Novel biomarkers for the management of chronic hepatitis B
Article information
Abstract
Hepatitis B virus (HBV) cannot be eliminated completely from infected hepatocytes because of the presence of intrahepatic covalently closed circular DNA (cccDNA). As chronic hepatitis B (CHB) can progress to cirrhosis and hepatocellular carcinoma (HCC), it is important to manage CHB to prevent HCC development in high-risk patients with high viral replicative activity or advanced fibrosis. Serum biomarkers are noninvasive and valuable for the management of CHB. Hepatitis B core-related antigen (HBcrAg) correlates with serum HBV DNA and intrahepatic cccDNA. In CHB patients with undetectable serum HBV DNA or loss of HBsAg, HBcrAg still can be detected and the decrease in HBcrAg levels is significantly associated with hopeful outcomes. Therefore, HBcrAg can predict HCC occurrence or recurrence. Measurement of the Mac-2 binding protein glycosylation isomer (M2BPGi) has been introduced for the evaluation of liver fibrosis. Because elevated M2BPGi in CHB is related to liver fibrosis and the prediction of HCC development, monitoring its progression is essential. Because alpha fetoprotein (AFP) has insufficient sensitivity and specificity for early-stage HCC, a combination of AFP plus protein induced by vitamin K absence factor II, or AFP plus Lens culinaris agglutinin-reactive fraction of alpha-fetoprotein might improve the diagnosis of HCC development. Additionally, Dickkopf-1 and circulating immunoglobulin G antibodies are the novel markers to diagnose HCC or assess HCC prognosis. This review provides an overview of novel HBV biomarkers used for the management of intrahepatic viral replicative activity, liver fibrosis, and HCC development.
INTRODUCTION
Hepatitis B virus (HBV) is a common cause of acute and chronic liver disease. The World Health Organization estimates that in 2015, 257 million people suffered from chronic hepatitis B (CHB), defined as hepatitis B surface antigen (HBsAg)-positive [1]. The majority of new HBV infections occur in highly endemic areas such as China, Southeast Asia, and sub-Saharan Africa [2]. HBV has been the most common cause of hepatocellular carcinoma (HCC), being responsible for >50% of cases worldwide [3]. Compared with uninfected patients, the relative risk of HCC development increases 15–20-fold in patients with HBV infection [4]. Meanwhile, Ho et al. [5] reported the clinical presentations of HCC have significantly changed over the past 12 years. In their report, hepatitis B and C virus-associated HCC became less common, and more patients were diagnosed at early cancer stage [5].
Unfortunately, although nucleos(t)ide analogues (NAs) or interferon (IFN) can efficiently suppress HBV replication, these are not curative treatments [6]. These drugs do not directly target covalently closed circular DNA (cccDNA), the key molecule responsible for intrahepatic viral persistence. cccDNA is a stable, extra-chromosomal transcriptional template for all HBV mRNAs such as pregenomic RNA [7-9]. The amount and transcriptional activity of cccDNA in hepatocytes is important for CHB progression and clinical outcomes [10]. The current aim of anti-HBV treatment is primarily to suppress complications associated with progressive inflammation and fibrosis, i.e., liver failure and decompensated cirrhosis [11,12].
HCC diagnosis and surveillance are mostly based on the detection of tumor markers and imaging techniques [11-13]. There is still a requirement for more reliable, non-invasive, and cost-effective biomarkers for CHB management. Especially, a significant number of CHB patients with non-cirrhotic liver develop HCC [14]. Current guidelines advise 6-monthly abdominal ultrasound surveillance for HCC in advanced fibrosis or cirrhotic CHB patients and in noncirrhotic patients depending on ethnic background and age [15,16]. Therefore, identifying biomarkers to better predict or diagnose HCC remains an important clinical and research priority.
In this review, we introduce novel biomarkers with great potential for CHB management and prognostic evaluation. The first is a surrogate marker of intrahepatic HBV replication, hepatitis B corerelated antigen (HBcrAg), which has revealed a good correlation with cccDNA [17]. The second, Mac-2-binding protein glycosylation isomer (M2BPGi), is a liver fibrosis marker that might predict HCC development [18]. Finally, the third is a tumor marker, alpha-fetoprotein (AFP), a protein induced by vitamin K absence or antagonist-II (PIVKA-II), Lens culinaris agglutinin-reactive fraction of alpha-fetoprotein (AFP-L3), Dickkopf-1 (DKK-1) and circulating immunoglobulin G (IgG) antibodies. We will focus on the clinical utility of these markers as predictors of HBV-related HCC development (Fig. 1).
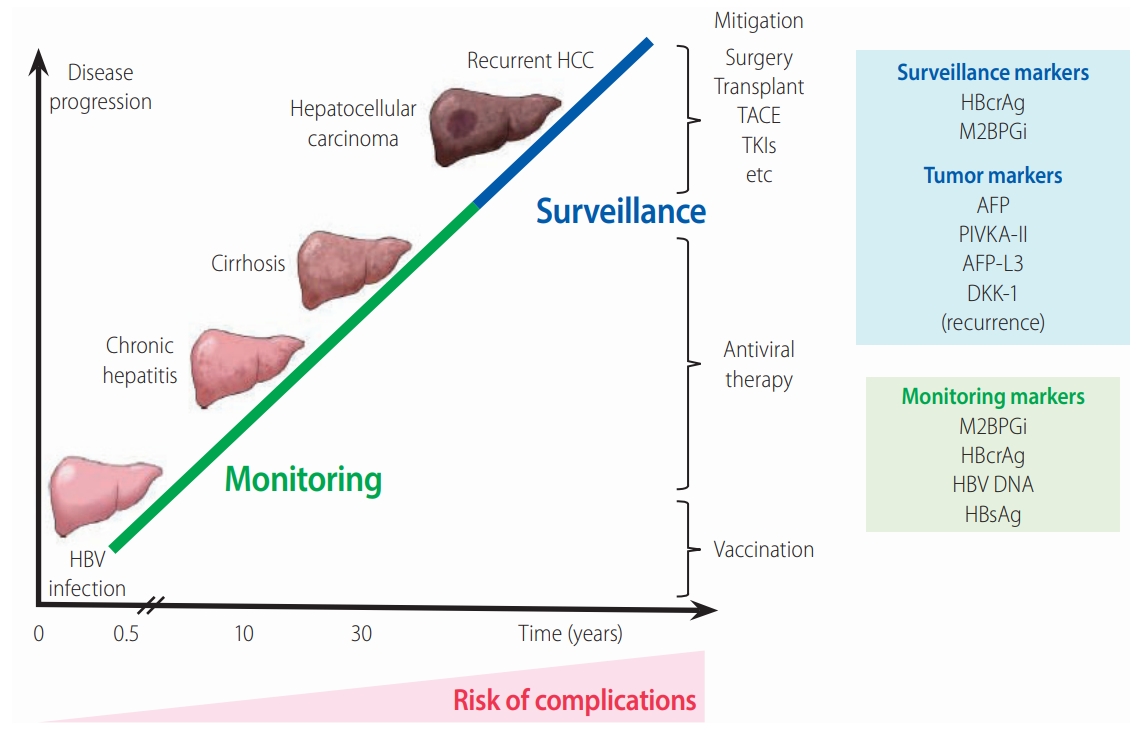
The relationship between chronic HBV infection and liver disease progression. The figure shows the clinical stages involved in the natural history of CHB. The serum biomarkers HBcrAg and M2BPGi provide valuable predictive data for the effective management of CHB. It is important to monitor patients at high risk and to treat them early to prevent liver complications, cirrhosis, and HCC development. AFP, PIVKA-II, and AFP-L3 are HCCspecific tumor markers summarized in this review. HBV, hepatitis B virus; HCC, hepatocellular carcinoma; TACE, transcatheter arterial chemoembolization; TKIs, tyrosine kinase inhibitors; AFP, alpha fetoprotein; PIVKA-II, protein induced by vitamin K absence or antagonist-II; AFP-L3, Lens culinaris agglutinin-reactive fraction of alpha-fetoprotein; DKK-1, Dickkopf-1; HBcrAg, hepatitis B core-related antigen; M2BPGi, Mac-2 binding protein glycan isomer; HBsAg, hepatitis B surface antigen.
MONITORING OF BIOMARKERS RELATED TO HBV INFECTION
With recent developments in molecular research methods, several biomarkers associated with the natural history of CHB and effectiveness of antiviral therapy have been identified [17,19]. Conventional serological biomarkers include serum HBV DNA levels and HBsAg titers, both of which predict the risk of cirrhosis and HCC [20,21]. Quantitation of HBsAg has been used as a predictive marker of liver disease, spontaneous HBsAg seroclearance, cirrhosis, and HCC development, when complementary to the measurement of HBV DNA [22-25]. However, cirrhosis and HCC can still occur in patients with undetectable HBV DNA [26] and HBsAg seroclearance [27,28]. Additionally, essential information of innovative and effective biomarkers including prediction of spontaneous or treatment-induced hepatitis B envelope antigen (HBeAg) seroconversion, and responses before/after cessation of NA treatment are still required.
SURROGATE MARKER OF INTRAHEPATIC HBV REPLICATION: HBcrAg
We recently described the clinical application of a new effective biomarker, HBcrAg, including the association of HBcrAg with other biomarkers, its competency for the prediction of clinical outcomes, and its role as a predictor of treatment [10] and HCC occurrence and recurrence [29]. Here, we introduce the unique features of HBcrAg and focus on its application for CHB management.
Components of HBcrAg (Fig. 2)

The lifecycle of HBV and HBcrAg. The schematic shows the steps involved in the lifecycle of HBV. Importantly, it shows the sources of various HBV-related molecules used routinely for diagnosis, clinical monitoring, and prognosis of HBV infection. These include the core-related antigens (p22cr, HBe, and core antigens), HBsAg, and HBV DNA measured in serum. cccDNA, although measurable in serum in association with liver injury, is mainly measured from liver biopsy samples. HBsAg, hepatitis B surface antigen; cccDNA, covalently closed circular DNA; HBcrAg, hepatitis B core-related antigen; HBeAg, hepatitis B envelope antigen; HBV, hepatitis B virus.
HBcrAg contains three products encoded by the precore/core gene. HBeAg is a circulating peptide derived from the precore protein by proteolysis and secreted from hepatocytes [7,30]. HBcAg is a component of the virion and forms the nucleocapsid surrounding the viral DNA. p22cr is a 22-kDa precore protein present in HBV DNA-negative empty Dane-like particles [31]. All three proteins share an identical 149 amino acid sequence [31,32]. HBcAg, p22cr, and HBeAg can all be measured as HBcrAg by serological testing [33,34].
Development of an HBcrAg assay
The first report of HBcrAg in 2002 concerned the development of a sensitive enzyme immunoassay specific for HBcAg and HBeAg [35]. Kimura et al. [35] designated the precore/core gene products, comprised of HBcAg and HBeAg, as HBcrAg. This assay detects HBcAg and HBeAg, even in anti-HBc or anti-HBe positive specimens. Because the level of HBcrAg reflects the serum HBV DNA level, this test can be used supplementary to HBV DNA to monitor CHB patients [35].
Further progress was achieved with the development of a chemiluminescence enzyme immunoassay for the detection of HBcrAg. The clinical performance of this assay was evaluated in CHB patients and the HBcrAg concentration correlated positively with HBV DNA concentration (P<0.001) over a 100,000-fold range [33]. The accuracy of HBV load measurement obtained by the HBcrAg assay was not affected by the absence of HBeAg in the sera or the presence of precore mutations in the HBV genome. More detailed information on the latest applications of HBcrAg measurement were recently reviewed [36]. Current quantitative HBcrAg assays have a lower limit of detection of 100 U/mL [37], but the current recommended cut-off point is 1,000 (3 log) U/mL. Compared with “prototype HBcrAg assays”, which measured only HBc, recently developed systems using p22cr (empty particle) and HBeAg [36] are automatic, and more sensitive and rapid by deactivating anti-HBc and anti-HBe in the test samples [10].
Japan was first to recommend HBcrAg in clinical guidelines for CHB management followed by the greater Asian region and then Europe [11,38,39].
Recent clinical assessments of HBcrAg
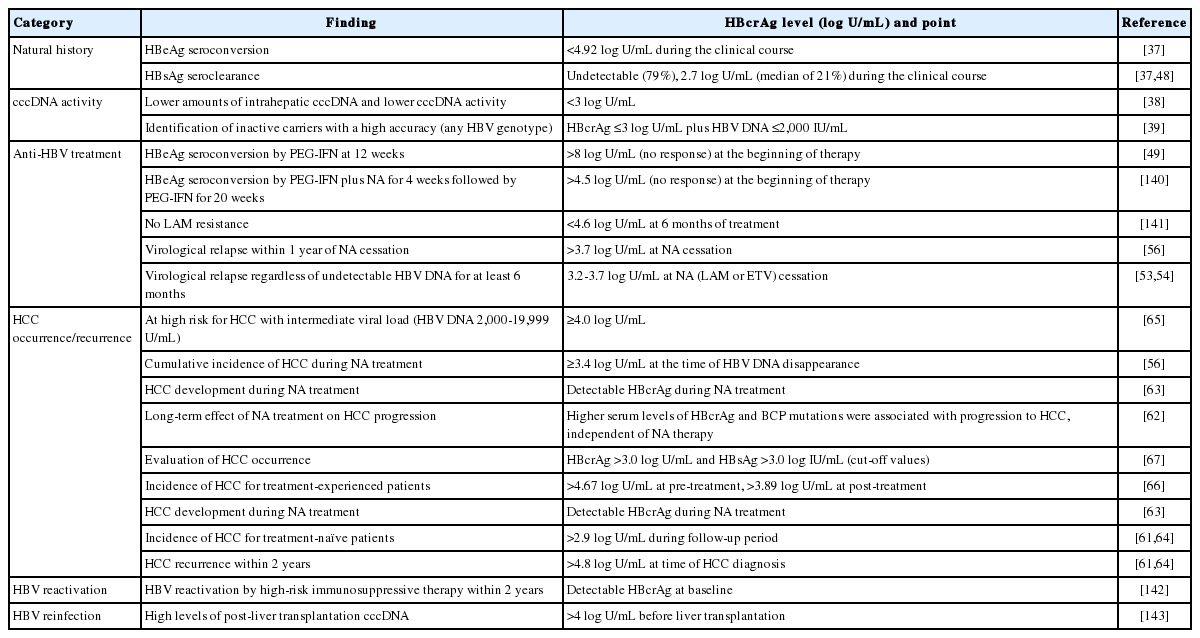
The novel biomarker, HBcrAg, has been used to support monitoring of CHB and the prediction of clinical outcomes. In this section, we describe briefly the recently reported clinical applications of HBcrAg (Table 1).
Serum HBcrAg levels are closely associated with intrahepatic cccDNA levels, as well as serum HBV DNA [38,40]. Riveiro-Barciela et al. [39] reported that HBsAg levels <3 log IU/mL were only valuable for detecting genotype D HBV inactive carriers. A single HBcrAg measurement ≤3 log U/mL plus HBV DNA ≤2,000 IU/mL was highly accurate in detecting HBV inactive carriers, regardless of their HBV genotype [39]. Testoni et al. [38] recently confirmed serum HBcrAg levels were significantly higher in HBeAg-positive patients than in HBeAg-negative patients without antiviral treatment and that they correlated with serum HBV DNA, intrahepatic HBV DNA, pgRNA and cccDNA levels, in addition to transcriptional activity. Patients negative for HBcrAg (<3 log U/mL) had lower amounts of intrahepatic cccDNA and lower cccDNA activity than HBcrAg-positive patients [38]. Hasegawa et al. [41] created a helpful prediction model for intrahepatic cccDNA levels in CHB patients. The method was named the fasting blood sugar (FBS)-cres score based on the variables used (FBS, HBcrAg, HBeAg, and HBsAg). The FBS-cres score is calculated by the following equation: 3.1686 – (0.0148 × FBS) + (0.1982 × HBcrAg) + (0.0008168 × HBeAg) + (0.1761 × log10 (HBsAg)). For example, in the training cohort, a significant correlation was shown between HBcrAg and cccDNA levels (P<0.0001, r=0.67), whereas the FBS-cres score was more closely correlated to cccDNA level (P<0.0001, r=0.81) [41].
In resource-limited areas such as Africa, HBV DNA quantification assays have limited availability and are expensive. Shimakawa et al. [42] evaluated the prospect of HBcrAg to identify Gambian patients qualified for treatment, using a new experimental algorithm that does not include HBV DNA. An easy treatment algorithm using HBcrAg without HBV DNA showed high area under the receiver operating characteristics (AUROC) (0.91; 95% confidence interval [CI], 0.88–0.95]), with a sensitivity of 96.6% and specificity of 85.8%. The measurement of HBcrAg, which is 5–10 times less expensive per test than the quantification of HBV DNA, might replace HBV DNA testing [42].
Change in HBcrAg and other HBV markers under NA therapy
A reduction in serum HBV DNA does not correlate with a decrease of intrahepatic cccDNA in patients receiving anti-HBV therapy. In a study of 43 patients treated with NA (median, 126 months) who are positive or negative for HBeAg, although 51% still had detectable intrahepatic cccDNA [43]. Similar findings were reported in 24 patients positive for HBeAg treated with sequential therapy of pegylated interferon (PEG-IFN) and adefovir (ADV) followed by ADV monotherapy—46% and 66% patients had undetectable serum HBV DNA and detectable intrahepatic cccDNA, respectively [44].
The difference between serum HBcrAg and HBV DNA can be explained by the action of NA on reverse transcription and the subsequent prevention of HBV DNA replication, while HBcrAg production remains persistently. The reduction of HBcrAg demonstrated a good correlation with the magnitude of change in intrahepatic cccDNA levels [45,46]. In contrast to serum HBV DNA, the reduction of HBcrAg was slower during NA treatment, with an increase in the ratio of serum HBcrAg:HBV DNA after 3 months of lamivudine [33]. Furthermore, in patients receiving NA treatment with undetectable serum HBV DNA, 78% had persistent HBcrAg [46]. Even in patients with documented HBsAg seroclearance, 21% had detectable serum HBcrAg, in contrast to detectable serum HBV DNA in only 2.1% [47,48].
Serum HBcrAg levels at baseline and changes while on anti-HBV therapy may also predict suitable indicators in CHB patients. Because HBV DNA is not detectable in most patients receiving NAs, HBcrAg will be used as a surrogate marker. For patients positive for HBeAg treated with PEG-IFN, a high baseline HBcrAg level >8 log U/mL conferred a >94.4% negative predictive value (NPV) for achieving HBeAg seroconversion and suppression of HBV DNA at 12 weeks (n=46) [49]. Furthermore, HBcrAg levels changed by treatment might predict clinical outcomes. In 58 patients positive for HBeAg treated with PEG-IFN, HBcrAg levels at week 12 predicted HBeAg seroconversion at 24 weeks after finishing treatment with an AUROC of 0.896 [50]. For patients treated with NA therapy (n=39), HBcrAg levels were lower in patients with NA-induced HBeAg seroconversion compared with patients who remained HBeAg-positive [45].
As an advanced technique, a combination of HBcrAg and HBsAg assays may be used to identify patients who are unlikely to achieve treatment end-points, i.e., HBeAg seroconversion [51]. Wang et al. [51] identified predictors of seroconversion using serum quantitative HBsAg and HBcrAg, in HBeAg-positive patients. Data and samples were obtained from 118 HBeAg-positive adults with HBV genotypes A–G treated with NA. Approximately 36.4% of patients achieved HBeAg seroconversion after NA treatment (median, 39 months). Regarding the treatment kinetics of HBV DNA, HBsAg and HBcrAg differed between patients with and without HBeAg seroconversion [51].
Additionally, baseline HBcrAg level is an independent predictor of long-term HBcrAg below the limit of detection [52]. Wang et al. [52] investigated the long-term kinetics of serum HBcrAg and its correlation with serum HBsAg in CHB patients positive or negative for HBeAg with NA therapy over 8 years. At 8 years from the start of NA treatment, 21.3% of patients achieved a serum HBcrAg level of <3 log10U/mL, and only baseline HBcrAg was an independent predictor [52].
HBcrAg in the prediction of NA cessation point
Once seroconversion to anti-HBe occurs, the reduction of HBcrAg and HBsAg followed by HBsAg loss is a major goal of current treatment efforts in CHB [11]. In HBeAg-negative CHB patients, the goal of antiviral therapy is a sustained off-treatment response and HBsAg clearance [49]. However, because it is difficult to obtain HBsAg clearance during NA therapy, the reduction of HBsAg and HBcrAg might be useful when judging the efficacy of NA therapy.
Therefore, HBcrAg might be more useful in the HBeAg-negative phase. With reference to the first published papers [53,54], the Japan Society of Hepatology (JSH) Guidelines for the Management of HBV Infection [13] described the criteria for the cessation of NA therapy (see Table 15) [13]. The three laboratory criteria for the cessation of NA therapy are: 1) at least 2 years administration of NAs; 2) undetectable serum HBV DNA levels (using real-time polymerase chain reaction); and 3) negative serum HBeAg at time of treatment cessation. When these criteria are met, the risk of relapse can be determined from HBsAg and HBcrAg levels at the time of cessation of therapy.
A decrease in HBcrAg was observed under NA therapy, and the pattern of reduction may provide predictive information on the risk of post-treatment HBV reactivation [55]. A serum HBcrAg level >3.7 log U/mL at NA cessation predicted virological relapse within 1 year of NA cessation [56]. Therefore, serum HBcrAg as a surrogate marker, may have a better influence on patients who are planning NA cessation.
Regarding entecavir (ETV) or tenofovir (TDF), the HBcrAg level at NA cessation is an independent relapse predictor, as well as HBsAg, age, alanine aminotransferase (ALT), and TDF use. Hsu et al. [57] enrolled 135 CHB patients who had stopped ETV or TDF after accomplishing viral reduction for a median of 25.2 months. All patients stopped NA with negative HBeAg and undetectable HBV DNA. During the follow-up period (median, 25.9 months), clinical relapse and HBsAg loss occurred in 66 and eight patients, respectively, with a 5-year cumulative incidence of 56.1% and 8.8%, respectively. A SCALE-B score was calculated using the equation 35 × HBsAg (log IU/mL) + 20 × HBcrAg (log U/mL) + 2 × age (years) + ALT (U/L) + 40 for TDF use. The concordance rates for clinical relapse were 0.87, 0.88, 0.87, 0.85, and 0.90 at 1, 2, 3, 4, and 5 years, respectively. Moreover, complete HBsAg loss occurred in low-risk patients predicted by the score [57].
HBcrAg in the prediction of HCC occurrence and recurrence
To predict which patients will develop advanced liver disease including HCC during NA treatment is difficult [58]. High levels of HBV DNA were correlated with increased risk of cirrhosis and HCC development [21]. Low or undetectable HBV viral load decreased the risk of HCC development but did not prevent it completely [59-61]. Several HBV markers are related to HCC development in CHB patients including serum HBcrAg level (Table 1) [62-64].
HBcrAg was superior to HBV DNA in terms of predictive power for HCC development in treatment-naïve CHB patients [64]. During follow-up (median, 10.7 years), 78 of 1,031 (7.6%) CHB patients without NA treatment developed HCC. HBcrAg >2.9 log U/mL (hazard ratio [HR], 5.05; 95% CI, 2.40–10.63) and basal core promoter (BCP) mutations (HR, 28.85; 95% CI, 4.00–208.20) were independently associated with HCC occurrence [64]. In another study, Tseng et al. [65] reported HBcrAg level was an independent risk factor of HCC in CHB patients with intermediate viral load (HBV DNA from 2,000–19,999 IU/mL). HBcrAg level of 4.0 log U/mL identified patients with an intermediate viral load who were at high risk for HCC [65].
For treatment-experienced patients, NA reduced, but did not eradicate, the risk of HCC occurrence [59-60]. Ando et al. [59] reported that the cumulative incidence of HCC at 1, 3, and 5 years was 0.0%, 13.6%, and 17.7%, respectively in patients with serum HBcrAg levels ≥3.4 log U/mL at the time of HBV DNA disappearance and 0.0%, 0.0%, and 2.4%, respectively in patients with serum HBcrAg levels <3.4 log U/mL (P=0.005). HBcrAg among 109 CHB patients receiving NA treatment for at least 2 years was an independent risk factor for HCC development (HR, 3.53) [63]. In addition, post-treatment HBcrAg >3.89 log U/mL predicted HCC with an odds ratio of 3.27. Considering only non-cirrhotic patients, a cutoff of >3.90 log U/mL predicted HCC with an odds ratio of 5.95 [66]. To investigate the long-term effect of NA treatment on HCC progression, Kumada et al. [62] compared CHB patients who received NA therapy with those who did not. They concluded that higher HBcrAg levels and BCP mutations were associated with HCC progression, independent of NA therapy [62].
A recent report showed that a combination of HBsAg and HBcrAg values was an effective biomarker for evaluating HCC occurrence in CHB patients [67]. When cut-off values of HBsAg and HBcrAg were defined as 3.0 log U/mL and 3.0 log U/mL, respectively, patients with HCC history were frequently found in the low HBsAg (P=0.002) and high HBcrAg (P<0.001) groups. When HBsAg and HBcrAg were combined, HCC history was most frequent in the subset with low HBsAg and high HBcrAg, among HBeAg-negative patients, despite NA therapy.
HBcrAg level before surgery might be a potential marker to stratify post-surgical surveillance approaches and to identify patients with a high risk of HCC recurrence. A recent study also confirmed the predictive value of HBcrAg for HCC recurrence after curative surgery. In a study of 55 patients, a serum HBcrAg level >4.8 log U/mL at the time of HCC diagnosis gave an HR of 8.96 for subsequent HCC recurrence within 2 years [61]. Finally, the HCC recurrence-free survival rates were significantly lower in HCC patients with high intrahepatic cccDNA and serum HBcrAg levels than those with low cccDNA/HBcrAg levels (P= 0.035 and P=0.003, respectively) [68].
LIVER FIBROSIS MARKER: M2BPGi
Innovative glycoproteomic studies revealed that fibrosis leads to a specific modification of the glycosylation and sugar chain structure on the Mac-2 binding protein (M2BP) [69,70]. Based on these observations, M2BPGi has been developed as a novel surrogate serum glycoprotein-based biomarker (glycobiomarker) that reflects fibrosis stage. Here, we describe the improvement and latest assessment of M2BPGi, as a surrogate marker of HBV-related liver fibrosis and as a predictor of HBV-related HCC occurrence.
M2BPGi and its features
In 2013, M2BPGi (also called Wisteria floribunda agglutinin [WFA]-positive M2BP [WFA+ -M2BP]) was introduced as a novel, noninvasive, rapidly assayed serological glycobiomarker for the evaluation of liver fibrosis [69]. Fibrosis results in specific modification of the glycosylation and sugar chain structure of M2BP, whereby levels of these modified M2BP proteins correlate significantly with the progression of fibrosis [69,70].
Quantification assays of serum M2BP with fibrosis-specific modified sugar chain structures, i.e., M2BPGi, were developed, evaluated, and approved for clinical use in Japan [69-71]. M2BPGi is detected using a specific lectin called WFA that recognizes the Nacetylgalactosamine residue of N-glycans and O-glycans on M2BP (Fig. 3A) [71]. An automated and high-throughput assay measures M2BPGi levels in 10 µL serum samples in 17 minutes (Fig. 3B) [69]. The M2BPGi assay (Sysmex Corp., Hyogo, Japan) has a reported cut-off index (C.O.I.) range of 0.1–20 and samples with less than 1.0 C.O.I. are considered negative.
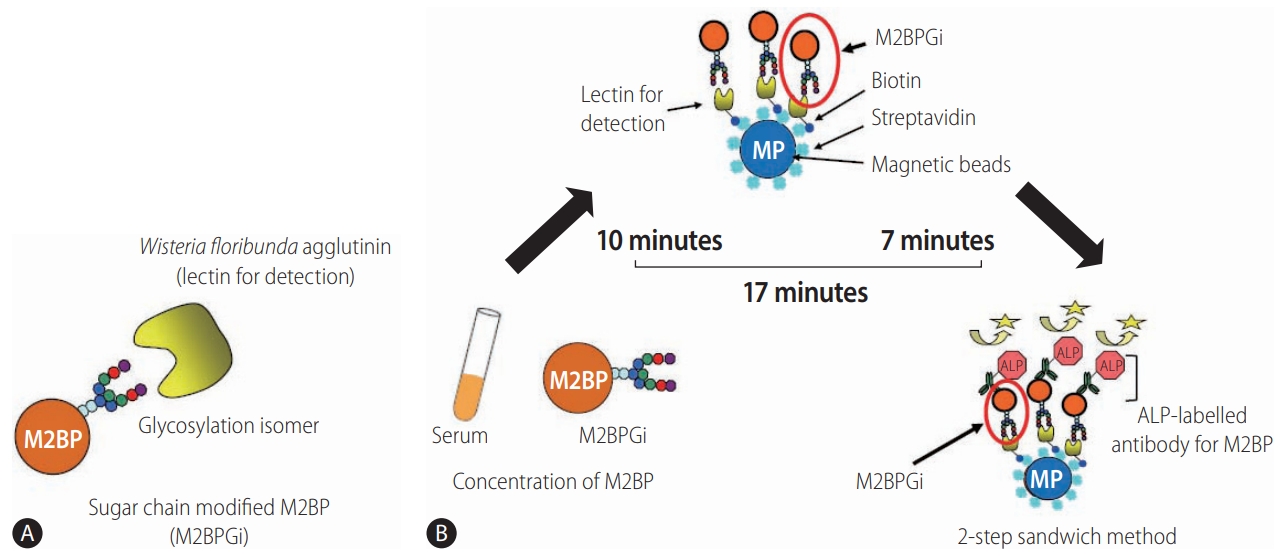
Detection of WFA+-M2BP [139]. (A) Structure of WFA+-M2BP. WFA+-M2BP, which was recently shown to be a liver fibrosis glycobiomarker with a unique fibrosis-related glycoalteration, has multibranching and sialylated N-glycans. WFA lectin binds specifically to the glycosylation isomer. (B) Quantification of serum WFA+-M2BP. WFA+-M2BP quantification is measured based on a lectin-antibody sandwich immunoassay using a fully-automatic immunoanalyzer HISCL-2000i (Sysmex Corp., Hyogo, Japan). The lectin-binding WFA+-M2BP recognizes ALP-labelled antibody in a chemiluminescence enzyme immunoassay (CLEIA). Every reaction is accustomed to the platform during the automatic assay, which is finished after 17 minutes. M2BP, Mac2 binding protein; M2BPGi, Mac-2-binding protein glycosylation isomer; ALP, alkaline phosphatase; WFA+-M2BP, Wisteria floribunda agglutinin-positive Mac-2 binding protein.
M2BPGi for HCC prediction in CHB
M2BPGi is predominantly used to evaluate liver fibrosis in CHC patients [72]. However, the diagnostic value of M2BPGi to assess liver fibrosis was demonstrated in patients with viral hepatitis [69], CHB [73], non-alcoholic fatty liver disease [74], and biliary atresia [75], as well as CHC [76]. In addition, M2BPGi levels are prognostic of the risk of HCC development in CHB [77,78], CHC [76] or non-alcoholic fatty liver disease [79]. Furthermore, post-sustained virological response M2BPGi is a predictive factor for the development of HCC in patients with no previous HCC history and those treated with DAAs for hepatitis C virus (HCV) infection [80]. In this section, we summarize studies showing M2BPGi has good performance for the assessment of liver fibrosis and HCC prediction in CHB.
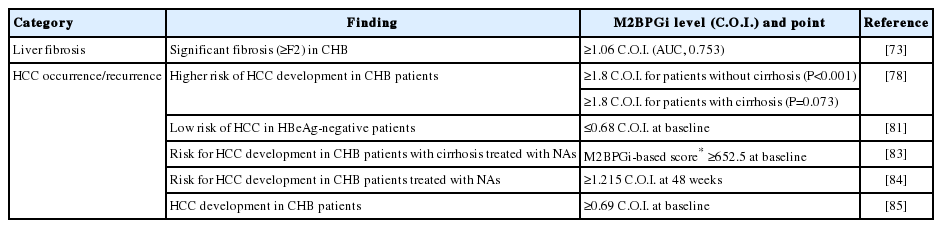
M2BPGi level predicted HCC development in CHB patients [78]. Kim et al. [78] reported that M2BPGi level was an independent predictor of HCC development (adjusted HR, 1.143; 95% CI, 1.139– 1.829), together with male gender and diabetes (all P<0.05) in multivariate analysis. In patients without cirrhosis (n=1,087), M2BPGi levels ≥1.8 were associated with a higher risk of HCC development (P<0.001), whereas M2BPGi levels ≥1.8 tended to be associated with a higher risk of HCC development in patients with cirrhosis (n=236) (P=0.073) [78].
Conversely, M2BPGi accurately identified CHB patients with a low risk of HCC. Mak et al. [81] evaluated M2BPGi for HCC prediction in 207 non-treated HBeAg-negative patients with recognized HBeAg seroconversion. Using a cut-off of 0.68 C.O.I., the baseline M2BPGi showed good performance (area under the curve [AUC], 0.88; sensitivity, 90%; specificity, 80%). In these HBeAg-negative patients, the NPV was very high (99.3%), while the positive predictive value was low (25.8%) [81].
Additionally, M2BPGi was more effective than AFP for predicting HCC occurrence and was an independent predictor of HCC [82]. Jun et al. [18] enrolled 947 treatment-naive patients mono-infected with HBV or HCV and without HCC at baseline. The median M2BPGi was significantly higher among patients with cirrhosis (2.67 vs. 0.80; P<0.001) and those who developed HCC (3.22 vs. 1.16; P<0.001). M2BPGi outperformed AFP for patients with CHB (0.84 vs. 0.75; P=0.02).
In CHB patients receiving NA treatment, M2BPGi level before treatment and 48 weeks after the start of NA treatment is an indicator of HCC occurrence. Hsu et al. [83] reported the baseline level can be factored into the risk prediction of HCC in NA-treated cirrhosis patients. Baseline M2BPGi level was associated with HCC risk (HR, 1.07 per C.O.I.; 95% CI, 1.01–1.14) in cirrhosis patients, whereas year 1 or 2 levels were not independently predictive [83]. Shinkai et al. [84] reported serum M2BPGi levels ≥1.215 C.O.I. at 48 weeks were associated with HCC development (HR, 5.73; P≤0.001).
In patients with undetectable HBV DNA during NA therapy, higher pre-treatment M2BPGi level was associated with increased risk of HCC development [83,85]. Cheung et al. [85] compared 57 NAtreated patients with undetectable HBV DNA and HCC development with 57 controls. There was a significant difference in median levels of pre-treatment M2BPGi between HCC and control groups (0.67 vs. 0.41 C.O.I.; P<0.001). Among patients with cirrhosis, the median level of M2BPGi was higher in the HCC group than the control group (0.74 vs. 0.47 C.O.I.; P=0.014). Among patients without cirrhosis, the median level of M2BPGi of the HCC group was also higher (0.48 vs. 0.28 C.O.I.; P=0.002). With a cutoff value of 0.69 C.O.I., the AUROC of pre-treatment M2BPGi to predict HCC development for the whole cohort was 0.70. With cut-off values of 0.69 and 0.34 C.O.I. for patients with and without cirrhosis, AUROCs that predicted HCC were 0.67 and 0.77, respectively [85].
In patients with HBV-related HCC, Kim et al. [86] showed that the level of M2BPGi is an independent predictive factor of HBV-related HCC recurrence after curative resection. Also, Heo et al. [87] evaluated its accuracy in assessing liver fibrosis and in predicting the risk of developing HCC in patients with CHB. They concluded the level of M2BPGi significantly reflected degree/extent of liver fibrosis and independently predicted the risk of developing HCC in patients with CHB [87].
Therefore, M2BPGi might be suitable for assessing fibrosis level and prediction of HCC in CHB patients. Clinical applications of M2BPGi in patients with chronic liver disease are summarized in Table 2.
TUMOR MARKERS: AFP, AFP-L3, PIVKA-II, DKK-1 AND CIRCULATING IgG ANTIBODIES
Measuring serum levels of tumor biomarkers for HCC is essential for CHB management. AFP, AFP-L3, and PIVKA-II are HCC-specific tumor markers [88]. Although tumor marker levels are not included in diagnostic criteria for HCC, screening recommendations in the guidelines of the American Association for the Study of Liver Diseases [89], or the European Association for the Study of the Liver [90], they provide valuable supportive information for diagnosing HCC.
Levels of AFP, AFP-L3, and PIVKA-II usually increase as HCC develops, i.e., with increased size and number of HCC lesions and progression to portal vein invasion [88]. Other studies reported increased tumor marker levels suggested a high degree of HCC malignancy regardless of morphological progression [91]. Therefore, increased levels of these tumor markers indicates an unfavorable prognosis after initial diagnosis [88].
An increased level of DKK-1 occurs in a wide variety of cancers, including multiple myeloma, prostate cancer and HCC [92,93]. Circulating anti-CD25 IgG antibodies may have prognostic rather than early diagnostic values for HCC [94]. Circulating IgG antibody to p16 protein might be a useful biomarker for HCC prognosis assessment rather than for early diagnosis of HCC [95].
In this section, we introduce three tumor markers, AFP, AFP-L3, and PIVKA-II, as indicators of HCC development and predictors of patient outcome. In addition, we provide information of DKK-1 and circulating IgG antibodies as novel markers of HCC.
AFP and AFP-L3
AFP was first discovered in the serum of HCC patients in 1964 and has since been the primary diagnostic biomarker for HCC [96]. It is normally produced at a low concentration by liver, yolk sac, and gastrointestinal tract during fetal and neonatal development [97]. High levels of serum AFP correlated with the presence and development of HCC and it is used as a diagnostic and prognostic factor [98].
Despite this, there are multiple problems using AFP as a diagnostic marker, such as elevation in non-HCC diseases including cirrhosis, hepatitis, cholangiocarcinoma, testicular germ cell tumor, and metastatic colon cancer [98]. Because of its low diagnostic accuracy, with sensitivities ranging from 18–60% and specificity of 85–90%, AFP has recently been excluded from American Association for Study of Liver Diseases (AASLD) HCC surveillance guidelines [99]. Indeed, only 60–80% of HCC have elevated AFP levels, leaving a large margin for false-negatives and missed diagnoses [100]. Therefore, AFP alone is not recommended as the main screening test for HCC [98]. To significantly improve the diagnostic accuracy of HCC, additional biomarkers are needed to complement AFP [100].
AFP exists as three glycoforms, each with different binding competence to lectin Lens culinaris agglutinin. AFP-L1 is a nonbinding fraction, AFP-L2 is a weak binding fraction, and AFP-L3 is a binding fraction [100]. AFP-L1 is increased in chronic hepatitis and liver cirrhosis, whereas AFP-L3 is specifically increased in HCC [100]. Because AFP-L3 has a high specificity of 92.0–99.4% for HCC and a low sensitivity of 18.8–37.0% [101-103], it is considered a more specific biomarker for HCC [104]. Moreover, because AFP-L3 is typically not detected when AFP levels are <20 ng/mL, AFP-L3 is not relevant for the diagnosis of HCC in individuals with a total AFP concentration <20 ng/mL. Thus, the sensitivity for AFP-L3 appears to be adversely affected by the total AFP concentration [100,105]. Furthermore, AFP and AFP-L3 levels were detected in blood 6months prior to a diagnosis of HCC [106].
Recent improvements in highly sensitive analytical methods have enhanced the sensitivity of the AFP-L3 immunoassay, referred to as “highly sensitive AFP-L3” (hs-AFP-L3) [107]. Toyoda et al. [107] examined patients with benign liver disease (n=44) including chronic hepatitis, cirrhosis, and HCC (n=54) and determined the performance of hs-AFP-L3. The sensitivity and specificity of hsAFP-L3 were 84.9% and 88.6%, respectively [107]. Additionally, postoperative AFP-L3% was a prognostic factor of HCC recurrence after curative treatment and detected small tumors and early stage HCC [108,109]. These results indicate hs-AFP-L3% might be a valuable biomarker for detecting early-stage HCC.
PIVKA-II
PIVKA-II, known as des-γ-carboxyprothrombin, is an abnormal prothrombin molecule that is increased in HCC [100]. During malignant transformation in hepatocytes, the vitamin K-dependent carboxylase system becomes damaged [110]: a deficiency in post-translational carboxylation leads to the production of PIVKA-II [111]. During this process, PIVKA-II loses its normal prothrombin function but may promote malignant proliferation in HCC. One benefit of PIVKA-II is that it is less likely to be elevated in non-HCC liver diseases than AFP [98]. In a screening test for HCC, when compared to cases of cirrhosis and chronic hepatitis, PIVKA-II yielded a sensitivity of 72.7% and a specificity of 90.0%, which was comparable to AFP [110].
Recently, the performance of AFP was compared with PIVKA-II for the diagnosis of early-stage HCC in France [112]. This study included 43 cirrhosis patients and 85 HCC patients including 32 patients with early HCC. PIVKA-II (threshold value, 42 mAU/mL) performed better than AFP (threshold value, 5.5 ng/mL) for earlystage HCC diagnosis (AUC, 0.81; 95% CI, 0.697–0.924 vs. AUC, 0.582; 95% CI, 0.443–0.722), and a PIVKA-II level >90 mAU/mL was an independent predictor of microvascular invasion (HR, 3.5; 95% CI, 1.08–11.8; P=0.043), a major prognostic factor in HCC [112].
As described above, PIVKA-II is a potential serum biomarker for the early diagnosis of HCC. Furthermore, Chen et al. [113] reported a meta-analysis where PIVKA-II had a better accuracy than AFP for the detection of HCC in CHB, regardless of tumor size, patient ethnic background (American, European, Asian, or African), or etiology of HCC (HBV-related or mixed).
Most importantly, serum PIVKA-II level is a suitable parameter for the development of portal venous invasion [114]. Koike et al. [114] enrolled 227 HCC patients who did not show portal venous invasion and who received percutaneous ethanol injection therapy and/or microwave coagulation therapy at the time of their first hospital admission. After their HCC was treated, the patients were followed for a mean of 19 months. Of the 227 patients, 24 (11%) later developed portal venous invasion, and the PIVKA-II level at the time of initial diagnosis of HCC had a significant correlation with the later development of portal venous invasion [114]. Yamashita et al. [115] reported that even in cases with small HCC (≤2 cm), patients with a high PIVKA-II level (>100 mAU/mL) were at risk for microinvasion. Therefore, in such patients, hepatic resection with a wide tumor margin should be recommended [115].
Moreover, Lee et al. [116] investigated whether pre- or post-ablation serum AFP and PIVKA-II levels predicted prognosis in patients with curative radiofrequency ablation (RFA) for HBV-related HCC. They retrospectively analyzed 412 patients with HBV-related single HCC treated with percutaneous RFA. AFP and PIVKA-II levels were measured before and 1 month after treatment. Among the tumor markers, post-ablation PIVKA-II was an independent prognostic factor for overall and recurrence-free survival (HR, 3.438; 95% CI, 1.331–8.877; P=0.011 and HR, 4.934; 95% CI, 2.761–8.816; P<0.001, respectively). They concluded that postablation serum PIVKA-II is a useful biomarker for predicting survival and recurrence after curative RFA in patients with HBV-related HCC [116].
Combination of AFP and PIVKA-II
Because PIVKA-II is more specific to HCC and has a reduced tendency to be elevated in other chronic liver diseases, a combination of AFP and PIVKA-II had a higher efficiency for the diagnosis of early HCC in cirrhosis patients. Lok et al. [117] reported a combination of AFP and PIVKA-II had increased sensitivity (65% to 87%) compared with AFP alone, but decreased specificity (84% to 69%). For screening, this increased sensitivity is obviously valuable [117]. Song et al. reported that in 120 patients with HCC, PIVKAII alone was inferior to the combination of AFP and PIVKA-II because of its lower sensitivity (53.3% vs. 78.3%) [118]. A recent metaanalysis including 11 studies by Caviglia et al. [119] also described that the use of AFP and PIVKA-II may improve the effectiveness of surveillance among patients at risk for HCC development. The weighted summary AUC (sAUC) of PIVKA-II and AFP for the discrimination between patients with HCC and those without was 0.791 (0.746–0.837) and 0.767 (0.732–0.803), respectively. The combination of PIVKA-II and AFP resulted in a sAUC of 0.859 (0.837–0.882), suggesting that the performance for HCC detection of PIVKA-II andAFP was significantly superior to each biomarker used alone (ΔsAUC, 0.068; P=0.032 and ΔsAUC, 0.092; P<0.001, respectively) [119].
Previous studies suggested specific biomarkers for HCC diagnosis, of which the combination of AFP and PIVKA-II appeared to be the best. Chen et al. [120] selected five representative biomarkers (AFP, AFP-L3, PIVKA-II, squamous cell carcinoma antigen, and centromere protein F autoantibody) with diagnostic potential for detecting HCC. Serum levels of the five biomarkers were simultaneously measured in a large sample set (n=846) including patients with HCC, cirrhosis, chronic HBV infection, and healthy controls.120 Overall, for two-marker combinations, a prediction algorithm including AFP and PIVKA-II had the best diagnostic value. The apparent AUC (without correction) of the two-marker algorithm in detecting early-stage HCC was 0.86 (95% CI, 0.81–0.90). Moreover, in another report of the diagnosis of HCC associated with alcoholic and non-alcoholic fatty liver disease, AFP and PIVKA-II appeared to be the best combination of biomarkers [121], suggesting that PIVKA-II might be a complementary biomarker for the diagnosis of HCC and to improve the identification of patients with AFP-negative HCC. Meanwhile, as the recent report, combined microRNA-122 (miR-122), AFP plus PIVKA-II had an adjusted HR for HCC development of 10.63 [122]. AUCs were 0.675 for miR-122, 0.791 for AFP and 0.846 for PIVKA-II, respectively, while their combination improved the discrimination power between cirrhosis and HCC (AUC, 0.918) [122].
DKK-1
DKK-1 is a glycoprotein which is a secretory antagonist of the Wnt/beta-catenin signaling pathway [123]. Although its detailed function is not completely understood, the increase of DKK-1 expression occurs in a wide variety of cancers, including multiple myeloma, prostate cancer and HCC [92].
Several studies have shown that higher DKK-1 levels in HCC patients than in healthy individuals [95,124-126] or than in patients with cirrhosis without HCC [126-128]. Shen et al. [126] reported serum DKK-1 and AFP levels in a cohort of 1,284 patients. DKK-1 was more useful for detection of AFP-negative HCC, while combining DKK-1 with AFP enhanced the detection rate of early-stage HCC to help in detection in HCCs, which do not produce AFP [126]. Another report from South Korea showed that the combination of AFP and DKK-1 was just slightly better than AFP alone for the detection of early-stage HCC (AUC, 0.693 vs. 0.691) in HCC patients with mainly HBV-infection (n=208) [129].
In addition, a meta-analysis published in 2014 containing four studies [130] and other recently published study [128] showed that higher levels of DKK-1 expression in patients with HCC were associated with the lower survival rate.
Circulating IgG antibodies
In recent studies, Wang et al. [94] found that serum IgG antibodies against linear peptide antigens derived from p16 protein, interleukin 2 receptor α-subunit (also called CD25) and forkhead/wingedhelix transcription factor box P3 (FOXP3) were significantly changed in the patients with HCC. Therefore, circulating IgG antibodies for these target molecules may be either diagnostic or prognostic values for solid tumors [131]. Serum levels of IgG antibodies for p16a, CD25a and FOXP3 were significantly higher in HCC patients than control subjects [131].
Further analysis showed that increased levels of plasma IgG for these three peptide antigens were mainly shown in patients with the intermediate and late-stage HCC. This study has confirmed that serum IgG antibodies to p16, CD25 and FOXP3 are significantly increased in HCC patients, especially in late-stage HCC. These autoantibodies may be useful biomarkers for assessment of HCC prognosis [131].
DISCUSSION
In this review, we described the characteristics and clinical applications of novel biomarkers. HBcrAg is appropriate for evaluating the amount of intrahepatic cccDNA in CHB patients. M2BPGi can assess the severity of liver fibrosis and the risk of HCC development in CHB patients. Regarding tumor markers including AFP, PIVKA-II, and AFP-L3, DKK-1 and circulating IgG antibodies, using only one had a restricted detection rate for HCC, but the combination by two showed improved detection rates.
Here, we discuss the future prospects of HBcrAg, M2BPGi, and tumor markers. First, the amount of HBcrAg is associated with that of HBV DNA in all CHB disease states. Even in patients who achieve a “functional cure” with undetectable serum HBV DNA and HBsAg, severe complications including HBV reactivation and HCC occurrence may be reported. Because some patients achieving a “functional cure” still have detectable serum HBcrAg, prospective studies comparing the long-term outcome between HBcrAg-positive and HBcrAg-negative patients are required. To date, most reports have been published from East Asia. For HBcrAg to be used more in clinical practice, large cohort studies should be completed in other areas, especially the United States and Europe.
To confirm a functional cure of HBV, higher sensitivity assays for HBsAg and HBcrAg are required. Recently, a new HBcrAg assay with approximately 10-fold higher sensitivity is being developed [29]. Furthermore, based on a fully-automated pretreatment technique before HBcrAg measurement, new highly sensitive HBcrAg measurements should be used to monitor HBeAg-negative patients.
Second, M2BPGi has been used as a valuable biomarker to evaluate liver fibrosis, especially in CHC patients. Although past studies have documented the accuracy of M2BPGi assay in patients with CHC, few have addressed CHB [73,78,87,132,133].
The M2BPGi cut-off value for predicting cirrhosis or HCC varies, depending on disease etiology. Ichikawa et al. [134] reported that M2BPGi ≥0.71 was a risk factor for HCC development in CHB patients. As described above, M2BPGi levels ≥1.215 48 weeks after starting NA treatment [84] were associated with HCC. Taken together, the M2BPGi levels of CHB patients seem to be lower than those of CHC patients. Conversely, in CHC independent of liver fibrosis, whether the M2BPGi level is increased is still controversial. Only one study has evaluated M2BPGi as a predictor of HCC in non‐Asian patients and patients outside East Asia [18]. Further studies should evaluate M2BPGi as an HCC biomarker in broader patient populations, including non‐Asians and those with severe liver fibrosis.
Third, although the diagnosis of HCC remains difficult, especially in the early stage, the early and accurate diagnosis of HCC is vital to improve CHB patient outcomes. Current information suggests no single biomarker is likely to have optimal sensitivity and specificity for the detection of HCC, particularly at the early stages of development. Many studies reported combinations of biomarkers improved the detection of early stage HCC. Additional studies are needed to evaluate further the effectiveness of combined biomarkers for HCC diagnosis.
Recent studies reported a combination of these three tumor markers had very high sensitivity and specificity for diagnosing HCC without the use of imaging studies [135], and improved the sensitivity and specificity of early HCC diagnosis [136]. In the JSH-HCC guidelines, simultaneous measurement of AFP, AFP-L3, and PIVKA-II with ultrasound examination is recommended for HCC surveillance of high-risk populations [16].
When considering HCC biomarker applications, there must be a careful and an accurate consideration as to the various metabolic profiles of variable ethnic groups [137]. Nguyen et al. [138] reported clear ethnic differences in the diagnostic value of AFP. Patients with HCV-related HCC in Asia, Europe, and Central and South America had a normal AFP level of 18%, whereas African-American patients had normal level of 43%. Furthermore, there was difference between the underlying etiologies of liver disease, where HCV-related HCC was more strongly associated with elevated AFP compared with HBV-related HCC [138]. Further studies are needed to evaluate PIVKA-II as an HCC biomarker in broader patient populations, including non‐Asians and those with mild liver fibrosis. In summary, etiological, dietary, genetic, and environmental factors that differ between populations suggest the need for validation studies in various regions to establish better diagnostic and screening tools.
CONCLUSIONS
HBcrAg and M2BPGi are useful novel biomarkers for the management of CHB including predicting HCC occurrence. AFP, AFPL3, PIVKA-II, DKK-1 and circulating IgG antibodies are HCC-specific tumor markers. Combinations of these biomarkers might have better prospects for application in clinical practice. Further global studies are required to increase the application of these useful biomarkers for many aspects of CHB clinical practice.
Notes
Authors’ contribution
Conceptualization, T.I. and Y.T.; Writing and Original Draft Preparation, T.I.; Writing, Review and Editing, Y.T.
Conflicts of Interest: Takako Inoue is currently supported by a research grant from Gilead Sciences and MSD.K.K. Yasuhito Tanaka is currently conducting research sponsored by Chugai Pharmaceutical Co., Ltd., Bristol-Myers Squibb, Fujirebio, Inc., and Gilead Sciences. Lecture fees are follows: Fujirebio, Inc. and Gilead Sciences.
Acknowledgements
This work was supported by a grant-in-aid from the Research Program on Hepatitis from the Japan Agency for Medical Research and Development (AMED JP19fk0310101) and the Ministry of Education, Culture, Sports, Science, and Technology (19H03640).
Abbreviations
AASLD
American Association for Study of Liver Diseases
ADV
adefovir
AFP-L3
Lens culinaris agglutinin-reactive fraction of alpha-fetoprotein
AFP
alpha fetoprotein
ALT
alanine aminotransferase
AUC
area under the curve
AUROC
area under the receiver operating characteristics
BCP
basal core promoter
C.O.I.
cut-off index
cccDNA
covalently closed circular DNA
CHB
chronic hepatitis B
CI
confidence interval
DKK-1
Dickkopf-1
ETV
entecavir
FBS
fasting blood sugar
FOXP
factor box P3
HBcrAg
hepatitis B core-related antigen
HBeAg
hepatitis B envelope antigen
HBsAg
hepatitis B surface antigen
HBV
hepatitis B virus
HCC
hepatocellular carcinoma
HCV
hepatitis C virus
HR
hazard ratio
hs-AFP-L3
highly sensitive AFP-L3
IFN
interferon
IgG
immunoglobulin G
JSH
Japan Society of Hepatology
M2BP
Mac-2 binding protein
M2BPGi
Mac-2- binding protein glycosylation isomer
miR-122
microRNA-122
NA
nucleos(t) ide analogue
NPV
negative predictive value
PEG-IFN
pegylated interferon
PIVKA-II
protein induced by vitamin K absence factor II
RFA
radiofrequency ablation
sAUC
weighted summary AUC
TDF
tenofovir
WFA
Wisteria floribunda agglutinin
WFA+ -M2BP
Wisteria floribunda agglutinin-positive Mac-2 binding protein