Use of glecaprevir/pibrentasvir in patients with chronic hepatitis C virus infection and severe renal impairment
Article information
Abstract
Background/Aims
Data on treatment efficacy and safety of glecaprevir/pibrentasvir (GLE/PIB) for chronic hepatitis C virus (HCV) infection in Asian patients with severe renal impairment are limited. This study aimed to study the treatment and side effects of GLE/PIB in these patients infected with non-1 genotype (GT) HCV.
Methods
We prospectively recruited patients with Child’s A cirrhosis and eGFR <30 mL/min/1.73 m2 in Hong Kong and Taiwan during 2017–2018 to receive GLE/PIB treatment.
Results
Twenty-one patients (GT2, n=7; GT3, n=6; and GT6, n=8) received GLE/PIB for 11.2±1.8 weeks. All except one were treatment-naïve. GLE/PIB was initiated in 16 patients while on dialysis (seven on peritoneal dialysis [PD] and nine on hemodialysis) and in five patients before dialysis. One patient died of PD-related peritonitis during treatment and two were lost to follow up. The SVR12 rate in the remaining 18 patients was 100%. All patients achieved undetectable levels at 4-, 12-, 24- and 48-week after treatment. Patients with deranged alanine aminotransferase showed normalization after 4 weeks and the response was sustained for 48 weeks. No significant adverse event was observed.
Conclusions
GLE/PIB treatment was associated with high efficacy and tolerability in HCV-infected patients with severe renal impairment.
Graphical Abstract
INTRODUCTION
Chronic hepatitis C virus (HCV) infection is an important cause of cirrhosis and hepatocellular carcinoma (HCC), and confers detrimental effects on the survival of patients undergoing regular dialysis and renal transplantation [1-3]. The conventional treatment for chronic HCV infection is interferon-based therapy, with or without the combination of ribavirin. Such regimens had suboptimal therapeutic efficacy in patients with advanced chronic kidney disease (CKD), and are often associated with substantial side effects which lead to treatment discontinuation [4]. The use of direct acting antiviral (DAA) have revolutionized the management of chronic HCV infection. In the general population, DAA-based treatments (with or without ribavirin) are associated with excellent efficacy and tolerability in HCV patients with or without cirrhosis [5-7], and yet clinical data on their use in patients with severe renal insufficiency remains relatively limited [1,8-11].
Glecaprevir/pibrentasvir (GLE/PIB) is a NS3/4A protease inhibitor/NS5A inhibitor combination drug which shows high anti-viral efficacy across six major genotypes (GT) of HCV and favourable pharmacokinetic properties in renal failure patients (once-daily oral dosing and with negligible (<1%) renal excretion) [12-18]. The use of GLE/PIB without concomitant administration of ribavirin is another merit for its application in patients with advanced CKD [19]. The data from EXPEDITION-IV suggested that GLE/PIB treatment was associated with good virological response and safety profile in patients with severe renal impairment [19]. Although this study reported that GLE/PIB was effective across all six major GT in patients with significant renal dysfunction, one should note that patients included mostly suffer from GT1 (~52%) and there was only one patient (1%) with GT6 HCV infection. In addition, only 9% of the study patients were of Asian origin. In this context, GT6 HCV infection is more prevalent in Southern China, Hong Kong, Taiwan and other Southeast Asian countries [20,21], and data for GLE/PIB treatment for non-1 GT (especially GT6) HCV infection in patients with severe renal impairment renal is lacking.
PATIENTS AND METHODS
Patients
We prospectively recruited patients with chronic HCV infection and severe renal impairment to receive GLE/PIB treatment. The inclusion criteria were: 1) patients who were at least 18 years of age and infected with HCV; 2) patients with GT2, GT3, GT5, or GT6 HCV infection, and compensated cirrhosis (Child-Pugh score ≤6) and/or extra-hepatic manifestations of HCV infection; and 3) CKD of Stage 4 (eGFR 15–29 mL/min/1.73 m2) or Stage 5 (eGFR <15 mL/min/1.73 m2 or requiring dialysis or kidney transplantation). Exclusion criteria were: 1) co-infection with chronic hepatitis B virus; 2) Child-Pugh B or C cirrhosis or history of hepatic decompensation (e.g., bleeding varices, encephalopathy, refractory ascites); 3) prior treatment with an NS5A or protease inhibitor; 4) life expectancy of less than 1 year; 5) patients with unknown HCV genotype; 6) patients with neoplasm, other than HCC, requiring chemotherapy during DAA treatment; 7) patients with newly diagnosed HCC or recurrent HCC within the past 12 months; 8) patients who required treatment with any of the following during DAA therapy, and could not be changed to an alternative medication: dabigatran, atazanvir, efavirenz, lopinavir, carbamazepine, rifampicin, atorvastatin, lovastatin, simvastatin, ethinylestradiol contraceptives and hormone replacement therapies, St. John’s wort; and 9) patients who were pregnant. All patients underwent ultrasonography and transient elastography (Fibroscan®; Echosens France, Paris, France) to document evidence of liver cirrhosis (≥F4 by Fibroscan® [Echosens France] and evidence of portal hypertension on ultrasonography). Fibroscan® (Echosens France) was performed in hemodialysis (HD) patients after a HD session and after peritoneal dialysate had been fully drained out in peritoneal dialysis (PD) patients. In patients who did not have fibroscan because of no reimbursement, the diagnosis of cirrhosis was based on compatible clinical/ultrasonographic features. The study was approved by the Institutional Review Board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster (Approval No. UW-18-370) and ethics committees of all participating centres.
Use of GLE/PIB treatment and follow-up schedule
Patient demographics, complete blood counts, liver and renal biochemistry, HCV GT and RNA levels and serum alpha-fetoprotein (AFP) levels were measured before the commencement of GLE/PIB treatment. GLE/PIB was initiated at 300 mg/120 mg daily for 12 weeks. Patients were followed at 4-, 12- and 24-week after the initiation of GLE/PIB treatment. During each study visit, patient were monitored for complete blood counts, liver and renal biochemistry, clotting profiles and HCV RNA levels. HCV GT and RNA levels were determined by COBAS 4800 system (Roche Diagnostic, Hong Kong, China), and the lower limit for detection was 15 IU/mL. Any clinically significant events or side effects were also documented. AFP was measured before commencement of treatment and also repeated at 24 weeks after treatment. To monitor for liver decompensation in PD patients, we also documented the number of exchanges, net ultrafiltration and residual urine volume before and after GLE/PIB treatment.
Statistical analysis
The primary outcome was the rate of sustained virological response at 12 weeks (SVR12, defined as an HCV RNA level of less than 15 IU/mL 12 weeks after the end of treatment). Secondary outcomes include the rate of sustained virological response at 24 weeks (SVR24, defined as an HCV RNA level of less than 15 IU/mL 24 weeks after the end of treatment), changes in HCV RNA levels, liver and renal function and adverse events. Continuous variables were expressed as mean±standard deviation and analysed with Student’s t test or Wilcoxon test where appropriate. Categorical variables were expressed as frequency (percentage), and analysed with chi-square test or Fisher-Exact test where appropriate. All statistical analysis were performed by statistical software SPSS (version 24; IBM, Armonk, NY, USA), and a P-value <0.05 was considered statistical significant.
RESULTS
Patients
A total of 21 patients were included for analysis (11 from Hong Kong and 10 from Taiwan) (Tables 1, 2). All patients were Asians and received GLE/PIB 300 mg/120 mg once daily. The mean duration of treatment was 11.2±1.8 weeks. Seven, six, and eight patients were infected with GT2, GT3, and GT6 HCV respectively. Two patients had history of HCV-related membranoproliferative glomerulonephritis (one received prednisolone 50 mg/day before GLE/PIB treatment and the other patient refused HCV treatment when renal biopsy was performed 2 years before the use of GLE/PIB). Twenty patients were treatment-naïve and one patient was treatment-experienced (previously received pegylated interferon [IFN] treatment).
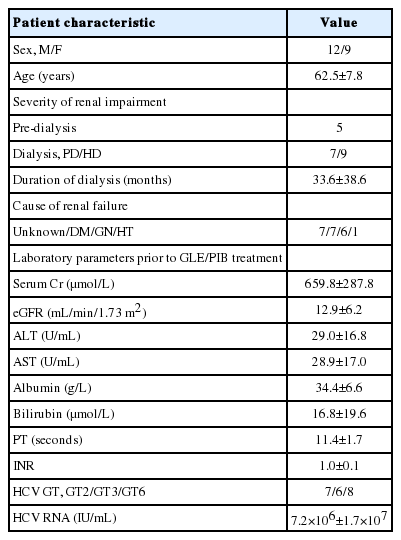
Clinical characteristics of patients with severe renal impairment and received glecaprevair/pibrentasvir treatment
Virological response
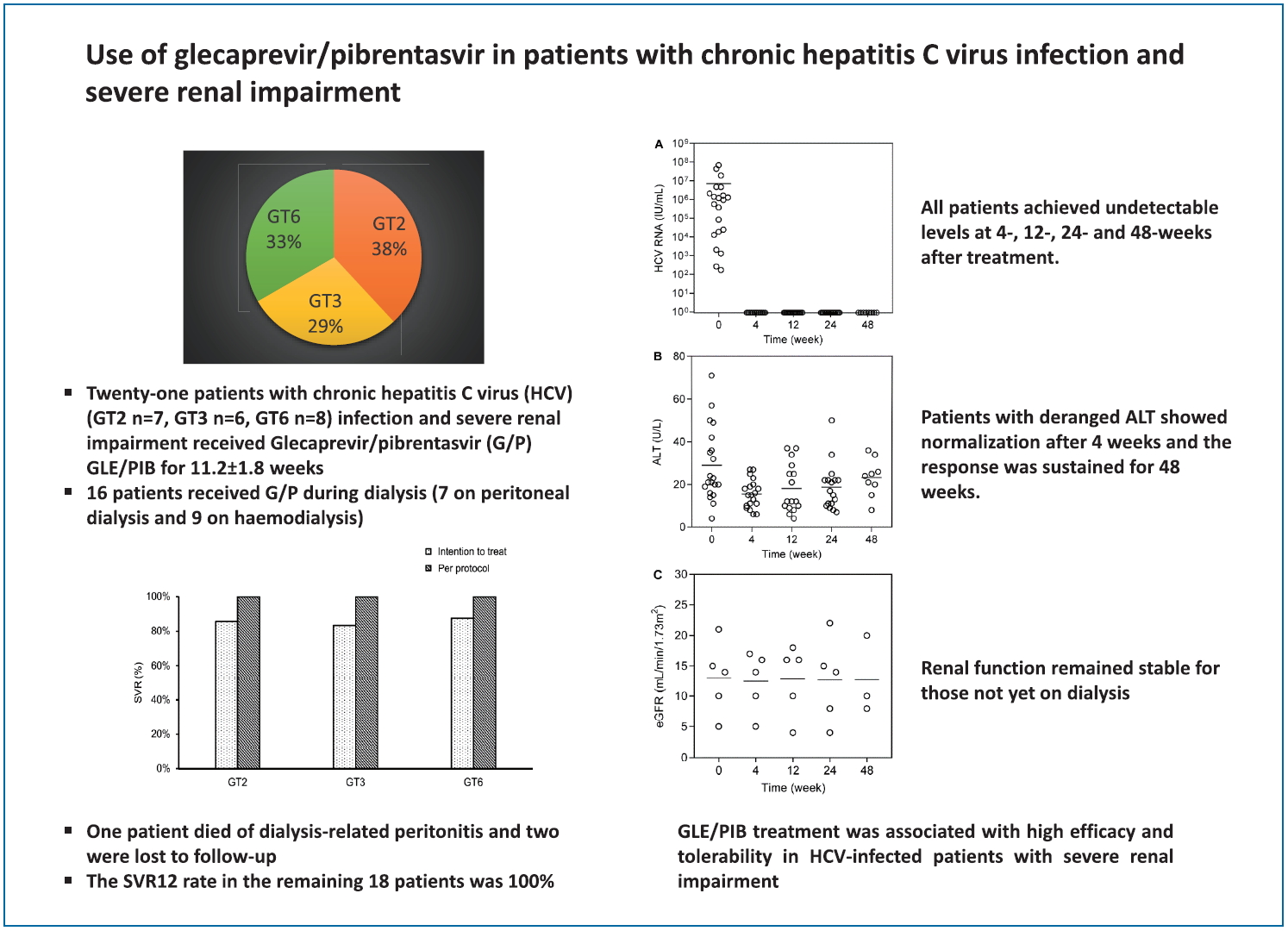
Of the 21 patients, one patient died of PD-related peritonitis during treatment and two were lost to follow up. The SVR12 in the remaining 18 patients was 100%. The baseline HCV RNA level before initiation of treatment was 7.2×106 ±1.7×107 IU/mL. All patients achieved undetectable HCV RNA at 4, 12, and 24 weeks after treatment initiation respectively (Fig. 1A). There was no difference in the SVR12 rates between GT2, GT3, and GT6 patients (100% for all, P>0.05 for all comparison). There was also no difference in SVR12 rates between patients who were treatment-naïve or treatment-experienced (100% for both, P>0.05) (Fig. 2). Three patients had elevated alanine aminotransferase (ALT) at baseline (59.3±10.7 U/mL), and the ALT level decreased to 23.0±4.0 U/mL, 23.0±2.8 U/mL and 24.0±8.9 U/mL at 4, 12, and 24 weeks after treatment (P=0.155, 0.027, and 0.039 compared with baseline value) (Fig. 1B). During a mean follow-up of 31.2±13.7 weeks, there was no virological breakthrough or increase in liver transaminase levels. Nine patients had followed up to 1 year, all showed undetectable HCV RNA during their last follow-up visits and the SVR24 rate was 100% in these nine patients.
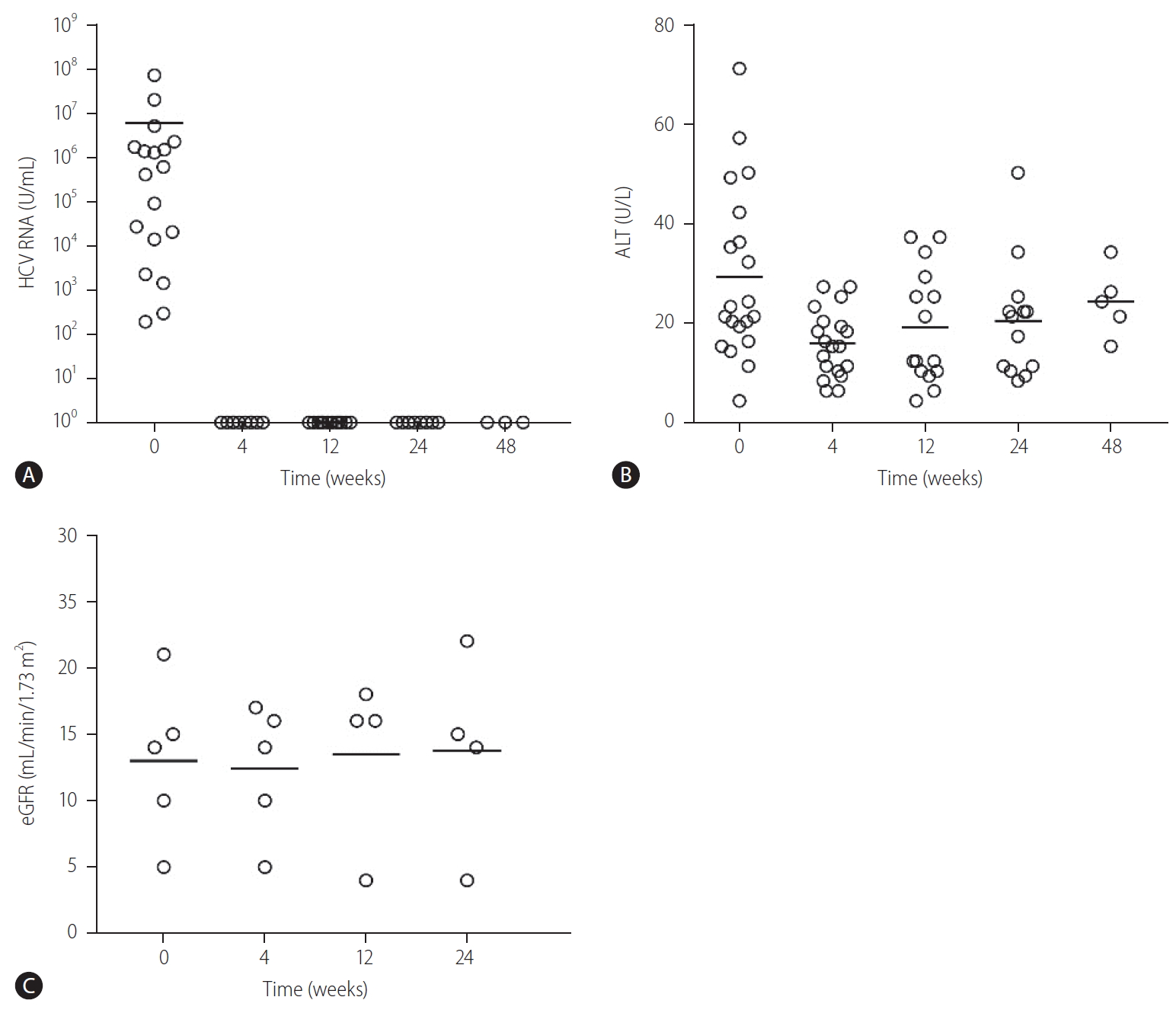
Changes in (A) HCV RNA, (B) ALT, and (C) renal function in patients with severe renal impairment and received glecaprevair/pibrentasvir treatment. HCV, hepatitis C virus; ALT, alanine aminotransferase; eGFR, estimated glomerular filtration rate.
Effect on renal function
Sixteen patients received dialysis (seven were on PD and nine were on HD). None of them required intensification of dialysis regimen after initiation of GLE/PIB. In PD patients, there was no difference in the mean number of PD exchanges (3.2±0.4 vs. 3.2±0.4 exchanges/day, P=1.00), volume of net ultrafiltration (780.0±204.9 vs. 720.0±192.4 mL/day, P=0.180) and residual urine volume (560.0±260.8 vs. 680.0±389.9 mL/day, P=0.317) before and after GLE/PIB treatment. Five patients were not on dialysis before the commencement of GLE/PIB, and all showed stable renal function for up to 24 weeks after initiation of treatment (Fig. 1C).
Drug tolerability and adverse events
One patient had early discontinuation of GLE/PIB because of patient preference and was unrelated to treatment side effects, and this patient also achieved SVR12 despite receiving only 4 weeks of GLE/PIB treatment. One patient died of PD-related fungal peritonitis, which occurred at 3 months after GLE/PIB treatment. GLE/PIB treatment was well-tolerated in other patients.
DISCUSSION
Our current data showed that GLE/PIB treatment was associated with high efficacy and tolerability in patients with chronic HCV infection and severe renal impairment. In this cohort, the rate of SVR12 was 100% and the suppression of HCV RNA to undetectable levels occurred as early as 4 weeks. Such excellent therapeutic response was in line with the data in general population and previous studies in patients with renal impairment [13-19]. While GLE/PIB was stated to show pan-genotypic efficacy in HCV infection, there was very limited data on some specific GT, for instance GT6. In this context, a previous study which investigated the use of GLE/PIB in patients with renal impairment only included one patient with GT6 [19]. In our study, we have included a considerable proportion of patients with GT6 (38.1%), and the SVR12 rate was 100% in these patients. The high SVR rates in this cohort were in line the data from previous pivotal studies (e.g., ENDURANCE-5, 6; SURVEYOR-2 and VOYAGE-1 and -2), which include considerable number of compensated cirrhotic/non-cirrhotic GT-6 patients who did not have significant renal dysfunction [22-25]. As in general population, we observed no significant difference in treatment response between patients of different GT [18]. There is also concern whether the therapeutic response of GLE/PIB is sustainable in patients with renal failure as they are recognized to have impaired innate and adaptive immunity [26]. In this study, nine patients had follow-up data up to 1 year and the rate of SVR24 was 100% with no virological breakthrough. The demonstration of sustainable HCV eradication had important clinical implications for infection control in dialysis units, allocation of dialysis machines and kidney transplantation. In the present cohort, patients were predominantly treatment-naïve (up to 95%) and all showed excellent therapeutic response, while the remaining patient who was previously treated with pegylated-IFN also showed successful viral clearance after GLE/PIB treatment.
Drug tolerability and renal safety is an important concern for the use of DAA in patients with severe renal impairment. Our current data suggested that GLE/PIB treatment was associated with favourable drug tolerability in patients with advanced CKD, which was in line with a previous study in patients with severe renal dysfunction [19]. In our cohort, only one patient had early discontinuation of GLE/PIB because of his own preference and yet he still achieved SVR12 despite receiving GLE/PIB for only 4 weeks. One patient died in this study due to PD-associated fungal peritonitis which was unrelated to GLE/PIB treatment. While previous studies had included predominantly HD patients and there was relatively limited data on the use of GLE/PIB in PD patients, the current guidelines recommend clinicians to follow practices in HD when treating HCV infection in PD patients [16,19]. Notwithstanding, pharmacokinetics and drug elimination are different in PD and HD patients [27]. Our current data, which included a significant proportion (33.3%) of PD patients, suggested that GLE/PIB was also effective and well tolerated in PD patients. The detection and monitoring of liver decompensation (e.g., increase in ascites) can be difficult in PD patients due to the presence of PD dialysate. Our present showed that there was no change in the number of exchanges, net ultrafiltration and residual urine volume in PD patients before and after GLE/PIB treatment. Such data is important as PD is a growing modality of renal replacement therapy in many localities, especially in developing countries and the Asia-Pacific region where HCV infection is still prevalent [28,29]. For patients who were not on dialysis, their renal function showed no significant deterioration during the course of treatment and had remained stable for 24 weeks.
GLE/PIB treatment was associated with high efficacy and tolerability in HCV-infected patients with severe renal impairment.
Notes
Authors’ contribution
Yap DYH: conception and conduction of study, analysis of data and preparation of manuscript
Liu KSH, Wong GLH, Tsai MC, Chen CH, Hsu CS, Hui YT, Li MKK, Liu CH, Kan YM, Yu ML: recruitment and care of patient, preparation of manuscript
Yuen MF: Conception and conduction of study, data analysis and preparation of manuscript
Conflicts of Interest: The authors have no conflicts to disclose.
Abbreviations
AFP
alpha-fetoprotein
ALT
alanine aminotransferase
CKD
chronic kidney disease
DAA
direct acting antiviral
GFR
glomerular filtration rate
GLE
glecaprevir
GT
genotype
HCC
hepatocellular carcinoma
HCV
hepatitis C virus
HD
hemodialysis
IFN
interferon
PD
peritoneal dialysis
PIB
pibrentasvir
References
Article information Continued
Notes
Study Highlights
1. Glecaprevir and pibrentasvir treatment is associated with high SVR in patients with non-genotype 1 HCV infection and severe renal impairment.
2. Glecaprevir and pibrentasvir treatment is safe and well-tolerated in patients with severe renal impairment, including those on hemodialysis and peritoneal dialysis.