Gadoxetic acid-enhanced magnetic resonance imaging: Hepatocellular carcinoma and mimickers
Article information
Abstract
Gadoxetic acid, a hepatocyte-specific magnetic resonance imaging (MRI) contrast agent, has emerged as an important tool for hepatocellular carcinoma (HCC) diagnosis. Gadoxetic acid-enhanced MRI is useful for the evaluation of early-stage HCC, diagnosis of HCC precursor lesions, and highly sensitive diagnosis of HCC. Furthermore, functional information provided by gadoxetic acid-enhanced MRI can aid in the characterization of focal liver lesions. For example, whereas lesions lack functioning hepatocytes appear hypointense in the hepatobiliary phase, preserved or enhanced expression of organic anion transporting polypeptides in some HCCs as well as focal nodular hyperplasia lead to hyperintensity in the hepatobiliary phase; and a targetoid appearance on transitional phase or hepatobiliary phase imaging can be helpful for identifying the histopathological composition of tumors. While gadoxetic acid-enhanced MRI may improve the sensitivity of HCC diagnosis and provide new insights into the characterization of focal liver lesions, there are many challenges associated with its use. This article reviews the pros and cons of HCC diagnosis with gadoxetic acid-enhanced MRI and discuss some clues in the radiological differentiation of HCC from HCC mimickers.
INTRODUCTION
Liver cancer is the fourth leading cause of cancer-related death globally [1]. Hepatocellular carcinoma (HCC) accounts for about 90% of primary liver cancers and its incidence is increasing worldwide [2]. In patients with liver cirrhosis, a noninvasive diagnosis of HCC can be established by a radiological hallmark exhibited on dynamic liver protocol computed tomography (CT) or magnetic resonance imaging (MRI) [2,3]. The classic radiological hallmark is arterial phase hyperenhancement (APHE) and portal venous phase (PVP) or delayed phase “washout” on extracellular contrast agent (ECA)-enhanced CT or MRI.
During the past decade, hepatocyte-specific contrast agents for MRI have been recognized as important tools for the detection of small HCCs and the characterization of focal liver lesions [4-6]. Gadoxetic acid (gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid; Gd-EOB-DTPA; Primovist, Bayer Schering Pharma, Berlin, Germany), a widely used hepatocyte-specific contrast agent, is excreted into the bile and urine at an approximate ratio of 1:1. The property of sufficient hepatobiliary excretion allows hepatobiliary phase (HBP) imaging, which is acquired approximately 20 minutes after contrast injection and exhibits excellent lesion-to-liver contrast [7].
As the diagnosis of HCC at an early stage is desirable, the use of gadoxetic acid-enhanced MRI for HCC diagnosis is recommended by several guidelines, including the Asia–Pacific clinical practice guidelines, the Japan Society of Hepatology guidelines, and the Korean Liver Cancer Study Group-National Cancer Center Korea Practice guidelines [8-10]. The Liver Imaging Reporting and Data System (LI-RADS) has also embraced the use of gadoxetic acid-enhanced MRI since 2014 [11]. The performance of HCC diagnostic criteria in gadoxetic acid-enhanced MRI is under continuous investigation [12-16].
This article first reviews the pros and cons of a radiological diagnosis of HCC with gadoxetic acid-enhanced MRI. Thereafter, some clues in the radiological differentiation of HCC from HCC mimickers will be discussed. Mimickers of HCC to be differentiated by imaging include 1) non-HCC malignancies, such as intrahepatic mass-forming cholangiocarcinoma (iCCA), combined hepatocellular carcinoma-cholangiocarcinoma (cHCC-CCA), and hypervascular metastasis; 2) benign hepatocellular lesions, including focal nodular hyperplasia (FNH), FNH-like nodules, and hepatocellular adenoma (HCA); and 3) benign non-hepatocellular lesions, such as hemangioma, hypervascular pseudolesions, and angiomyolipoma.
HCC DIAGNOSIS USING GADOXETIC acid-enhanced MRI
The major advantage of using gadoxetic acid-enhanced MRI is the improved detection of small (≤2 cm) HCCs compared with ECA-enhanced MRI or CT [17-19]. The detection sensitivity can be further heightened by the use of diffusion-weighted imaging (DWI) [20]. Furthermore, it has been demonstrated that the combined use of gadoxetic acid-enhanced MRI and CT can diagnose additional HCCs compared with the use of CT alone, which may change the tumor stage and lead to improved survival outcomes [21]. Therefore, gadoxetic acid-enhanced MRI is considered an important diagnostic tool, particularly in patients with early-stage HCC.
Another advantage of using gadoxetic acid-enhanced MRI is that it facilitates the diagnosis of borderline hepatic nodules (i.e., early HCC and high-grade dysplastic nodule [HGDN]), which are considered precursors of progressed, distinctly nodular HCC [12,22]. Early HCC is histopathologically defined as vaguely nodular, small (≤2 cm), well-differentiated HCC and is distinguished from HGDNs in terms of the presence of stromal invasion [23]. While progressed HCCs are typically hypervascular on imaging, early HCCs rarely exhibit arterial hypervascularity and thus are not diagnosed as HCC by this radiological hallmark [24]. On the other hand, the expression of organic anion transporting polypeptide 1B3 (OATP1B3), the transporter of gadoxetic acid, starts decreasing prior to neo-arterialization during hepatocarcinogenesis [24,25]. Consequently, early HCCs and approximately one-third of HGDNs appear as non-hypervascular HBP hypointense nodules (NHHNs) on gadoxetic acid-enhanced MRI, contrary to benign cirrhosis-associated nodules or low-grade dysplastic nodules which are usually isointense in the HBP due to preserved OATP1B3 expression [26,27]. About one-third of NHHNs undergo hypervascular transformation within 3 years [28]. In addition, the presence of an NHHN is regarded as a cause of intrahepatic recurrence after surgical resection or radiofrequency ablation [29,30]. The management strategy for these borderline lesions is not yet established and therefore needs further investigation [31].
Concerns have been raised regarding the decrease in the specificity of HCC diagnosis when using gadoxetic acid-enhanced MRI [31]. The radiological hallmark (i.e., APHE and PVP or delayed phase “washout”) used in ECA-enhanced CT or MRI provides lower specificity on gadoxetic acid-enhanced MRI, which can be attributed to the “pseudo-washout” phenomenon observed in HCC mimickers (i.e., the relative hypointensity of a non-HCC lesion in the transitional phase [TP] due to adjacent liver parenchymal contrast uptake) [5,32-34]. Therefore, the 2018 European Association for the Study of the Liver guidelines and LI-RADS require that “washout” be assessed only in the PVP when using gadoxetic acid-enhanced MRI, not in the TP or HBP [2,35]. However, PVP “washout” in gadoxetic acid-enhanced MRI has been regarded as less sensitive for HCC than TP or HBP hypointensity, especially for small HCCs, and is not independently predictive of HCC [12,13]. Studies have also demonstrated that use of the HBP improves the detection of small HCCs and provides near-perfect sensitivity for HCC diagnosis [13,36].
Recent studies have suggested that the exclusion of HCC mimickers may overcome the hurdle of “pseudo-washout” and enable a more sensitive diagnosis of HCC on gadoxetic acid-enhanced MRI [12-14,37]. When benign lesions, such as hemangioma (i.e., LI-RADS category 1 or 2) and possible non-HCC malignancy (i.e., LI-RADS category M) are properly excluded by the LI-RADS algorithm, an evaluation of “washout” in the TP as well as the PVP can diagnose HCC without impairing specificity (Fig. 1) [37]. Furthermore, the evaluation of “washout” in the PVP, TP, or HBP in lesions that are not LI-RADS category 1, 2, or M identifies HCC with acceptable specificity [14]. When hemangiomas are excluded from nodules detected by surveillance ultrasound, the presence of APHE and HBP hypointensity can provide comparable specificity to that of APHE and PVP or TP hypointensity; the satisfaction of APHE, HBP hypointensity, and restricted diffusion criteria can result in similar specificity to that of APHE and PVP “washout” criteria [12,13]. These diagnostic criteria may be especially applicable when improved sensitivity for HCC diagnosis is prioritized over an “acceptable” decrease in specificity [31].

A small hepatocellular carcinoma (HCC) in a 58-year-old man with chronic hepatitis B. A 19-mm nodule in hepatic segment VIII depicts (A) non-rim arterial phase hyperenhancement and “washout” in the (B) portal venous phase, qualifying it as Liver Imaging Reporting and Data System category 5 (i.e., definite HCC). Hypointensity in the (C) transitional phase and (D) hepatobiliary phase are also noted on gadoxetic acid-enhanced magnetic resonance imaging.
MIMICKERS OF HCC
Non-HCC malignancy
Non-HCC malignancy frequently exhibits a targetoid appearance on imaging, namely targetoid dynamic enhancement, targetoid diffusion restriction, and a targetoid TP or HBP appearance [35]. The appearance results from the histopathological composition of the tumor with dense fibrous stroma at the core and hypercellularity at the periphery. The peripheral cellular component shows hyperenhancement in the arterial phase and hypoenhancement in the following phases, while the central fibrous stroma enhances progressively throughout dynamic imaging. A targetoid appearance on TP or HBP imaging as observed on gadoxetic acid-enhanced MRI refers to mild contrast retention in the fibrous core and a lack of contrast uptake in the peripheral cellular portions; the feature can sometimes be the sole identifier of an iCCA on imaging [37,38]. Pitfalls in radiological differentiation between non-HCC malignancy and HCC lie in the facts that HCC with atypical imaging features can exhibit targetoid features as well (Fig. 2) and that some non-HCC malignancies, especially cHCC-CCA and small-sized iCCAs, can mimic HCC by demonstrating a non-targetoid appearance.

A surgically diagnosed hepatocellular carcinoma (HCC) with abundant fibrous stroma mimicking a non-HCC malignancy. In a 74-year-old man with alcoholic liver disease, a 40-mm mass in hepatic segment IV shows a targetoid appearance with rim-like hyperenhancement in the (A) arterial phase, and peripheral “washout” and progressive central enhancement throughout the (B) portal venous phase and (C) transitional phase. The targetoid appearance is also demonstrated in the (D) hepatobiliary phase with mild contrast retention in the fibrous core and on (E) diffusion-weighted imaging with peripheral diffusion restriction. Histopathologic confirmation is often required for a conclusive diagnosis of a targetoid mass, which rendered the diagnosis of scirrhous HCC for this lesion.
Similar to ECA-enhanced MRI, rim APHE, being a targetoid feature, is the most important imaging feature distinguishing iCCA from HCC on gadoxetic acid-enhanced MRI [33,39,40]. However, HCC with abundant fibrotic stroma or a central scar can also be observed as a targetoid mass [41]. Features of the enhancing “capsule,” an intratumoral septum, and multifocal T2 hyperintense foci can be clues for identifying HCC when it mimics iCCA on imaging [41,42]. Owing to the overlaps in targetoid features among HCC and non-HCC malignancies, histopathologic confirmation can be considered for a targetoid mass [3]. On the other hand, iCCA can also depict non-rim APHE, particularly when the tumor is small in size [39,43]. Histopathologically, “small duct type” iCCAs arise commonly from the peripheral small bile duct in a cirrhotic liver or chronic hepatitis and have a tendency to possess less stromal fibrotic tissue than “large duct type” iCCAs, which originate from chronically diseased perihilar bile ducts [44,45]. Because of their histopathologic characteristics, “small duct type” iCCAs depict a larger area of APHE and diffusion restriction on imaging and thus are likely to mimic HCC (Fig. 3) [46].
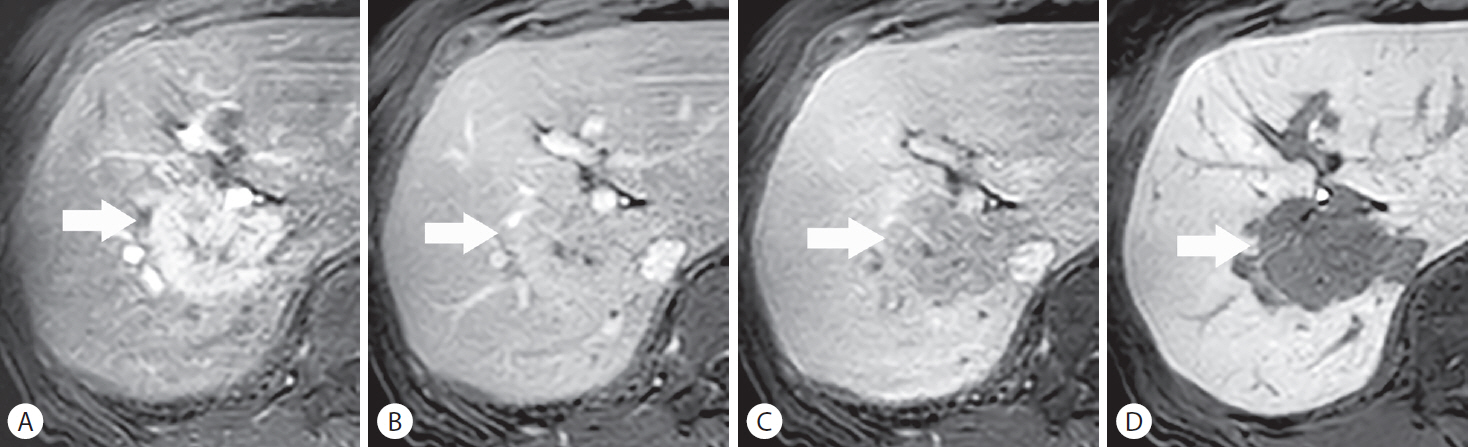
An intrahepatic mass-forming cholangiocarcinoma (iCCA) mimicking hepatocellular carcinoma (HCC) in a 79-year-old woman with no underlying liver disease. A 46-mm lobulated mass (arrows) in hepatic segment VII shows non-rim hyperenhancement in the (A) arterial phase and persistent contrast enhancement in the (B) portal venous phase. The nodule depicts mild hypointensity in the (C) transitional phase (i.e., “pseudo-washout”) and hypointensity in the (D) hepatobiliary phase from background liver parenchymal enhancement. The histopathological examination after surgical resection revealed a diagnosis of small duct type iCCA, which is likely to mimic HCC on imaging.
The discrimination of cHCC-CCA from HCC by imaging has also been considered challenging. The histopathologic components of cHCC-CCA are presumed to determine its radiological appearance [47]. While cHCC-CCA with predominant cholangiocarcinoma components is more likely to demonstrate a targetoid mass, cHCC-CCA with predominant HCC components is more likely to mimic HCC by showing non-rim APHE or a strong degree of rim APHE (Fig. 4) [48,49]. Furthermore, the combination of non-rim APHE and PVP “washout” is not infrequently observed among cHCC-CCAs, especially among tumors smaller than 2 cm [50]. As the histopathological terminology for cHCC-CCA has been recently re-defined by expert consensus [47], future studies are warranted to elucidate the radiological and clinical characteristics of cHCC-CCA in a large-scale cohort.
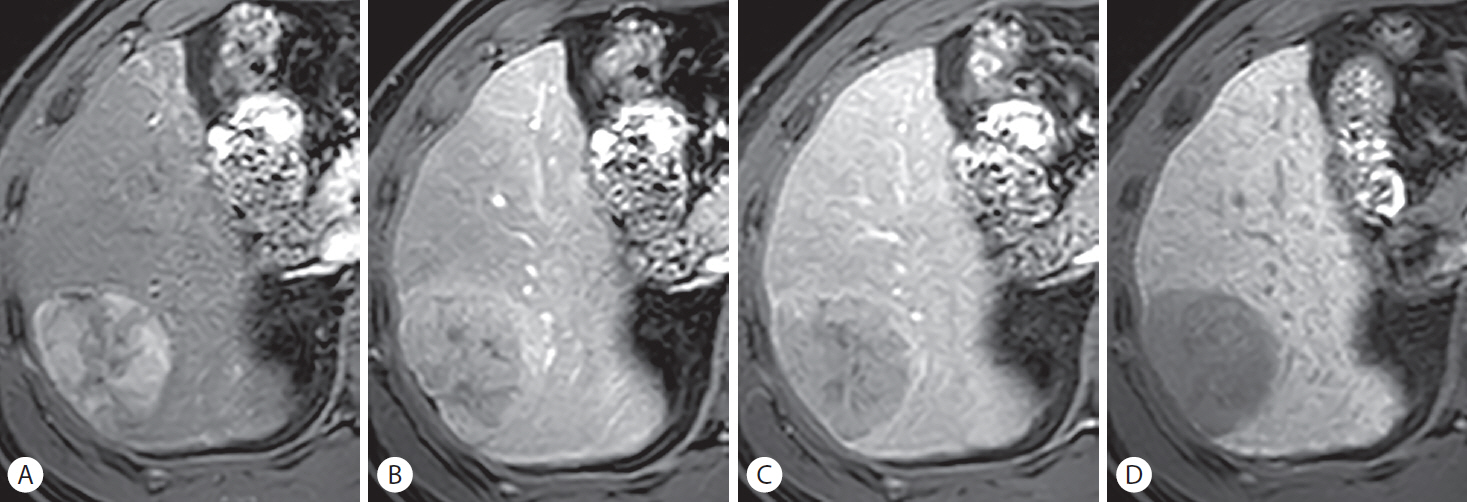
A combined hepatocellular carcinoma-cholangiocarcinoma (cHCC-CCA) mimicking hepatocellular carcinoma (HCC) in a 49-year-old man with hepatitis B-related liver cirrhosis. A 56-mm mass in hepatic segment VI depicts non-rim hyperenhancement in the (A) arterial phase. Partially hypointense areas are noted in the (B) portal venous phase, suggesting the presence of “washout.” A hyperintense rim around the mass is noted in the (C) transitional phase, suggesting the presence of an enhancing “capsule.” The nodule appears as a diffusely hypointense mass in the (D) hepatobiliary phase. The histopathological examination after surgical resection revealed cHCC-CCA with an HCC component comprising 80% of the mass. The cholangiocarcinoma component comprising 20% of the mass is not well appreciated on imaging.
The possibility of hypervascular metastasis needs to be prioritized over that of HCC when a patient has an active extrahepatic malignancy and no underlying liver disease. Neuroendocrine tumor, renal cell carcinoma, choriocarcinoma, melanoma, breast cancer, thyroid cancer, and other hypervascular tumors could metastasize to the liver [51]. On the contrary, when an active extrahepatic malignancy and liver cirrhosis are present at the same time, radiological clues indicating HCC, such as intratumoral fat (Fig. 5) or blood products, may properly prompt a histopathologic confirmation of the liver mass.
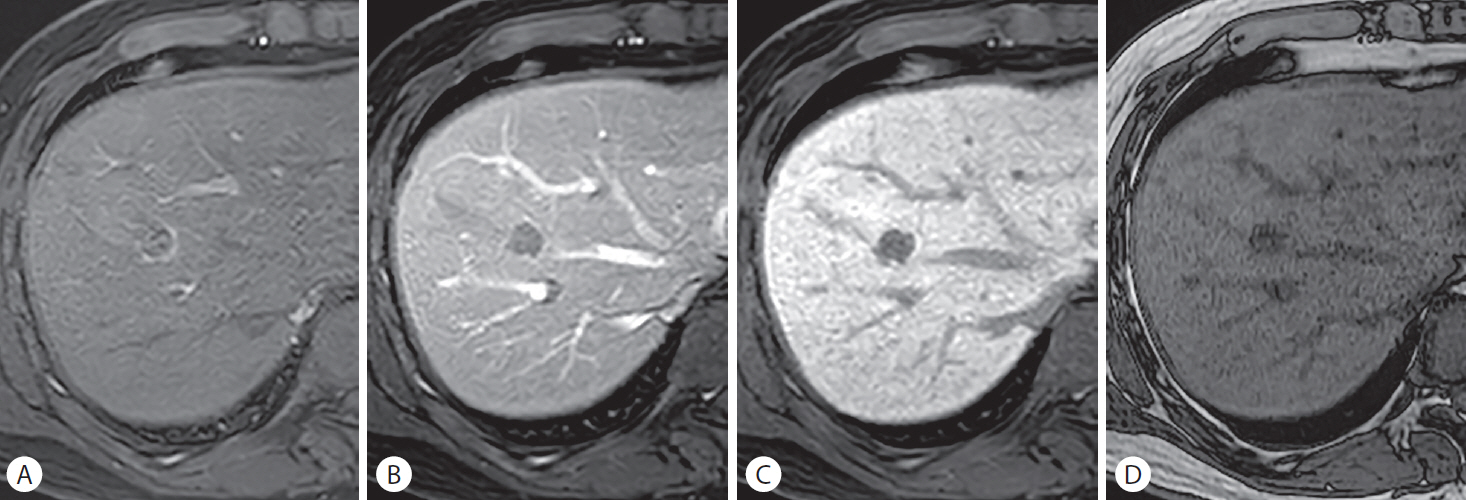
A surgically diagnosed hepatocellular carcinoma (HCC) in a 53-year-old man with a history of rectal cancer and hepatitis B-related liver cirrhosis. A 19-mm nodule in hepatic segment IV/VIII demonstrates rim-like hyperenhancement in the (A) arterial phase, and diffuse hypointensity in the (B) portal venous phase and (C) hepatobiliary phase. (D) The out-of-phase image depicts diffuse hypointensity of the lesion, suggesting the presence of a fat component. Although rim arterial phase hyperenhancement can be seen in metastasis from rectal cancer, the presence of a fatty component and liver cirrhosis favors the diagnosis of HCC over metastasis and necessitates a histopathological diagnosis.
Benign hepatocellular lesions
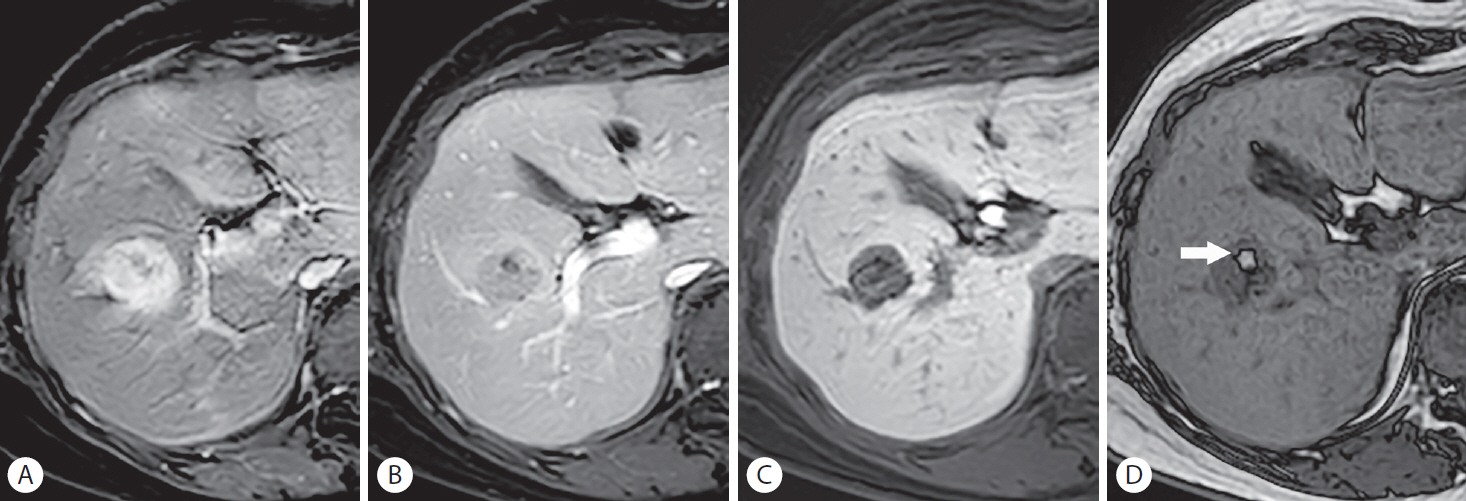
Gadoxetic acid-enhanced MRI can help characterize benign hyperplastic hepatocellular lesions, such as FNH in non-cirrhotic liver and an FNH-like nodule in cirrhotic liver, which can be managed conservatively. These lesions, consisting of hyperplastic hepatocytes, typically appear iso- or hyperintense in the HBP with a central hypointense scar and lobulated margins [26]. However, because of the hypervascular nature of FNH or an FNH-like nodule, these lesions can resemble HCC; FNH-like nodules which are frequently observed in patients with alcoholic liver cirrhosis can be misinterpreted as HCCs. Furthermore, about 5% to 20% of HCCs have preserved or enhanced OATP1B3 expression and appear iso- or hyperintense in the HBP, mimicking FNH (Fig. 6) [25,52]. Moreover, these HCCs frequently do not exhibit PVP or TP hypointensity, similar to FNH, because of active contrast uptake by the tumor [25]. The presence of HBP hypointense foci or a smooth hypointense rim in the HBP are features differentiating HCC from FNH or benign cirrhosis-associated nodules [53]. In addition, dynamic CT can be helpful in the differentiation by depicting “washout” of HCC in the PVP or delayed phase regardless of the OATP1B3 expression level [54].
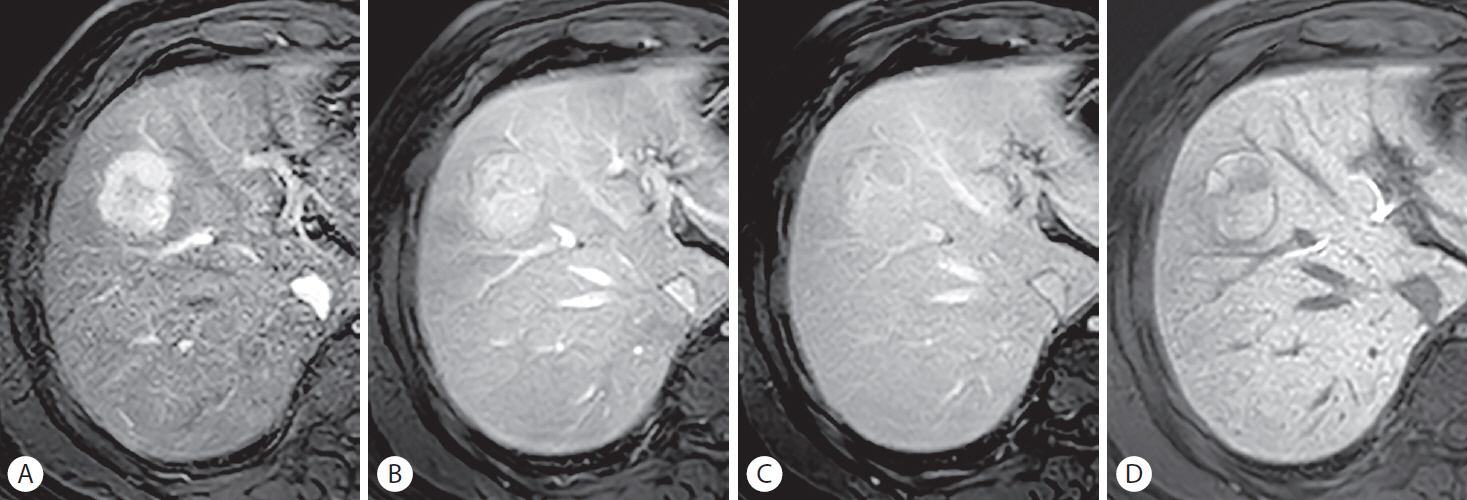
Hepatocellular carcinoma (HCC) with preserved organic anion transporting polypeptide expression in a 60-year-old man with chronic hepatitis B. A 35-mm mass in hepatic segment VIII shows (A) non-rim arterial phase hyperenhancement and persistent enhancement throughout the (B) portal venous phase, (C) transitional phase, and (D) hepatobiliary phase (HBP), showing iso- to hyperintensity to the adjacent liver. The smooth, hypointense rim shown in the (D) HBP favors the diagnosis of HCC over that of a benign lesion. The histopathological examination after surgical resection revealed Edmondson grade I to II differentiation of the tumor.
Gadoxetic acid-enhanced MRI can aid in the characterization of HCA, especially for the subtyping [26,55,56]. On HBP imaging, about 75% of HCAs appear hypointense to the adjacent liver, which is useful for differentiation of these tumors from FNH; hepatocyte nuclear factor 1 alpha–inactivated HCAs, most inflammatory HCAs, and unclassified HCAs are characterized by HBP hypointensity [26,55]. On the other hand, β-catenin–activated HCAs and some inflammatory HCAs with β-catenin mutation may exhibit HBP hyperintensity primarily due to OATP1B3 overexpression (Fig. 7) [26,56]. The risk of malignant transformation is high for HCA with β-catenin mutation, which may merit surgical resection [55]. MRI can also help in HCA subtyping by depicting the fat components of hepatocyte nuclear factor 1 alpha–inactivated HCA as well as the telangiectatic features of inflammatory HCA as a T2 high-signal area in the lesion periphery (i.e., the atoll sign) [56].
Hepatocellular adenoma with β-catenin mutation in a 48-year-old man with no underlying liver disease. A 21-mm nodule in hepatic segment II depicts non-rim arterial phase hyperenhancement on (A) gadoxetic acid-enhanced magnetic resonance imaging (MRI). Persistent enhancement is demonstrated throughout the (B) portal venous phase (PVP), (C) transitional phase, and (D) hepatobiliary phase, which is a typical enhancement pattern for the β-catenin–activated subtype. The nodule also shows non-rim arterial phase hyperenhancement on (E) computed tomography (CT) image. However, contrary to the finding on MRI, the nodule shows hypoenhancement in the (F) PVP of CT.
Benign non-hepatocellular lesions
Hemangioma, the most common benign tumor of the liver, needs to be differentiated from HCC. The phenomenon of “pseudo-washout” can make the differentiation difficult on gadoxetic acid-enhanced MRI (Fig. 8), especially for rapidly enhancing or high-flow hemangioma [57,58]. Nonetheless, markedly bright signal intensity comparable to that of the cerebrospinal fluid on a heavily T2-weighted image (a fluid-sensitive protocol with a long echo time), a high apparent diffusion coefficient (ADC) value, and signal intensity equivalent to that of the portal vein suggests hemangioma [59-61].

Discrepant imaging features of hemangioma according to the magnetic resonance imaging (MRI) contrast media used. A 14-mm nodule (arrows) was incidentally found in hepatic segment IV/V in a 45-year-old woman. On both gadoxetic acid-enhanced MRI and extracellular contrast agent (ECA)-enhanced MRI (not shown), the nodule shows peripheral nodular enhancement in the (A) arterial phase and slight progressive enhancement along the lesion periphery in the (B) portal venous phase, which are indicative of a slowly enhancing hemangioma. The nodule appears as a defect in contrast uptake in the (C) hepatobiliary phase due to a lack of functioning hepatocytes, while diffuse contrast enhancement is demonstrated in the (D) delayed phase of ECA-enhanced MRI as a result of its prominent vasculature. (E) Bright signal intensity in a heavily T2-weighted image and (F) a high apparent diffusion coefficient value are suggestive of hemangioma.
Hypervascular pseudolesions or arterioportal shunts are frequently observed in cirrhotic liver, mimicking HCC [62]. The differentiation of these findings from HCC can be aided greatly by the HBP of gadoxetic acid-enhanced MRI. Hypervascular pseudolesions are typically wedge-shaped and found in a subcapsular location, while some are nodular in appearance [6]. The majority of hypervascular pseudolesions are isointense or only slightly hypointense in the HBP, contrary to HCCs, which are generally hypointense in the HBP [63,64]. A lack of hypointensity in the HBP combined with isointensity on DWI can properly identify hypervascular pseudolesions [63].
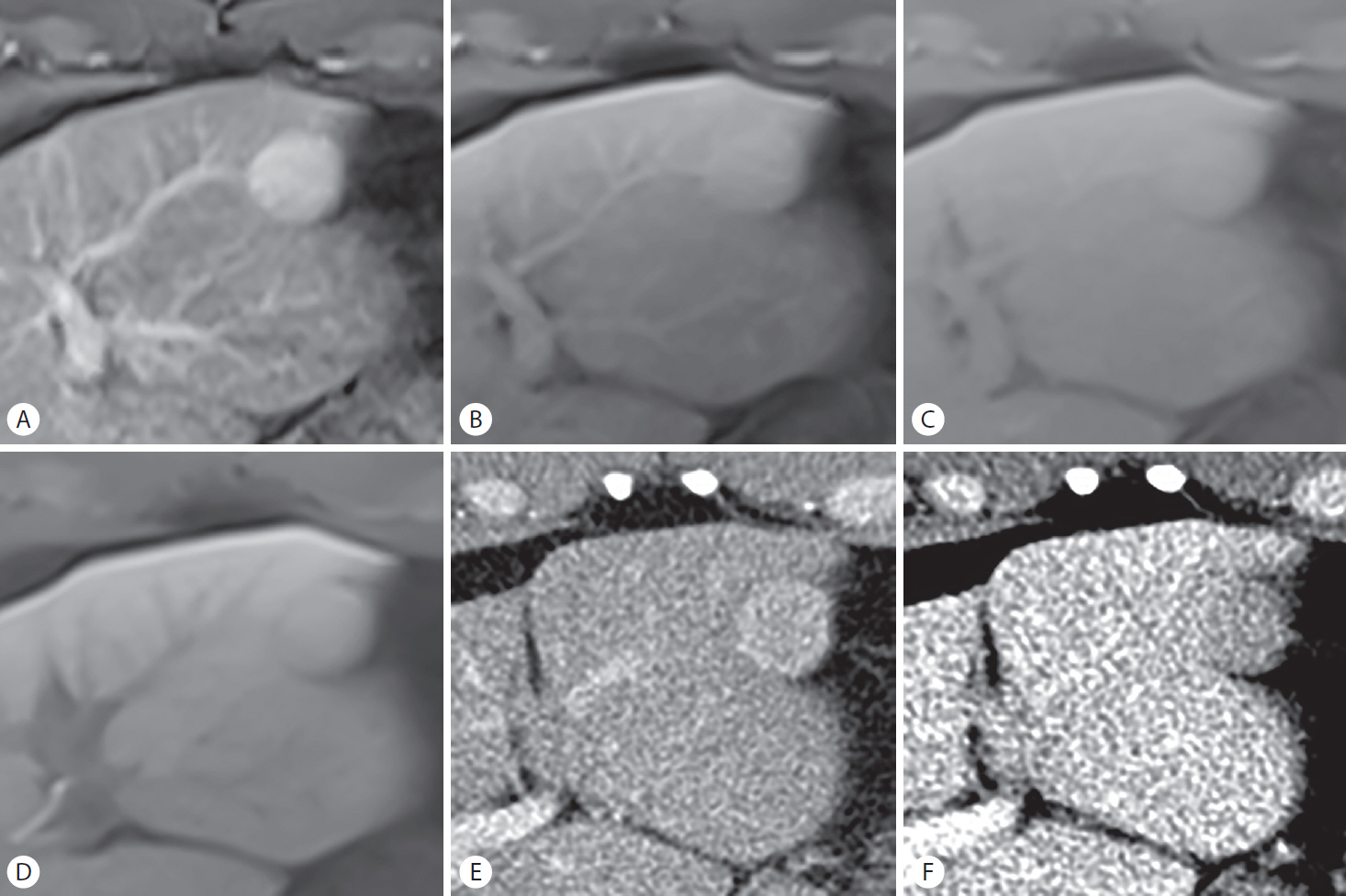
The radiological distinction between angiomyolipoma and HCC is challenging because of overlapping imaging features, such as the contrast enhancement profile (APHE and PVP or TP hypointensity), diffusion restriction, and intratumoral fat (Fig. 9) [6,65,66]. This differentiation is particularly problematic in non-cirrhotic liver and commonly requires histopathologic proof. On gadoxetic acid-enhanced MRI, the hypointensity of angiomyolipoma in the HBP tends to be marked and homogeneous compared to that of HCC [65,66]. This can be attributed to a complete lack of hepatocytes in angiomyolipoma in contrast to HCC, which may contain dysplastic foci. In addition, persistent enhancement in the PVP, presence of an early draining vein, and absence of an enhancing “capsule” favor the diagnosis of angiomyolipoma over HCC [65]. Last but not least, angiomyolipoma tends to occur more frequently in female patients, patients younger in age, and in normal liver [65,66].
Biopsy-proven angiomyolipoma in a 55-year-old woman with no underlying liver disease. A 27-mm mass in hepatic segment V shows non-rim hyperenhancement in the (A) arterial phase and mild hypointensity in the (B) portal venous phase, mimicking hepatocellular carcinoma (HCC). Marked and homogeneous hypointensity in the (C) hepatobiliary phase may favor a diagnosis of angiomyolipoma over that of HCC. (D) The out-of-phase image depicts diffuse hypointensity of the lesion, suggesting the presence of a fat component; a small, hyperintense, fatty focus (arrow) appears hypointense on other fat-suppressed images (A-C). As both angiomyolipoma and HCC possibly contain intratumoral fat and are hypervascular, the exact radiological differentiation remains challenging.
CONCLUSIONS
The strengths of using gadoxetic acid-enhanced MRI include the facilitated diagnosis of small HCCs and precursor lesions of progressed HCC. The exclusion of HCC mimickers may help overcome the diagnostic hurdle of “pseudo-washout” on gadoxetic acid-enhanced MRI and enable the highly sensitive diagnosis of HCC without significant specificity impairment. The differentiation of HCC from HCC mimickers can be aided by functional information provided by gadoxetic acid. However, pitfalls in the characterization of focal liver lesions unique to gadoxetic acid-enhanced MRI needs to be recognized for a more accurate diagnosis of HCC.
Notes
Author contributions
YY Kim and MS Park contributed to this paper with conception and design of the study; YY Kim and KS Aljoqiman performed literature review and drafting; MS Park, JY Choi, and MJ Kim performed critical revision and editing; All authors approved the final version of the manuscript.
Conflict of Interest
The authors have no conflicts of interest to disclose.
Abbreviations
ADC
apparent diffusion coefficient
APHE
arterial phase hyperenhancement
cHCC-CCA
combined hepatocellular carcinoma-cholangiocarcinoma
DWI
diffusion-weighted imaging
ECA
extracellular contrast agent
FNH
focal nodular hyperplasia
HBP
hepatobiliary phase
HCA
hepatocellular adenoma
HCC
hepatocellular carcinoma
HGDN
high-grade dysplastic nodule
iCCA
intrahepatic mass-forming cholangiocarcinoma
LI-RADS
Liver Imaging Reporting and Data System
NHHN
non-hypervascular hepatobiliary phase hypointense nodule
OATP
organic anion transporting polypeptide
PVP
portal venous phase
TP
transitional phase
