Drug induced liver injury: East versus West – a systematic review and meta-analysis
Article information
Abstract
Drug induced liver injury (DILI) may be different in the East compared to the West due to differing disease prevalence, prescribing patterns and pharmacogenetic profiles. To review existing literature on causative agents of DILI in the East compared to the West, a comprehensive literature search was performed on electronic databases: MEDLINE/PubMed, Embase, Cochrane Library and China National Knowledge Infrastructure without language restrictions. Studies which involve patients having DILI and reported the frequency of causative agents were included. A random effects model was applied to synthesize the current evidence using prevalence of class-specific and agent-specific causative drugs with 95% confidence intervals. Of 6,914 articles found, 12 showed the distribution of drugs implicated in DILI in the East with a total of 33,294 patients and 16 in the West with a total of 26,069 DILI cases. In the East, the most common agents by class were anti-tuberculosis drugs (26.6%), herbal and alternative medications (25.3%), and antibiotics (15.7%), while in the West, antibiotics (34.9%), cardiovascular agents (17.3%), and non-steroidal anti-inflammatory drugs (12.5%) were the commonest. For individual agents, the most common agents in the East were isoniazid-rifampicin-pyrazinamide (25.4%), phenytoin (3.5%), and cephalosporin (2.9%) while in the West, amoxicillin-potassium clavulanate combination acid (11.3%), nimesulide (6.3%), and ibuprofen (6.1%) were the commonest. There was significant heterogeneity due to variability in single-centre compared to multi-centre studies. Differences in DILI in the East versus the West both in drug classes and individual agents are important for clinicians to recognize.
INTRODUCTION
Drug induced liver injury (DILI) is defined as liver damage due to drugs, herbal medications or supplements. The pathogenesis of DILI is extremely heterogenous and causes vary from intrinsic hepatotoxicity, mitochrondrial toxicity, to immune mediated liver damage related to variations in drug metabolism, genetic differences and HLA susceptability [1].
DILI has the potential to be fatal with a wide spectrum of liver damage that ranges from mild abnormality to liver fibrosis to acute liver failure. Severe events are relatively rare, but can be catastrophic, particularly once jaundice occurs. This is reflected in Hy’s law [2] in which alanine aminotransferase in serum is increased to over three times the upper limit of normal, and bilirubin is also increased to over twice the upper limit of normal. Those who fulfill this “rule” have over 10% risk of mortality or liver transplantation.
The mainstay of treatment is withdrawal of the offending agent. In mild to moderate cases, this is followed by eventual resolution of the liver damage, but in severe cases, liver transplantation may be necessary.
There are very few studies on the incidence of DILI worldwide. The main difficulty is to acertain the demoninator, i.e., the number of individuals receiving a culprit drug. True prevalence studies are prospective community based studies with well characterised reporting systems, low dropout and loss to follow up. Consequently, there are very few population based studies.
So far the ‘best’ available population based survey was performed in Nievre, France, 2002 where the local population had only a few hospitals for their healthcare, and almost all the doctors participated in the study using a standard protocol following the International Consensus Meeting Proposal. The annual incidence rate of DILI was 13.9±2.4 per 100,000 inhabitants [3]. In a population based study in Iceland recently [4], the crude annual incidence rate of DILI was 19.1 per 100,000 inhabitants, with a crude hospitalization rate of 4.4 per 100,000. A Spanish study in 2005 estimated the annual incidence of hepatotoxicity to be 3.4 per 100,000 inhabitants with crude hospitalization rate of 1.6 per 100,000 inhabitants [5]. In the East, a prospective study from Korea in 2012 involving 17 referring university hospitals with defined criteria based on World Health Organisation-Uppsala Monitoring Center (WHO-UMC) extrapolated crude annual incidence rate to be 12 per 100,000 [6]. Based on the above limited data available, it appears that there is not a major difference in incidence of DILI between the East and the West. However it is difficult to make firm conclusions based on the scanty data available, and there is a definite need for well conducted prospective population studies, particularly from the East.
Is drug induced liver injury different in the East compared to the West? One may speculate that Asians have a different pharmacogenetic and immunological profile, and may therefore handle drugs differently. An additional problem in Asia is the widespread use of herbal and alternative medicines, the safety of which is poorly defined.
Consequently, there is an unfilled knowledge gap with regards to differences in Eastern compared to Western DILI with regards to causative agents. We aimed to determine whether agents causing DILI in the East was different from the West by performing meta-analyses of studies of DILI reported in the East, and those in the West, with regards to the most frequently reported agents. Since the reporting frequencies of DILI are most affected by reporting bias, we took measures to reduce bias.
METHODS
Literature search and eligibility criteria
A comprehensive literature search was performed on electronic databases: MEDLINE/PubMed, Embase, Cochrane Library and China National Knowledge Infrastructure until 31 March 2016. The specific concepts used in the search strategy were “drug induced liver injury”, “incidence” and “prevalence”. We searched these terms in combination with the conducted literature search by Medical Subject Headings or Emtree terms, and free text terms. There were no restrictions on language. All the bibliography listed in review papers and included publications were also checked.
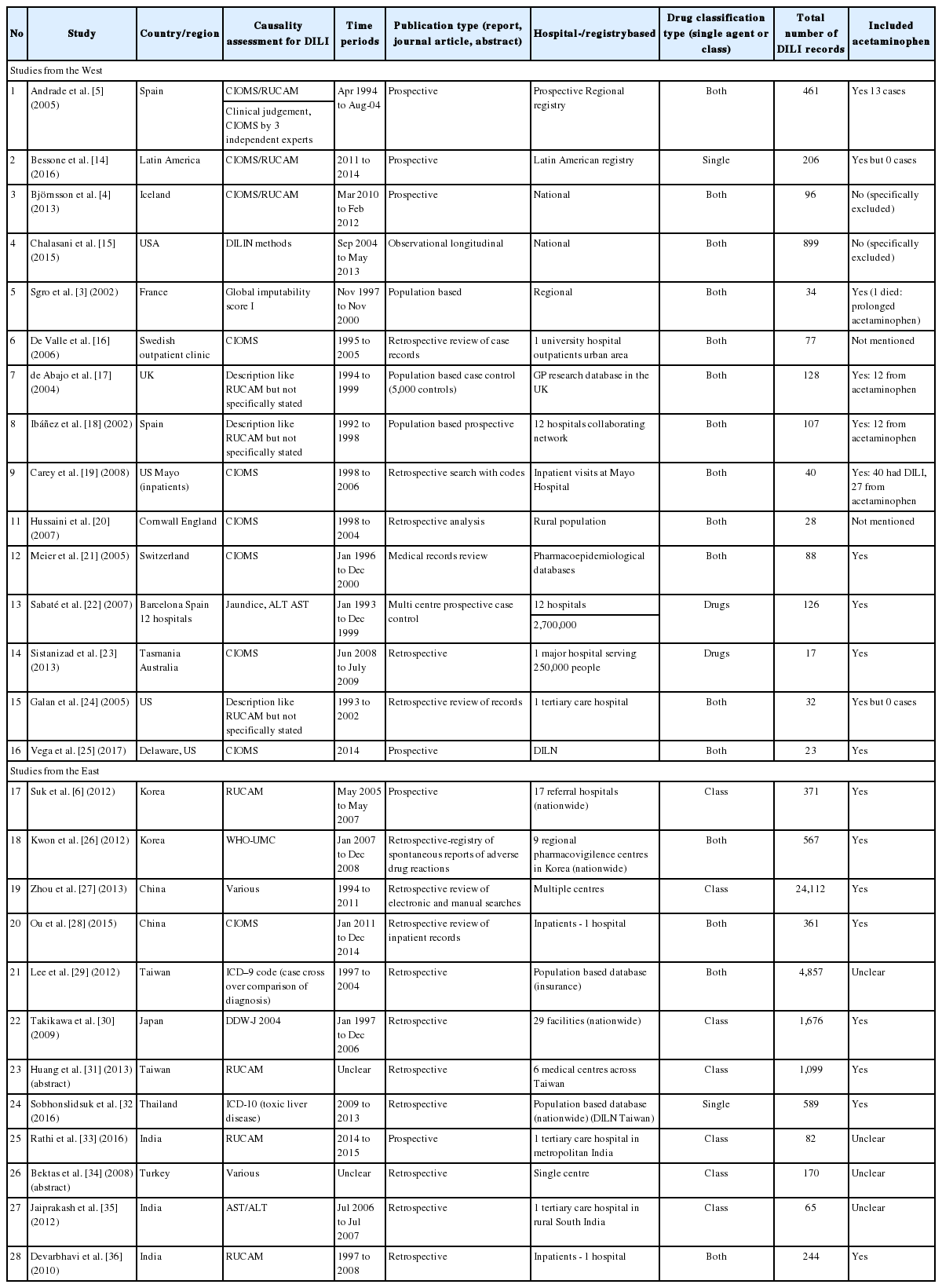
Two investigators (E.X.S.L. and S.G.L.) independently screened for eligible studies based on pre-defined eligibility criteria. Inclusion criteria were cross sectional or cohort studies which involve patients having DILI and which reported the frequency of the causative agents. For studies that had published duplicate results with accumulating numbers of patients, only the most recent or complete reports were included. Exclusion criteria were studies which sampled less than 200 subjects, did not provide sufficient information, technical reports, editorials, letters to the editor, and case reports. Any discrepancies regarding whether articles met selection criteria were resolved by consensus.
Data extraction and risk of bias assessment
The following data were extracted (E.X.S.L. and S.G.L.) from the included studies: 1) study characteristics (publication year, country of population, causality assessment criteria and study design); and 2) frequency of class-specific and agent-specific causative drugs. To reduce bias, frequency of agent specific causative drugs were only included if they were present in at least three studies.
The quality of each study was evaluated by two independent investigators (Z.Q. and E.C.). Quality of sampling (i.e., source of sampling and sampling methods) and quality of measurement (i.e., causality assessment criteria and consistency of criteria application) were assessed to determine the study quality. Any disagreement in quality assessment was resolved by discussion and consensus.
Statistical analysis
A random effects model was applied to synthesize the current evidence using prevalence of class-specific and agent-specific causative drugs with 95% confidence interval (CI). To assess the potential heterogeneity, we calculated the I2 for each of analysis. We also planned a sub-group analysis on single-center and multicenter study to explore the potential source of heterogeneity, as it was possible that single-center studies may lead to higher risk of bias compared to multi-center studies. Potential small-study effects and publication bias was assessed by a funnel plot. The estimated prevalence was plotted against the inverse of the standard error of the prevalence as a measure of precision reflecting the effect size. The Egger’s test was conducted with the null hypothesis that symmetry exists in the funnel plot. Statistical analyses were performed using Comprehensive Meta Analysis 3.3 (Biostat, Englewood, NJ, USA) and StataMP 15.1 (StataCorp LLC, College Station, TX, USA).
RESULTS
A total of 6,914 studies were found using the search strategy but after exclusions only a total of 28 studies (Fig. 1, Table 1) were included for analysis. The causative agents were listed by class or as individual agents. The results were reported as event rates and 95% CIs, then were ranked based on the frequency of events, either as a drug class or as individual class. The frequencies and event rates were shown between studies reported from Western countries and Eastern countries which were present in at least three studies. Since the studies were independently conducted no direct differences could be compared.
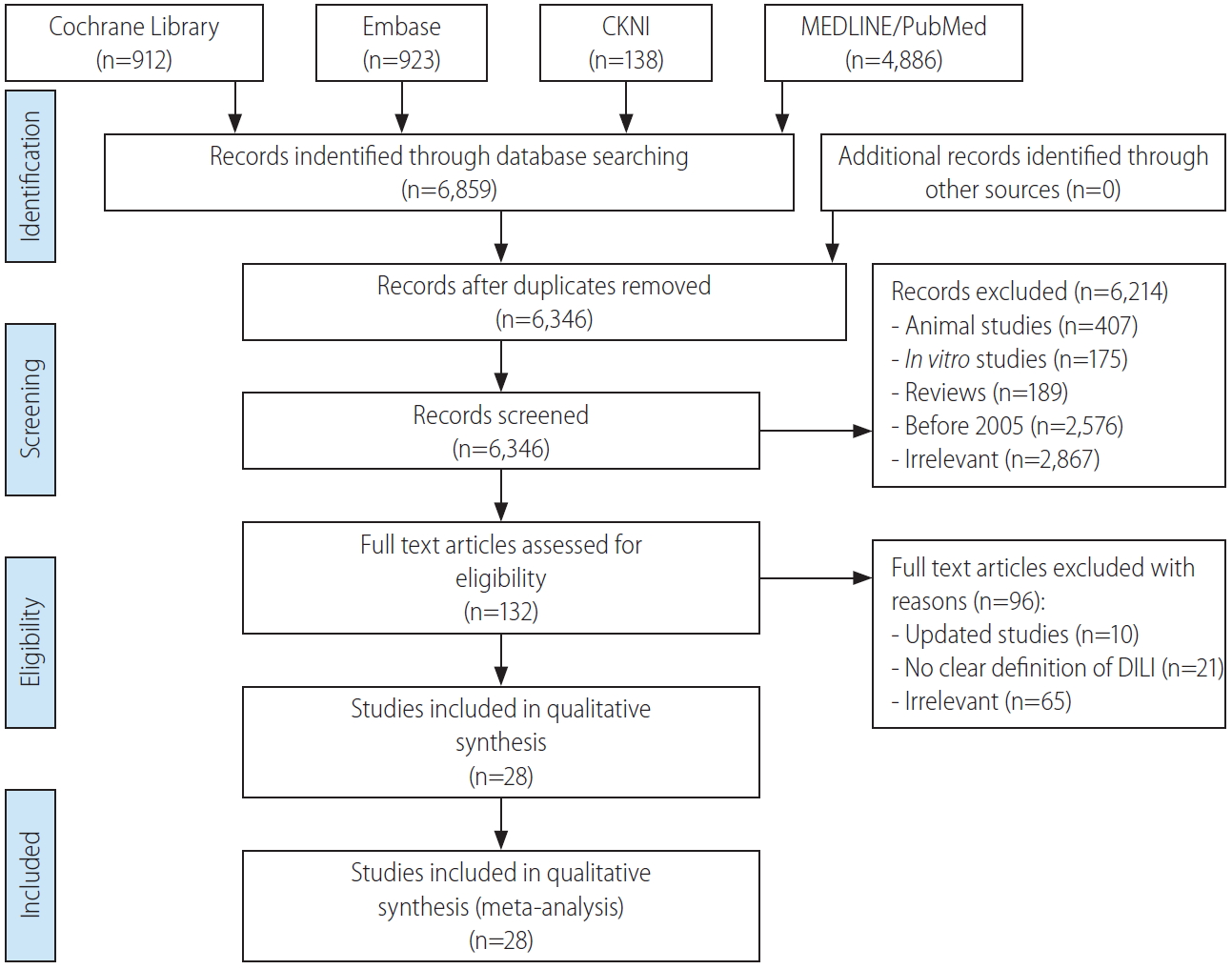
Flowchart of the identification, screening, eligibility, and included studies. CKNI, China Knowledge Network CKNI Database; DILI, drug induced liver injury.
Frequency of DILI by class and individual agents: West versus East
In Figure 2, summary pooled estimates of the frequency or causative agents reported in Eastern and Western studies were shown by drug class in a descending order of number of causative events present in at least three studies. Forrest plot of classes of agents can be viewed in the supplementary material (Supplementary Fig. 1, 2). Drug classes were listed as antibiotics, anti-epileptics, anti-tuberculosis (TB) drugs, cardiovascular (CVD) agents, gastrointestinal agents, herbal and supplements, lipid-lowering agents, non-steroidal anti-inflammatory drugs (NSAIDs), and psychotropic drugs. The event rate and 95% CIs for each study and the pooled analysis for each drug class was shown. Significant heterogeneity was shown for pooled analysis of some drug classes and in other drug classes, when the number of studies was less than four, it was not possible to generate statistical heterogeneity (Fig. 2).

(A) DILI by drug class of Eastern studies. Summary pooled estimates are shown as ES and 95% CIs. Where I2 and P-values are not shown, this indicates <4 studies and statistical heterogeneity could not be assessed. (B) DILI by drug class of Western studies. Summary pooled estimates are shown as ES and 95% CIs. Where I2 and P-values are not shown, this indicates <4 studies and statistical heterogeneity could not be assessed. ES, estimate of proportion; CI, confidence interval; DILI, drug induced liver injury.
In Figure 3A, summary pooled estimates of the frequency of individual agents reported in Eastern studies and in Figure 3B, summary pooled estimates of individual agents reported in Eastern studies were shown. Forrest plots of the individual agents can be viewed in the supplementary material (Supplementary Fig. 3, 4). The event rate and the 95% CIs are shown for each study and the pooled estimate. Only four drugs as individual agents were found from a search of Eastern studies, as most of the studies reported only drug classes in contrast to Western studies where there were many more reports of individual agents. Significant heterogeneity was seen for acetaminophen, amoxicillinclavulanate acid, diclofenac, flucloxacillin, heparin, and minocycline for Western studies, while in Eastern studies, heterogeneity was seen in acetaminophen, and isoniazid-rifampicin-pyrazinamide (INH_RIF_PZA).

(A) DILI by individual agents in Eastern studies. Summary pooled estimates are shown as ES and 95% CIs. Where I2 and P-values are not shown, this indicates <4 studies and statistical heterogeneity could not be assessed. (B) DILI by individual agents in Western studies. Summary pooled estimates are shown as ES and 95% CIs. Where I2 and P-values are not shown, this indicates <4 studies and statistical heterogeneity could not be assessed. ES, estimate of proportion; CI, confidence interval; INH_RIF_PZA, isoniazid-rifampicin-pyrazinamide; DILI, drug induced liver injury.
Ranking of DILI in West and East
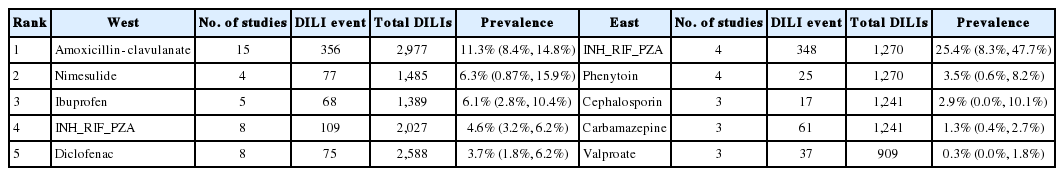
In Table 2, a summary table of the ranking of number of causative events of the class of agents implicated in DILI from Western studies and Eastern studies are shown. In Western studies antibiotic DILI ranked the highest with 1,161 events and a prevalence of 34.9% (95% CI, 25.4–45.1%) while anti-TB drugs was ranked the highest for Eastern studies with 563 events and a prevalence of 26.6% (95% CI, 13.1–42.9%). For the second ranked DILI in the West, CVD agents had 392 events and a prevalence of 17.3% (95% CI, 7.8–29.5%), while in the East, herbs and supplements ranked second with 914 events and a prevalence of 25.3% (95% CI, 12.5–40.6%). In the West, psychotrophic drugs with 161 events and a prevalence of 13.1% (95% CI, 6.8–21.0%) ranked third, while in the East, antibiotics with 554 events and a prevalence of 15.7% (95% CI, 9.0–23.9%) ranked third.
While anti-TB agents are also antibiotics, they were classed separately since they were often quite specific and used in combination.
In Table 3, the summary table of the ranking of the number of causative events of the individual agents implicated in DILI from Western studies and Eastern studies are shown. There was a much larger number of individual agents described in Western studies (n=9) compared to Eastern studies (n=4). This is mainly due to Eastern studies describing classes of agents rather than individual agents. The top ranked individual agent in the West was amoxicillin-clavulanate with 356 events and a prevalence of 11.3% (95% CI, 8.4–14.8%) while in the East was INH_RIF_PZA with 25.4% (95% CI, 8.3–47.7%). Nimesulide with 77 events and prevalence of 6.3% (95% CI, 0.87–15.9%) was ranked second in the West and phenytoin with 25 events and a prevalence of 3.5% (95% CI, 0.6–8.2%) in the East.
Potential heterogeneity and publication bias
Almost all the forest plots showed significant statistical heterogeneity. One source of heterogeneity was whether the data source was a multi-centre or single centre study. It is possible that single centre studies may lead to higher risk of bias compared to multi-centre studies. There were significant differences in prevalence for individual drugs. This was especially true for Western studies in particular, such as the case of acetaminophen where multi-centre studies reported an event rate of 7% (95% CI, 0.9– 37.1%) compared to 67.5% (95% CI, 51.7–80.1%) for single centre studies (Supplementary Table 1). For Eastern studies, only amoxicillin-clavulanate and INH_RIF_PZA were represented as both multi-centre and single centre studies, and differences were relatively minor. When we examine the differences in class of drugs comparing multi-centre versus single centre studies in Western studies, there were notable differences in prevalence of antibiotics 27.1% (95% CI, 19.0–36.9%) for multicentre studies, and 42.1% (95% CI, 27.6–58.2%) for single centre studies and NSAIDs 9.1% (95% CI, 2.8–25.5%) for multicentre studies, and 17.7% (95% CI, 7.9–35.1%) for single centre studies. In Eastern studies, notable differences were seen in prevalence of antibiotic related DILI, 11.7% (95% CI, 6.9–19.3%) for multicentre studies, and 26.1% (95% CI, 6.5–64.1%) for single centre studies, and lipid-lowering agents 2.4% (95% CI, 0.7–8.1%) for multicentre studies, and 11.4% (95% CI, 0.4–78.9%) for single centre studies (Supplementary Table 1). Given that funnel plots generally require at least 10 studies for Egger’s test, we selected Antibiotics for the plot generation as it was the most reported medication class among the included studies. From the Egger’s tests, we found that both P-values were larger than 0.05, indicating that current evidence cannot detect any statistically significant small-study effects or publication bias (Supplementary Fig. 5, 6).
DISCUSSION
Studies of DILI have reported varying frequency of implicated agents. Almost all of these studies come from the West where there are well established networks and infrastructure for DILI. From our systematic review, we can conclude that the agents commonly implicated in the East are quite different to those from the West. Ranking of number of causative events show that in the West, amoxicillin-clavulanate, nimesulide and ibuprofen are the common agents implicated in DILI while in the East, INH_RIF_PZA, phenytoin and cephalosporins are the commonly reported. INH_RIF_PZA is common in Eastern DILI, but is less frequent in the West.
Examining the event frequencies of classes of DILI agents are also revealing. Antibiotics 34.9%, are the most frequent class of agent in the West but only the third most frequent 15.7% in the East, and is reflected in the lower frequency by almost half. An increasingly important DILI group is herbs and supplements, for which Eastern studies show a frequency of 25.3% compared to Western studies showing a significantly lower frequency of 6.7%.
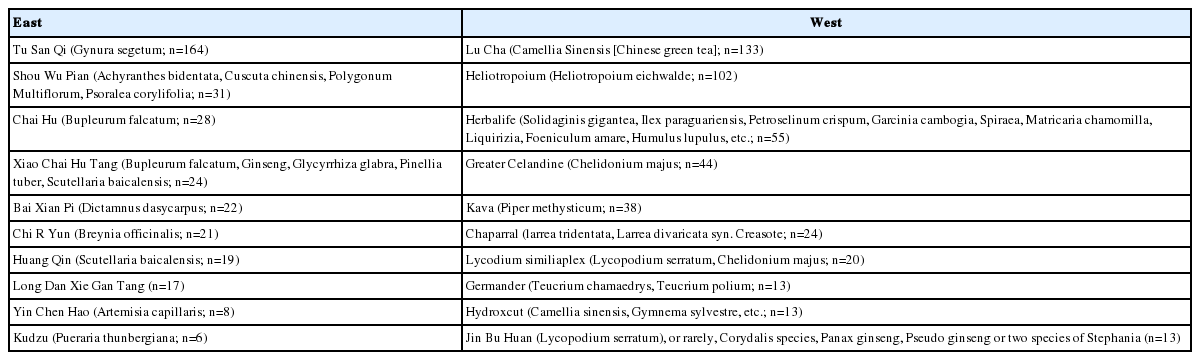
The herbs implicated are also likely to be different in the West compared to the East. In a review of herbal hepatotoxicity, Teschke and Eickhoff [7] compiled a comprehensive overview of herbal preparations implicated. By extracting the source reference, we compiled the top 10 common herbs implicated in the East versus the West (Table 4). The most common herb causing hepatotoxicity in the East was Tu San Qi (Gynura segetum, n=164 cases) while the most common herbal hepatoxocixity in the West was Lu Cha (Camellia Sinensis, n=133 cases) or Chinese Green Tea (Table 4). Unfortunately, in the case of most herbal DILI, it may be impossible to identify the particular chemical that is causing the liver damage. Moreover, many herbal preparations may contain undeclared adulterants [8], which could also contribute to liver damage.
By using the frequency of reported DILI comparing Western reports to that of Eastern reports we are able to obtain an overview of the common drugs implicated and a pooled estimate of their frequency. Nonetheless, the study’s main limitation is that of reporting bias. Potential sources of bias were reduced by examining subgroups using multicentre rather than single centre studies. These were sources of heterogeneity with particular drugs such as acetaminophen. In the case of acetaminophen, this may be due to a classification problem since it may be considered a drug causing liver toxicity and tends to be treated separately from DILI as this has been excluded from the prospective DILIN study in the USA [9].
The certainty of pooled estimates are reflected by the narrowness of the CIs. Although this can be a little subjective, we can be more certain when CIs are relatively narrow. In this regard, Western reports of antibiotics show an event frequency of 34.9% (95% CI, 25.4–45.1%) but when examining single agents of antibiotics, amoxicillin-clavulanate 13.1% (95% CI, 10.6–16.2%), and nitrofurantoin 5.1% (95% CI, 3.3–7.8%) provide more reliable estimates while flucloxacillin 12.2% (95% CI, 4.9–27.5%), and minocycline 5.9% (95% CI, 1.4–21.0%) have wider CIs. In other drug classes of Western DILI, anti-TB drugs 8.2% (95% CI, 6.3– 10.5%) and anti-neoplastic drugs 4.7% (95% CI, 2.8–7.7%) have reasonably narrow CIs. With regards to Eastern DILI by drug class, NSAIDs 4.8% (95% CI, 2.2–8.2%) has reasonable CIs and for the single agents, carbamazepine 1.8% (95% CI, 0.7–4.7%) has reasonable CIs. Nonetheless, despite the wider CIs in some instances, the overall findings and rankings of both classes of agents and individual agents provides a picture of differences in DILI between East and West.
Could the differences between Western and Eastern DILI be explained by pharmacogenetic differences? One well studied field is TB drug related DILI. A meta analysis by Cai et al. [10] studied the association between drug metabolizing enzyme (DME) gene polymorphisms and the risk of TB drug related DILI. Four DME genes were studied (NAT2, CYP2D1, GSTM1, and GSTT1) through 38 studies involving more than 2,000 patients. TB drug related DILI was associated with NAT2 slow acetylator genotype (odds ratio [OR], 3.18; 95% CI, 2.49–4.07) as well as GSTM1 null genotype (OR, 1.43; 95% CI, 1.08–1.88), amongst East Asians, Indian, Middle Eastern and Caucasians. In Asians, all three risk genes were significantly associated to risk of TB drug related DILI compared to Caucasians; CYP2E1*1A 1c/1c wildtype (OR, 1.35; 95% CI, 1.01–1.81), GSTM1 null genotype (OR, 1.55; 95% CI, 1.12– 2.13), NAT2 slow acetylator (OR, 3.32; 95% CI, 2.43–4.53). These findings suggest that genetic factors involved in drug handling play an important role in TB drug related DILI susceptibility.
The strength of our study is that it provides for the first time frequencies and ranking of DILI comparing East and West, despite the limitations of the quality of the DILI reports, differences in disease prevalence and patterns of drug prescribing. Overall, the data syntheses show differences in DILI reporting and prevalence between East and West, both in drug class and individual agents. How should we use this information? The first clinical utility is that clinicians should be aware that certain classes and individual agents are more susceptible in the East to DILI, and monitor such patients more carefully, and the second is that certain classes of agents should be avoided (herbs and supplements) if they provide no clear benefit but come with increased risk, and finally drugs that are potentially hepatotoxic in the West, may not be so in the East. In the first instance, TB drug related DILI in the East has a 26.6% frequency amongst all DILI (but only 8.2% in the West, see Supplement Fig. 1-6) which makes these crucial agents to monitor for DILI. Liver failure due to TB drug related DILI is still a major but preventable problem that occurred in 14% of DILI cases in one report [11]. This could be avoided if proper monitoring was in place with the knowledge that patients treated for TB have a high risk of DILI. The second important finding is that herbal DILI contributes 25.3% of DILI, and that many Asians are taking herbs and supplements without knowledge of their toxicity. One report of 177 cases acute liver failure from China, DILI accounted for 43.5%, and herbal DILI was the predominant cause in 39% of DILI cases [12]. Patients should be forewarned that herbs and supplements pose a substantial risk without clear benefit. Lastly, certain drugs which have increased frequency of DILI in the West such as amoxicillin-clavulanate, is a rare cause of DILI in the East.
As discussed previously, limitations of our study are that the reported differences in prevalence of DILI may be confounded by the frequency of the disease in the countries concerned and consequently the frequency of the drugs prescribed, and not just explained by pharmacogenomics differences. It is beyond the scope of our study to examine such limitations, which can only be addressed by prospective collection of data in country specific databases. An example of differences in disease prevalence and incidence that could impact DILI reporting is that of TB. The World Health Organisation Tuberculosis Global Report [13], provides an overview of the incidence of TB and Asian countries contribute to 11 of the top 30 burden of disease countries with India (2,790,000) and Indonesia (1,020,000) contributing the largest burden. Overall Western countries (Americas, Eastern Mediterranean, and Europe) contribute to 1,330,000 cases but Asia (South-East Asia and Western Pacific) contributes 6,470,000 cases. In this setting, there are far more cases of TB in the East compared to the West and this may be a contributing reason for the high frequency of TB drug related DILI.
In conclusion, this study has shown clear differences in frequency of drugs implicated in DILI reported in Western studies and compared to Eastern studies, both by drug class (antibiotics are frequent DILI in the West while anti-TB drugs are frequent in the East), and for specific agents (Amoxicillin-clavulanate related DILI is the common in the West while INH_RIF_PZA is the common in the East). Knowledge of such differences and their pooled estimates of frequency, now provides clinicians of more precise dangers of DILI during prescribing, and to advise patients of potential DILI when taking herbs and supplements. This field is also an area where research into genetic polymorphisms drug handling are much needed.
Notes
Authors’ contribution
Overall concept and study design: SGL
Search, retrieval and evaluation of articles: SGL, EXSL
Analysis of quality and statistics: QZ, EC
Manuscript draft and editing: SGL, EXSL, QZ, EC
Final approval: SGL, EXSL, QZ, EC
Conflicts of Interest: The authors have no conflicts to disclose.
SUPPLEMENTAL MATERIAL
Supplementary material is available at Clinical and Molecular Hepatology website (http://www.e-cmh.org).
Western DILI by class of agents: forest plots. CI, confidence interval; TB, tuberculosis; CVD, cardiovascular; NSAIDs, non-steroidal anti-inflammatory drugs; DILI, drug induced liver injury.
Eastern DILI by class of agents: forest plots. CI, confidence interval; TB, tuberculosis; CVD, cardiovascular; NSAIDs, non-steroidal anti-inflammatory drugs; DILI, drug induced liver injury.
Western DILI by single agents: forest plots. CI, confidence interval; INH_RIF_PZA, in the East was isoniazid-rifampicin-pyrazinamide; DILI, drug induced liver injury.
Eastern DILI by single agents: forest plots. CI, confidence interval; INH_RIF_PZA, in the East was isoniazid-rifampicin-pyrazinamide; DILI, drug induced liver injury.
Funnel plot for antibiotics in the East.
Funnel plot for antibiotics in the West.
INH_RIF_PZA, in the East was isoniazid-rifampicin-pyrazinamide; TB, tuberculosis; NA, not available; CVD, cardiovascular; NSAID, non-steroidal anti-inflammatory drug.
Abbreviations
CI
confidence interval
CVD
cardiovascular disease
DILI
drug induced liver injury
DME
drug metabolizing enzyme
INH_RIF_PZA
isoniazid-rifampicin-pyrazinamide
NSAIDs
non-steroidal anti-inflammatory drugs
OR
odds ratio
TB
tuberculosis
WHO-UMC
World Health Organisation-Uppsala Monitoring Center