is reported to be only 18%, and 71.3–84% of acute VH-A cases occur in this age group. Actually, the age in which the infection is most prevalent is 33.3 years, and this is the age group with the highest risk for symptomatic acute VH-A.
Additionally, the recent seropositive rates in the 1–14 age group have considerably increased compared with those in 2001–2003 [8]. However, the rates in the 25–44 age group have significantly decreased at the same time, showing a horizontal shift of the seropositivity graph to the right [7-10]. Based on the data reported in 2011–2013, the 25–34 age group has become vulnerable to infection. Hence, a catch-up vaccination in the special age groups would be helpful in VH-A prevention in these ages.
Cases of VH-A in the military
Several outbreaks were reported in military population since 1998. In 1998, 102 epidemic cases were reported from the northwest region of Gyeonggi Province. Additionally, in May–August 2007, an acute VH-A epidemic was reported in 73 cases out of 332 military personnel from Gangwon Province [11]. The incidence rate of acute VH-A was 1.6–9.8 cases per 100,000 military personnel [12]. However, this rate rose to 34.5 cases in 2009 [13]. A new vaccination strategy consisting of the administration of single-dose HAV vaccination to new recruits on the frontline was introduced since 2013 [14].
Direct and indirect medical costs of acute VH-A
Considering the low anti-HAV seropositive rates in the 10–39 age group, more than 80% of the patients with symptomatic acute VH-A are in their 20s and 30s. Patients older than 30 years have higher complication rates of renal failure or prolonged cholestasis than those younger than 30 years. Therefore, as many as 60% of patients in the 20–39 age group have been admitted to the hospital due to acute VH-A (Fig. 3) [15]. The mean medical cost for all patients who had VH-A is reported to be 643,194 won in 2009 (Table 3). The medical cost for admitted patients is 1,221,159 won, which is approximately 10 times more expensive than that for patients who were treated in the outpatient clinic. From a socioeconomic perspective, the total medical cost for acute VH-A actually increases, considering that people in their 20s and 30s are the most active group in working places (Table 4) [15].
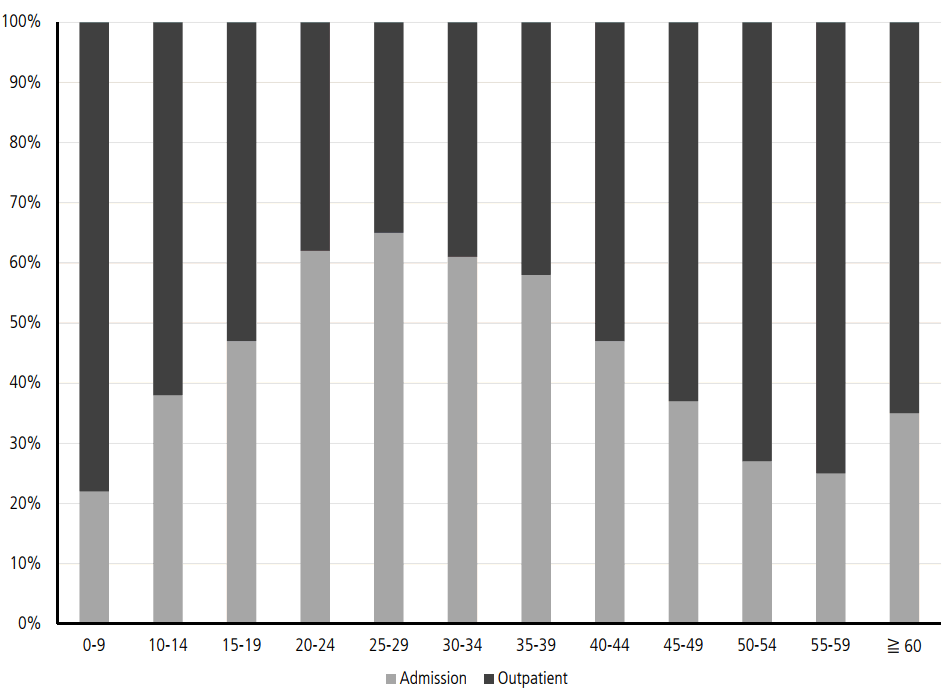
The ratios of admitted patients and outpatients by ages in 2009 [15]. The most common age groups who require admission for treatment of VH-A are p eople aged 20 -39 as shown. People older than 30 years have chance for high rates of complications. Also, they are the most actively working group in their work places. Therefore, the total cost for treating VH-A is rapidly increasing in socioeconomic perspective.
Genotypic shift of HAV and imported cases of acute VH-A
A HAV genotypic shift from genotype IA to IIIA was recorded in the mid-2000s. The genotype of HAV isolated from the samples of patients in 1998–2006 was IA [16]. However, genotype IIIA accounted for 51.0–54.6% of the HAV isolates between 2007 and 2008, and was reported to be the predominate HAV genotype, forming up to 93% of the total, in 2009 [16-18]. Therefore, HAV genotypes IA and IIIA seem to coexist in Korea, and both are endemic from 2007 to 2008. The possibility of the presence of genotype IIIA imported from foreign countries as an effect of globalization was suggested to be the reason for the abrupt epidemiologic shift in mid-2000s in Korea [17]. Among the 7,585 cases reported to KCDC through the National Infectious Diseases Surveillance System and National Health Insurance Review and Assessment Service from 2011 to 2013, as many as 58 cases were infections acquired from foreign countries. Among the 1804 cases reported in 2015, 25 were infections acquired outside of Korea, and the associated countries were Vietnam (1 case), Singapore (1 case), Uzbekistan (1 case), India (3 cases), Japan (1 case), China (2 cases), Kazakhstan (1 case), Cambodia (5 cases), Thailand (2 cases), Thailand–Cambodia–Vietnam (1 case), Philippines (6 cases), and Hong Kong (1 case).
The question of whether older patients are affected by genotype IIIA, or if the genotype itself is correlated with the clinical outcomes remains controversial [17]. However, more severe clinical manifestations are reported to be correlated with genotype IIIA than IA [16,18]. Therefore, careful monitoring for imported VH-A cases and possible outbreaks is needed.
STRATEGIES FOR VH-A CONTROL IN KOREA
Designation of VH-A as one of the nationally notifiable infectious diseases and operation of surveillance system
VH-A was designated as a nationally notifiable infectious disease in 2000, and a sentinel surveillance system for this disease was established. The number of reported cases has steeply increased between 2007 and 2009 and reached its peak in 2009 (15,231 cases reported by the sentinel surveillance system). Given the high chance of the disease becoming epidemic, VH-A has been redesignated as one of the group 1 nationally notifiable infectious diseases, which are acquired through ingestion of contaminated food or water. A mandatory surveillance system has been operating since 2011. Every physician is required to mandatorily report patients presenting with typical VH-A manifestations and identified to be infected with HAV to the KCDC through the National Infectious Diseases Surveillance System.
Although the total number of cases identified by the surveillance system has considerably decreased until 2013, the number of cases is re-increasing recently. Particularly the reported number of VH-A in 2016 was 4,679, which was 260% higher than the recorded 1804 cases in 2015. There is no available official data of the VH-A cases in 2016 to check specific characteristics. Instead, looking at the data for 2015, the infection was more prevalent in males than in females, which constituted 58.4% of the total cases. In terms of age distribution, 87% of the patients were in their 30s to 50s (1560 out of 1804 cases). Gyeonggi, Seoul, and Incheon were the top three provinces with the highest incidence rates (Table 1). The monthly incidence was predominantly distributed from March to April (Table 5). The numbers of cases of infection acquired outside the country are 22, 18, 18, 21, and 25 for the years 2011–2015, respectively, with a sustained number of approximately 20 cases per year. Philippines and Cambodia were the most common sources of imported VH-A.
Universal vaccination for newborns
HAV infection has been designated as one of the diseases covered under the national immunization program. Universal vaccination program for newborns has been started since 2015. Nationwide vaccination program reimburse vaccination costs for hepatitis A by the government, but is limited to newborns who were born after 2012. Generally, two doses of HAV vaccines are administered to children at 12–23 months after birth, and 6–12 months after the first shot. The number of vaccinated cases among the 12–36-month-old children increased from 60,000–70,000/month to 225,835 in May 2015 after the introduction of the universal vaccination. However, this number dropped to 104,955 in June 2015, after the first month of the universal vaccination introduction. Actually, 640,237 doses were administered to children for the first dose during January 2015 to September 2016 after the universal vaccination introduction. The number of newborns that were candidates for the vaccination from Jan 2012 to Dec 2015 is 1,816,441. However, the actual number of candidates should exceed this figure because the number of newborns from January 2016 to September 2016 were not included. Nevertheless, the rate for the first dose of HAV vaccine is just 35.2% based on this data (Fig. 4). Therefore, sustained monitoring of vaccinated cases and development of action plans to improve vaccination rates are needed.

The number of monthly-vaccinated cases after the introduction of universal vaccination in the children who were born after 2012. The number of monthly-vaccinated cases as either a 1st dose or a 2nd dose are monitored by KCDC. The rate of vaccinated cases out of the born neonates may prove the result of the universal vaccination.
In one study, the accessibility of HAV vaccine among the 800 parents of children aged 14–17 years has been evaluated through a telephone survey [9]. The seropositive rates in the groups that reported to have vaccinated and unvaccinated children were at most 42.2% and 9.1%, respectively. The low seropositive rate among the vaccinees might be related to recall bias. Additionally, they might have missed the second dose that affects the long-term protective antibody formation because of the low HAV awareness rate among the parents in the early 2000s. The recommendation of HAV vaccination by the healthcare providers was the most significant factor for parents to have their children vaccinated. Furthermore, the parents reported that the most crucial source of information was the promotion of the public health organization. Another study conducted a telephone survey among the parents of children aged 7–18 years on their willingness to vaccinate their children at the current cost of the HAV vaccine either in an epidemic or promptly [19]. Sixty-two percent of the parents expressed willingness to have their children vaccinated at the current cost. Only 32% replied that they wished to have prompt vaccination for their children regardless of epidemicity. However, 92% of the parents expressed willingness to vaccinate their children at half the price of the current cost for HAV vaccination. In this regard, the current cost of the vaccine might be one of the important barriers preventing accessibility to HAV vaccination.
PROPOSALS FOR VH-A ERADICATION
Catch-up vaccination program
For people who live in high-risk area
Even with the introduction of universal vaccination for infants, the seroprevalence rate is not high among the people aged 20–39 years. In particular, Seoul, Gyeonggi, and Incheon are the areas with high population density, low anti-HAV antibody rate, and high VH-A incidence. Therefore, promoting and sponsoring catch-up vaccination programs among adolescents and young adults in these areas is important.
For new recruits in military
The seroprevalence rate of people aged 20–24 years is at most 11%–18%. Therefore, testing for anti-HAV antibody in total cases for new recruits and supplying doses for HAV vaccination in seronegative recruits are crucial.
For students in school age
Cervical cancer vaccination has been provided free of charge to 12–13 year old girls since 2016. Moreover, routine medical checkup is now available upon entrance to high school as sponsored by the Ministry of Education. The determination of the seropositive rate for anti-HAV antibody can be included in the checkup, and school-aged students can be evaluated to find out if they need a catch-up vaccination. The number of 17-year-old population is 620,509 in 2016, and the HAV vaccination cost for children is currently 15,000 won. The cost for catch-up vaccination without performing antibody test in this population would be 9,300,000,000 won. Therefore, the cost-effectiveness of the antibody test or catch-up vaccination should be further evaluated.
For people who travel to highly endemic areas
The number of imported VH-A infection acquired abroad remained constant in the last 5 years. Therefore, recommending HAV vaccination and educating people who are planning to travel to Southeast Asia, Eastern Europe, and Africa on hand sanitation and being cautious of contaminated food and water would be helpful.
Down-regulation of cost for vaccination and government assistance for securing supply
The cost for HAV vaccine seems to be one of the important barriers in increasing the vaccination rate, especially in families with low income. Therefore, the down-regulation of cost would considerably improve the vaccination rate in school-aged students.
Four kinds of HAV vaccines, namely, HAVRIX®, VAQTA®, Epaxal®, and AVAXIM®, are available in Korea. All these vaccines are imported as finished goods. In 2015, those vaccines were used in a large scale for post-exposure prophylaxis during the outbreak that occurred in one domestic area. However, the supply of those vaccines could not meet the demand during and after the outbreak for some duration. Therefore, government sponsorship is needed for domestic production to secure the supply of HAV vaccines.
Nationwide campaign for improving sanitation and raising arousals for VH-A
Personal HAV awareness should be improved among the general population and healthcare providers. Hand hygiene should be routinely practiced along with the use of hand sanitizer. The general population should be educated about the necessity of HAV vaccination in adolescents and young adults. Korea is gradually becoming a low endemic area for HAV. Furthermore, foods that are imported from foreign countries as a result of globalization can be an important source of a VH-A epidemic. In fact, contaminated frozen blueberries led to the mass infection of 1589 people in the European Union in 2013 [20]. Moreover, contaminated clams infected 290,000 people with VH-A in China [21]. Therefore, imported food from highly-endemic areas should be monitored and controlled.
Post-exposure prophylaxis
Post-exposure prophylaxis is the most important approach toward the prevention of HAV spread after the occurrence of VH-A. Two post-exposure prophylaxis methods are available, that is, serum immune globulin and vaccination. A single-dose HAV vaccine is safe and has comparable effects to immune globulin in protecting the recipient against clinical hepatitis A. Persons who have not been vaccinated and recently exposed to HAV shall be given a single-dose HAV vaccine within 2 weeks of exposure [22-24]. The administration of a second dose of HAV vaccine may ensure long-term immunity and may have cost-saving effects when the standard vaccination schedule is followed [3].
Establishment of viral hepatitis sector and central lab system in KCDC
A genotypic shift from IA to IA+IIIA has been recorded in Korea recently and genotype IIIA has become autochthonous. Considering the effect of globalization, another genotypic shift might possibly occur. Therefore, a reliable lab system for genotypic and phylogenetic analyses should be established. Currently, these analyses are conducted under the water-borne disease sector of KCDC. Furthermore, the epidemiologic studies are handled by the infectious disease control sector. VH-A is manifested as acute hepatitis, which is significantly different from other water-borne diseases. Therefore, an independent viral hepatitis sector in KCDC that can organize epidemiologic surveillance, carry out laboratory studies, and make integrative decisions for viral hepatitis control should be established.
CONCLUSION
Korea has put considerable efforts to reduce the VH-A incidence through universal vaccination of the newborns and operation of mandatory surveillance system. However, the number of cases has gradually re-increased since 2013. The two outbreaks in several regions and the low seroprevalence in people aged 20–39 years may be the reasons for the recent rebound. Moreover, acute VH-A is seem to be significantly increasing again in 2016. Therefore, interest and attention to hepatitis A is necessary both in physician and public health institute. Raising VH-A awareness and educating the public on the preventive methods (i.e., vaccination and routine use of hand sanitizer) should be the bases of these efforts. Monitoring the results of universal vaccinations in newborns and meticulous investigation of the surveillance system report should be continued. The cost-effectiveness of catch-up vaccination in special groups should be evaluated to reduce the seropositive rates among people in their 20s and 30s.
Abbreviations
HAV
Hepatitis A virus
KCDC
Korea Centers for Disease Control and Prevention
VH-A
viral hepatitis A
Notes
Authors’ contribution
Concept and design: Ji Hoon Kim, Hyun Woong Lee, Dong Hyun Sinn Acquisition and analysis of data: Ji Hoon Kim, Hyun Woong Lee,
Dong Hyun Sinn, Eileen L. Yoon
Drafting of manuscript: Eileen L. Yoon
Peer review and critical revision of the manuscript: Ji Hoon Kim, Hyun Woong Lee, Dong Hyun Sinn
Study supervision: Ji Hoon Kim
Financial support
This research was supposed by a fund (4851-308-260-01) by Research of Korea Centers for Disease Control and Prevention.
Conflicts of Interest: The authors have no conflicts to disclose.