Influence of hepatic steatosis on the outcomes of patients with chronic hepatitis B treated with entecavir and tenofovir
Article information
Abstract
Background/Aims
The influence of hepatic steatosis (HS) on chronic hepatitis B (CHB) is unclear. We evaluated the influence of the degree of HS, assessed using the controlled attenuation parameter (CAP) of transient elastography (TE), on treatment outcomes in CHB patients initiated on antiviral therapy.
Methods
A total of 334 patients who were initiated on entecavir or tenofovir between 2007 and 2016 with available TE results were recruited.
Results
Of the total study population, 146 (43.7%) patients had HS (CAP > 238 dB/m). Three-hundred-three patients (90.7%) achieved complete virological response (CVR) (hepatitis B virus DNA<12 IU/L), and 25 patients (7.5%) developed hepatocellular carcinoma (HCC). Among hepatitis B e antigen (HBeAg)-positive patients (n=172, 51.5%), 37 (21.5%) experienced HBeAg loss. On univariate analysis, CAP value was not associated with the probability of HCC development (P=0.380). However, lower CAP value was independently associated with higher probability of HBeAg loss among HBeAg-positive patients (hazard ratio [HR]=0.991, P=0.026) and with CVR achievement in the entire study population (HR=0.996, P=0.004). The cumulative incidence of HBeAg loss among HBeAg-positive patients was significantly higher in patients without HS than in those with HS (log-rank, P=0.022).
Conclusions
CAP values were not correlated with HCC development in patients initiated on entecavir and tenofovir. However, CAP values were negatively correlated with the probability of HBeAg loss among HBeAg-positive patients and with CVR achievement.
Introduction
More than 250 million people are infected with hepatitis B virus (HBV) worldwide, including >6% of the Asian and Western Pacific adult population [1]. Additionally, more than 20% of patients with chronic hepatitis B (CHB) developed hepatocellular carcinoma (HCC) within 10 years [2,3]. Thus, to prevent poor outcomes in patients with HBV viremia due to uncontrolled replication, the primary treatment strategy is to suppress replication with antiviral therapy (AVT) [4]. Furthermore, newer AVT agents such as entecavir and tenofovir, with little to no resistance, introduce new methods of blocking HBV replication [5].
Hepatic steatosis (HS) is frequently found in patients with chronic liver disease and its prevalence in HBV infected patients was reported to be between 14% and 67% [6]. However, the influence of HS on HBV infection has been controversial. First, no association between HS and HBV has been reported. Some studies showed no significant correlation between the presence of hepatitis B e antigen (HBeAg) and HS [7,8] and between HBV DNA level and HS in genotypes B and C HBV [9]. Moreover, a recent study demonstrated that HS diagnosed with sonography showed no significant correlation with virological response or HBeAg seroconversion in CHB patients treated with entecavir [10]. In contrast, pooled data from seven studies showed a strong protective influence of HS on HBV viral load [11]. The unfavorable influence of HS on the clinical outcomes of patients with HBV infection has been also reported. Recent studies strongly suggested that severe HS is associated with severe fibrosis in HBV infection [12,13] and that HS lowered the efficacy of AVT [10]. Furthermore, a previous study showed that the presence of metabolic syndrome, which is closely correlated with HS, was an independent risk factor linked with an increased risk of liver cirrhosis and HCC in CHB patients [6,14].
Liver biopsy is considered the gold standard for evaluating HS [15]. However, this process cannot be sufficiently used repetitively and its value as a screening method is limited by its drawbacks ranging from invasiveness to sampling errors [16]. As a noninvasive tool for HS diagnosis, ultrasonography is commonly used; however, it has limitations such as observer variability and low sensitivity of mild HS [17]. Recently, transient elastography (TE), equipped with a controlled attenuation parameter (CAP), is a proven effective method for determining the degree of HS and fibrosis simultaneously. The accuracy of CAP in detecting HS has been considered acceptable [18].
Thus, because it is not yet confirmed whether HS can influence the outcome of AVT, we aimed to evaluate the influence of HS, assessed using CAP, on the treatment outcomes in CHB patients initiated on AVT using entecavir and tenofovir.
Materials and methods
Between 2007 and 2016, treatment-naïve CHB patients with available TE results who were initiated on AVT with entecavir or tenofovir, from the database of Severance Hospital, Yonsei University College of Medicine, Seoul, South Korea, were considered eligible for this retrospective study. CHB was defined as the persistent presence of serum HBV surface antigen for >6 months. The exclusion criteria were as follows: liver stiffness (LS) measurement failure; unreliable LS value; >1 month interval between AVT initiation and TE assessment; HCC at enrollment or history of HCC; hepatic decompensation or liver transplantation at enrollment, or history of either condition; Child-Pugh class B or C; alanine aminotransferase (ALT) >5× the upper normal limit; co-infection with human immunodeficiency virus or hepatitis C virus; alcohol ingestion in excess of 40 g/day for >5 years; right-sided heart failure; ascites or pregnancy; any other serious medical comorbidities; or follow-up duration <12 months.
The study methodology conforms to the ethical guidelines of the Declaration of Helsinki and was approved by the institutional review board of Severance Hospital. Informed consent was waived owing to the retrospective nature of the study.
Enrollment and follow-up
Patients initiated on AVT with either entecavir or tenofovir were selected according to our inclusion and exclusion criteria. AVT was administered according to the treatment guidelines of the Korean Association for the Study of the Liver [19] and the reimbursement guidelines of the National Health Insurance Service of the Republic of Korea. After AVT initiation, each patient was regularly followed up every 3 or 6 months with ultrasound examination and laboratory tests. These included tests for the levels of alpha-fetoprotein and virological markers such as HBV DNA and HBeAg and anti-HBe antibody. In case of virological breakthrough (defined as >1 log IU/mL increase in serum HBV DNA level from nadir on 2 consecutive tests) or genotypic mutation, rescue therapy was applied [20].
Fibrosis and steatosis assessment with TE
TE was performed on the right lobe of the liver through the intercostal spaces, with the patient lying down in the dorsal decubitus position with the right arm in maximal abduction. Experienced nurses blinded to clinical information performed TE (FibroScan®; EchoSens, Paris, France). The results were expressed in kilopascals (kPa) for fibrosis and decibels per meter (dB/m) for steatosis. Interquartile range (IQR) was used as an index of intrinsic variability of TE readings, corresponding to the central interval containing 50% of valid measurements between the 25th and 75th percentiles. Only examinations with at least 10 validated measurements and a success rate of at least 60% and an IQR to median ratio <30% for LS were accepted as reliable [15,19]. The cutoff LS value for significant fibrosis was 13 kPa [21]. The cutoff CAP value to diagnose the presence of HS was ≥238 dB/m [22].
End points and definitions
Complete virological response (CVR), HBeAg loss among HBeAg-positive patients, and HCC development were monitored. CVR was defined as undetectable HBV DNA level <12 IU/L. HBeAg loss was defined as HBeAg negativity among HBeAg-positive patients at enrollment. HCC diagnosis was based on the guidelines of the American Association for the Study of Liver Diseases [23]. Liver cirrhosis was diagnosed on the basis of ultrasonographic findings suggestive of cirrhosis, including a blunted, nodular liver edge with splenomegaly (>12 cm) [24].
Statistical analysis
Continuous variables were expressed as median with IQR, as appropriate. When comparing baseline characteristics between two groups, a chi-square test (or Fisher’s exact test) and Student’s t -test (or Mann-Whitney U -test) were used. Actuarial rates of clinical outcomes were calculated using the Kaplan-Meier method and compared with the log-rank test. Independent risk factors for each clinical outcome were estimated using multivariate Cox proportional hazard regression analysis. Multivariate analysis was performed using variables with a P -value of <0.2 in univariate analysis. Statistical analyses were performed using SPSS software 24.0 (IBM Corp., Armonk, NY, USA), and statistical significance was considered for comparisons with a 2-tailed P -value of <0.05.
Results
Baseline characteristics
Among 1,658 treatment-naïve patients with CHB who were initiated on AVT with entecavir or tenofovir between 2007 and 2016, 476 patients with available TE results were evaluated. Twenty-one patients were excluded owing to LS measurement failure or unreliable LS value. Of 455 patients with reliable LS value, 121 were excluded according to our exclusion criteria. Finally, 334 patients were selected for this retrospective study (Supplementary Fig. 1).
The baseline characteristics of the whole study population are described in Table 1. The median age of the study population (210 men and 124 women) was 51.0 years. HBeAg positivity was found in 172 (51.5%) patients, and the median HBV DNA level was 5.84 log IU/mL. The median CAP value was 229.0 (IQR 202.8–255.0) dB/m. Most patients (n=292, 87.4%) received tenofovir.
Comparison between patients with and without HS
Of the study population, 146 (43.7%) patients showed HS (Table 1). When patients with and without HS were compared, patients with HS had significantly higher body mass index (BMI), serum albumin level, platelet count, total cholesterol level, and CAP values than those without HS (all P<0.05) However, LS values and the proportion of patients with liver cirrhosis (45.9% vs. 51.6%) were not significantly different between patients with and without HS (all P >0.05) (Table 1).
Treatment outcomes of AVT
The median follow-up period was 38.6 (IQR 28.1–47.6) months (median 38.4 [IQR 28.0–46.7] in patients with HS vs. 38.6 [IQR 28.1–48.4] months in patients without HS, P =0.757).
The AVT outcomes are listed in Supplementary Table 1. During the follow-up period, 303 (90.7%) and 25 (7.5%) patients achieved CVR and developed HCC at 5 years, respectively. Among 172 HBeAg-positive patients, 37 (21.5%) experienced HBeAg loss. the proportion of patients with HBeAg loss among HBeAgpositive patients at 5 years was significantly higher in those without HS (28.3% vs. 13.8%; P =0.022, log-rank test) (Supplementary Table 1, Fig. 1).
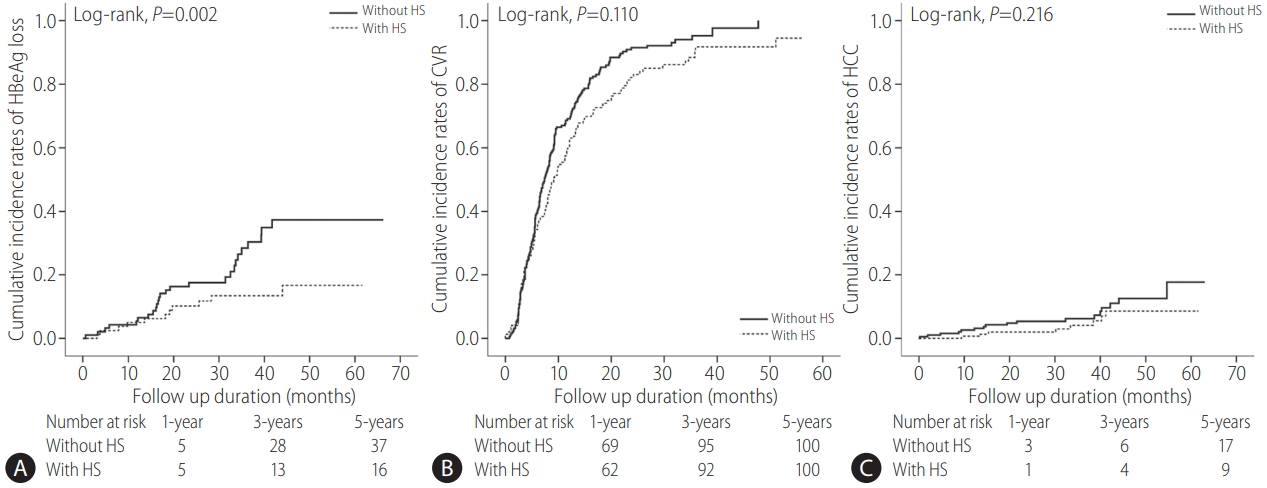
Cumulative incidence rates of HBeAg loss (A) (n=172), CVR (B), and HCC (C) (n=334) in patients with and without HS (Kaplan-Meier plot). The cumulative incidence rates of HBeAg loss were calculated among HBeAg-positive patients. HBeAg, hepatitis B e antigen; CVR, complete virological response; HCC, hepatocellular carcinoma; HS, hepatic steatosis.
Among patients treated with entecavir (n=42, 12.6%), the probability of HBeAg loss among HBeAg-positive patients was not statistically significant between the groups with and without HS (P =0.139, log-rank test), whereas among patients treated with tenofovir (n=292, 87.4%), the probability of HBeAg loss was significantly higher in the group without HS (P =0.046, log-rank test) (Supplementary Fig. 2).
The follow-up period was statistically similar between HBeAgpositive and HBeAg-negative patients (median 38.7 [IQR 28.3–48.0] vs. 38.6 [IQR 27.9–47.4] months, P =0.461). In contrast, the follow-up duration of patients treated with entecavir and tenofovir was significantly different (median 47.0 [IQR 39.1–59.0] vs. 36.7 [27.3–46.7] months, P<0.001). When the study population was stratified according to HBeAg status, the proportion of patients with CVR achievement was significantly higher at 5 years among HBeAg-negative patients than in HBeAg-positive patients (96.3% vs. 85.5%; P<0.001, log-rank test) (Supplementary Table 2, Supplementary Fig. 3).
Comparisons between patients with and without HBeAg loss, CVR achievement, and HCC development
Because the rate of HBeAg loss was significantly different according to the presence of HS, we selected 172 HBeAg-positive patients and compared the baseline characteristics between those with and without HBeAg loss (Table 2). Patients with HBeAg loss showed significantly lower CAP value (median 214.0 vs. 239.0 dB/m, P=0.013), whereas the proportion of CVR achievement was significantly higher in patients with HBeAg loss (97.3 vs. 82.2%, P =0.021) (Table 2).
When the study population was stratified according to CVR achievement (Table 3) and HCC development (Supplementary Table 3), BMI (median 23.3 vs. 24.9 kg/m2), ALT level (median 54 vs. 66 IU/L), platelet count (median 148×109/L vs. 182×109/L), HBeAg positivity (48.5 vs. 80.6%), HBV DNA level (median 5.66 vs. 8.23 log IU/mL), CAP value (median 228 vs. 244 dB/m) were significantly lower in patients with CVR achievement than in those without (all P<0.05), whereas the proportion of liver cirrhosis was significantly higher in patients with CVR achievement than in those without (51.2 vs. 27.3%, P =0.019). Additionally, patients who developed HCC were significantly older (median age 57.0 vs. 50.0 years) and had a significantly higher proportion of liver cirrhosis (92.0% vs. 45.6%) than those who did not (all P<0.001).
Independent predictors of treatment outcomes
First, univariate and subsequent multivariate analyses were performed to identify independent predictors of HBeAg loss among HBeAg-positive patients (Table 4). When CAP values were adjusted as continuous variables, higher ALT level (hazard ratio [HR]=1.017, 95% confidence interval [CI] 1.008–1.027; P<0.001), lower HBV DNA level (HR=0.814, 95% CI 0.687–0.964; P =0.017), and lower CAP values (HR=0.991, 95% CI 0.983–0.999; P =0.026) were independently associated with a higher probability of HBeAg loss among HBeAg-positive patients. Furthermore, CAP value was selected as an independent predictor of CVR achievement (Table 5, Supplementary Table 4).
When the cutoff value of 238 dB/m was used to define HS (S1-3 [n=146, 43.7%] vs. S0 [n=188, 56.3%]) [22], the presence of HS tended to reduce the probability of HBeAg loss (HR=0.494, 95% CI 0.233–1.047, P =0.066); however, both CVR achievement and HCC development were not significantly associated with HS after adjustment (all P >0.1). In addition, when HS was further divided as S0-1 (n=259, 77.5%) vs. S2-3 (n=75, 22.5%) using the cutoff value of 269 dB/m,22 S2-3 was not associated with the probability of HBeAg loss and HCC development (all P >0.1). S2-3 was, however, independently associated with a reduced probability of CVR achievement (HR=0.676, 95% CI 0.500–0.914, P =0.011).
Discussion
In this study, around half of the study population with CHB had concomitant HS (146 of 334, 43.7%) at the time of initiating AVT. During the study period, 303 (90.7%) and 25 (7.5%) patients achieved CVR and developed HCC, respectively. Moreover, among 172 HBeAg-positive patients, 37 (21.5%) experienced HBeAg loss. Multivariate analysis indicated that lower CAP value was independently associated with a higher probability of HBeAg loss among HBeAg-positive patients (HR=0.989) and with CVR achievement in the entire study population (HR=0.996, P =0.004), whereas the probability of HCC development was not significantly associated with CAP value (P =0.380). Finally, the cumulative incidence of HBeAg loss among HBeAg-positive patients was significantly higher in patients without HS than in those with HS (P =0.022, log-rank test).
In accordance with the rising prevalence of fatty liver worldwide, the prevalence of concomitant HS in chronic viral hepatitis and its influence on the natural course or treatment outcomes have recently gained medical interest [6]. Furthermore, recent potent antiviral agents such as entecavir and tenofovir have been reported to suppress viral replication and the progression of liver cirrhosis, in of patients treated with AVT [25]. Thus, our study naturally focused on the remaining factors of concomitant HS that could not be controlled using antiviral agents. According to previous studies in CHB patients, the prevalence of concomitant HS has ranged from 15% to 48.8% [6,26]. The prevalence of HS affects 20–46% of the general Western and Asian population [12,27], which is not so different from that estimated in CHB patients with HS. In our cohort, 43.7% of the CHB population had coexisting HS, which is similar to the previous prevalence.
Studies on the interaction between HS and HBV have provided controversial results. Pooled data of seven studies found an inverse association between HBV DNA levels and fatty liver in HBV patients [11], whereas Wong et al. reported a negative association between CHB and nonalcoholic fatty liver disease prevalence [28]. Our study found a significant influence of HS on the long-term treatment outcomes of AVT in CHB patients; higher CAP values were associated with a lower probability of HBeAg loss among HBeAgpositive patients. Our results are supported by a recent metaanalysis showing that the efficacy of AVT declined in CHB patients with HS [10]. This study suggested that virological and biochemical responses were significantly different between subgroups with and without HS diagnosed using sonography [19]. This negative effect of HS on HBeAg loss in HBeAg-positive patients might be explained by the decreased bioavailability of AVT due to fatty liver load and its fatty acid deposits leading to a significant loss in contact area between hepatocytes and antiviral agents [29]. In contrast, other studies have indicated that HS would not be significantly associated with virological response or HBeAg seroconversion [26]. Differences in antiviral agents, range of BMI, or diagnostic tools for diagnosing HS (TE in our study vs. ultrasonography in previous studies) might explain this discrepant result. Indeed, TE has been considered a more reliable tool for the diagnosis and grading of HS in chronic viral hepatitis [17].
Furthermore, CAP value was selected as an independent negative predictor of CVR achievement in the entire study population. Cell histological changes in fatty liver, such as the nucleus being pushed to the periphery, could prevent the interaction between the AVT agent and viral nucleic acids and explain the negative correlation between CAP value and the probability of CVR achievement [30]. After adjustment, HBeAg positivity and HBV DNA levels were both significantly associated with a lower probability of CVR achievement, which has been a well-known disadvantage [4].
Although the clear relation between HCC in HS patients and HBV is unclear, many factors, including metabolic factors, that are significantly associated with fatty liver, are considered to increase the risk of HCC development in HBV-infected patients [9]. However, fatty liver and CAP value did not influence the risk of HCC development in our study. The reason for this discrepancy is unclear, but can be explained in part by the proportion of CHB patients with accompanying nonalcoholic steatohepatitis with fibrosis progression. Indeed, although a recent study by Seto et al. showed a significant correlation between the degree of liver steatosis and fibrosis assessed using TE [12], the association was not significant in our study. Although histological information is lacking, the different proportions of CHB patients with concomitant insult from fatty liver-related inflammation and fibrosis may have been a factor [11]. Thus, the exact association between steatotic burden and the risk of HCC development should be further investigated.
Our study has several advantages that strengthen its clinical implications. First, our study is the first of its kind to comprehensively analyze the effects of HS on the clinical outcomes of CHB patients undergoing treatment with potent AVT agents according to recent guidelines [4]. Thus, our results can be applied to CHB patients in this era of potent and active AVT. Second, in contrast to previous research with subjective ultrasonography assessment of steatotic burden, our study adopted TE with CAP to assess HS, which has been widely used to assess the degree of fatty liver [12,18]. Finally, we focused on the prognostic clinical influence of accompanying HS in CHB patients undergoing AVT beyond the crosssectional analysis. Although the influence of dynamic change in steatotic burden during AVT could not be assessed, our results might provide the rationale to reduce liver steatosis, to increase the probability of HBeAg seroconversion for HBeAg-positive patients.
Our study also has several limitations. First, although the overall sample size was acceptable, the proportion of end points such as HCC was relatively small. Thus, the lack of association between steatotic burden and the risk of HCC development might stem from statistical bias. Indeed, cirrhosis being selected as one of the independent predictors of HCC development in our study whereas LS value assessed using TE was not may be attributable to the small number of patients who developed HCC. Additionally, the high proportion of patients with liver cirrhosis (49.1%) and high ALT levels at the time of starting AVT, in spite of the exclusion of ALT >5× the upper limit of normal, might also have influenced our results. Second, our study was limited by the lack of data on detailed histological characteristics related to the risk of end-point achievement. We do not know whether pure steatotic burden was significantly associated with the treatment outcomes or whether a combination of steatotic burden and additional characteristics such as fibrosis are involved. Lastly, the skewed distribution of patients with different steatotic burden might have biased our results. Owing to the retrospective nature of this study, it was inevitable to have more patients with lesser steatotic burden. Likely due to the same reason, the results from binary stratification of HS using several previous cutoff values were similar, but not consistent with those from continuously expressed CAP values [22].
In conclusion, we demonstrated that CAP value was not correlated with HCC development in patients initiated on AVT with entecavir and tenofovir. However, CAP value was negatively correlated with the risk of HBeAg loss among HBeAg-positive patients and with CVR achievement in the entire study population.
Notes
Author’s contribution
S.U. Kim designed this study.
D.S. Kim, M.Y. Jeon, and S.U. Kim, carried out the data analysis and wrote the manuscript.
D.S. Kim, M.Y. Jeon, H.W. Lee, B.K. Kim, J.Y. Park, D.Y. Kim, S.H. Ahn, K.H. Han, and S.U. Kim contributed to inclusion of patients, acquisition and analysis of data.
All authors contributed to the interpretation of results, critical revision of the manuscript and approved the final manuscript.
S.U. Kim is the guarantor.
Financial support
This study was supported by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Science, ICT & Future Planning (2016R1A1A1A05005138). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Illustration assistance The authors are grateful to Dong-Su Jang, (Medical Illustrator, Medical Research Support Section, Yonsei University College of Medicine, Seoul, Republic of Korea) for his help with the figures.
Notes
Conflicts of Interest: None to declare for all authors.
SUPPLEMENTARY MATERIALS
Supplementary materials are available at Clinical and Molecular Hepatology website (http://www.e-cmh.org)
Flow of study population selection. A total of 1,658 treatment-naïve patients with CHB who were initiated on antiviral therapy with ETV or TDF between 2007 and 2016 were considered eligible. Of them, 476 patients received TE assessment at enrollment. Twenty-one patients were excluded owing to LS measurement failure or unreliable LS value. Of 455 patients with reliable LS value, 121 were excluded according to our exclusion criteria. Finally, 334 patients were selected for the statistical analysis. CHB, chronic hepatitis B; ETV, entecavir; TDF, tenofovir; TE, transient elastography; LS, liver stiffness; AVT, antiviral therapy; HCC, hepatocellular carcinoma; HIV, human immunodeficiency virus.
Cumulative incidence rates of HBeAg loss according to the presence of hepatic steatosis among HBeAg-positive patients treated with entecavir (A) or tenofovir (B). Among patients treated with entecavir (n=42, 12.6%), the probability of HBeAg loss among HBeAg-positive patients was not statistically significant between the groups with and without hepatic steatosis (P=0.139, log-rank test), whereas among patients treated with tenofovir (n=292, 87.4%), it was significantly higher in the group without hepatic steatosis (P=0.046, log-rank test). HBeAg, hepatitis B e antigen; HS, hepatic steatosis.
Cumulative incidence rates of HBeAg loss, complete virological response, and HCC in patients with and without HBeAg (A–C) and in patients treated with entecavir or tenofovir (D–F) (Kaplan-Meier plot). The cumulative incidence rates of HBeAg loss were calculated among HBeAg-positive patients (n=172). The cumulative incidence rates of CVR achievement were significantly higher in HBeAg-negative patients than in HBeAg-positive patients (P<0.001, log-rank test) (n=334). The cumulative incidence rates of HCC were significantly higher in HBeAg-negative patients than in HBeAg-positive patients (P=0.027, log-rank test) (n=334). In contrast, the cumulative incidence rates of CVR achievement, HBeAg loss among HBeAg-positive patients, and HCC were statistically similar (all P>0.05, log-rank test). HBeAg, hepatitis B e antigen; HCC, hepatocellular carcinoma; CVR, complete virological response.
Treatment outcomes of antiviral therapy in entire population and subgroups with and without hepatic steatosis
Treatment outcomes of antiviral therapy in the subgroups with and without HBeAg positivity and in the subgroups treated with entecavir and tenofovir
Comparison between patients who developed HCC and those without
Independent predictors of HCC development
Abbreviations
ALT
alanine aminotransferase
AVT
antiviral therapy
BMI
body mass index
CAP
controlled attenuation parameter
CHB
chronic hepatitis B
CI
confidence interval
CVR
complete virological response
dB/m
decibels per meter
HBeAg
hepatitis B e antigen
HBV
hepatitis B virus
HCC
hepatocellular carcinoma
HS
hepatic steatosis
HR
hazard ratio
IQR
interquartile range
kPa
kilopascals
LS
liver stiffness
TE
transient elastography
References
Article information Continued
Notes
Study Highlights
• There is a lack of evidence to support the influence of hepatic steatosis (HS) on antiviral therapy with entecavir and tenofovir.
• The controlled attenuation parameter (CAP) values, measured by transient elastography, can assess the degree of HS.
• CAP values were negatively correlated with the probability of hepatitis B e antigen (HBeAg) loss among HBeAg-positive patients.
• CAP values were associated with the achievement of complete virological response, whereas they were not associated with the risk of developing hepatocellular carcinoma.





