| Clin Mol Hepatol > Volume 24(2); 2018 > Article |
|
ABSTRACT
Background/Aims
Methods
Results
FOOTNOTES
Figure 1.
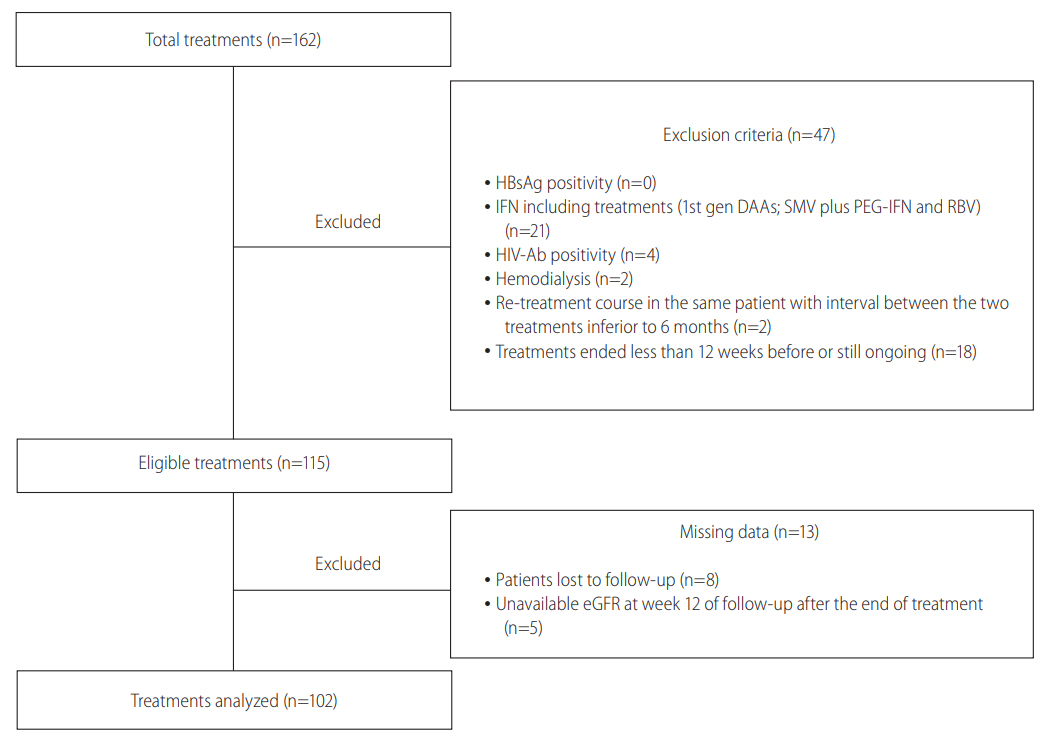
Figure 2.
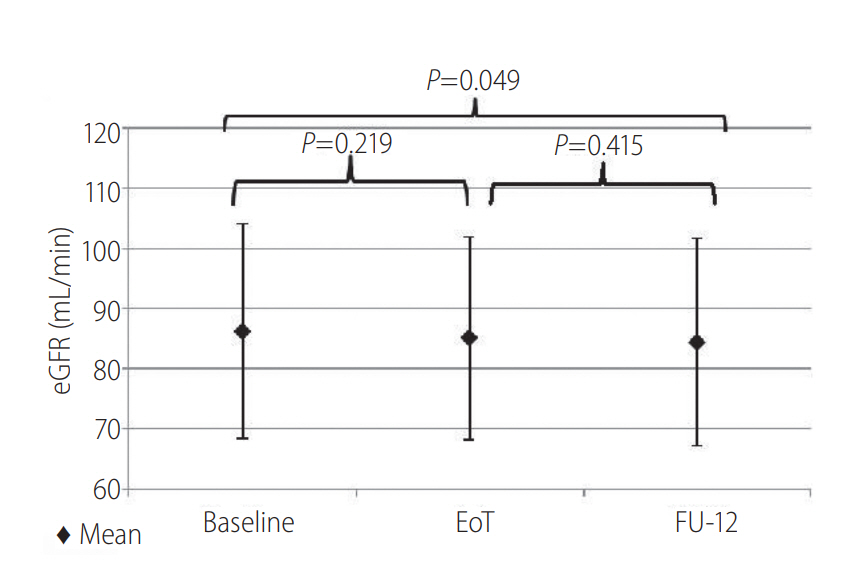
Figure 3.
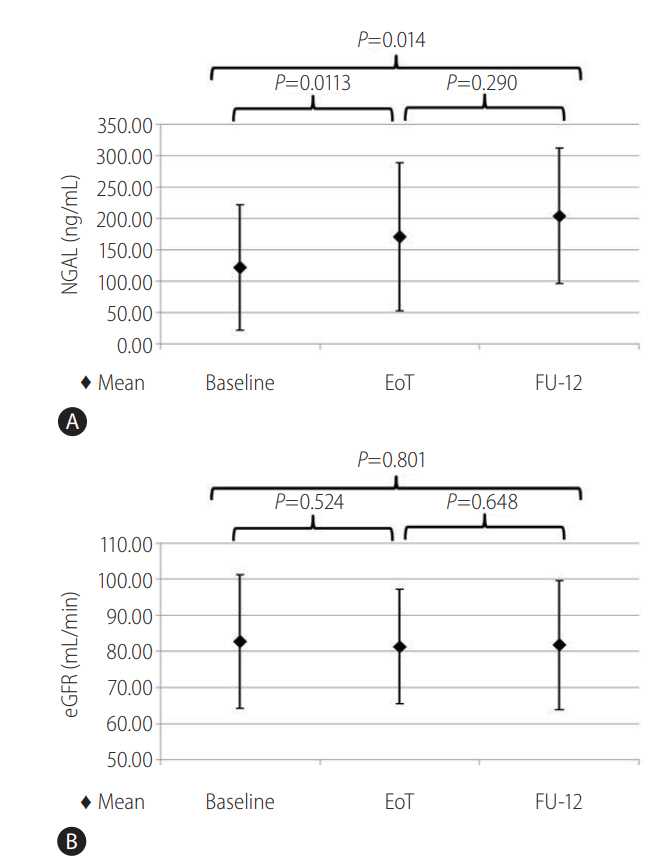
Table 1.
Values are presented as n (%) or mean (SD) unless otherwise indicated.
eGFR, estimated glomerular filtration rate; NGAL, neutrophil gelatinase associated lipocalin; BMI, body mass index; HCV, hepatitis C virus; IFN, interferon; HBcAb, hepatitis B core antibody; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; HBsAb, hepatitis B surface antibody; HIV, human immunodeficiency virus; NSAIDs, non-steroidal anti-inflammatory drugs; CKD, chronic kidney disease; SD, standard deviation; RNA, ribonucleic acid; INR, International normalized ratio; AST, aspartate aminotrasferase; ALT, alanine aminotransferase; FIB-4, Fibrosis-4; APRI, AST to platelet ratio index.
Table 2.
Values are presented as n (%).
eGFR, estimated glomerular filtration rate; NGAL, neutrophil gelatinase associated lipocalin; DAAs, directly acting antivirals; SOF, sofosbuvir; LDV, ledipasvir; DCV, daclatasvir; SMV, simeprevir; PTV, paritaprevir; OVB, ombitasvir; r, ritonavir; DSB, dasabuvir; IFN, interferon; Gen. DAA, generation directly acting antivirals; SVR, sustained virological response.
Table 3.
Values are presented as n (%) or mean (SD) unless otherwise indicated.
RBV, ribavirin; eGFR, estimated glomerular filtration rate; NGAL, neutrophil gelatinase associated lipocalin; HCV, hepatitis C virus; IFN, interferon; CKD, chronic kidney disease; AST, aspartate transaminase; ALT, alanine transaminase; FIB-4, Fibrosis-4; APRI, AST to platelet ratio index.
Abbreviations
REFERENCES
- TOOLS
-
METRICS

- ORCID iDs
-
Alessio Strazzulla

https://orcid.org/0000-0003-0199-3449 - Related articles



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print



