| Clin Mol Hepatol > Volume 26(4); 2020 > Article |
|
ABSTRACT
Severe alcoholic hepatitis (AH) is an acute and often devastating form of alcohol-associated liver disease. Clinically, AH is characterized by elevated bilirubin, model for end stage liver disease scores >20, and nonspecific symptoms that are caused by underlying inflammation, hepatocyte injury, and impaired intestinal barrier function. Compromised immune defense in AH contributes to infections, sepsis and organ failure. To date, corticosteroids are the only recommended treatment for severe AH, however it does not provide survival benefits beyond 1 month. Recent preclinical and early clinical studies in AH aided understanding of the disease and presented opportunities for new therapeutic options targeting inflammation, oxidative stress, liver regeneration and modification of intestinal microbiota. In this comprehensive review, we discuss promising preclinical results and ongoing clinical trials evaluating novel therapeutic agents for the treatment of severe AH.
Alcohol-associated liver disease (ALD) is one of the top 30 causes of death worldwide. The most serious form of this condition is acute AH, that may present as acute-on-chronic liver failure (ACLF) and result in high short-term mortality [1]. While research has long focused on the pathogenesis of AH, translation of those findings to clinical trials is only in the early stages. To date, corticosteroid therapy remains the most effective treatment option even though it is beneficial in only about 60% of AH patients and only on 1 month survival [1]. While several other treatment options demonstrated initial promise, most failed efficacy and some were even harmful (Table 1). Liver transplantation in AH is available to a very limited group of patients [2], costly, and requires life-long immunosuppression. Thus, medical treatment of AH continues to be an unmet need. Ongoing pre-clinical studies and clinical trials are actively searching for curative approaches to AH. Here, we aim to describe the current state of research on AH and discuss the implication for potential treatment options.
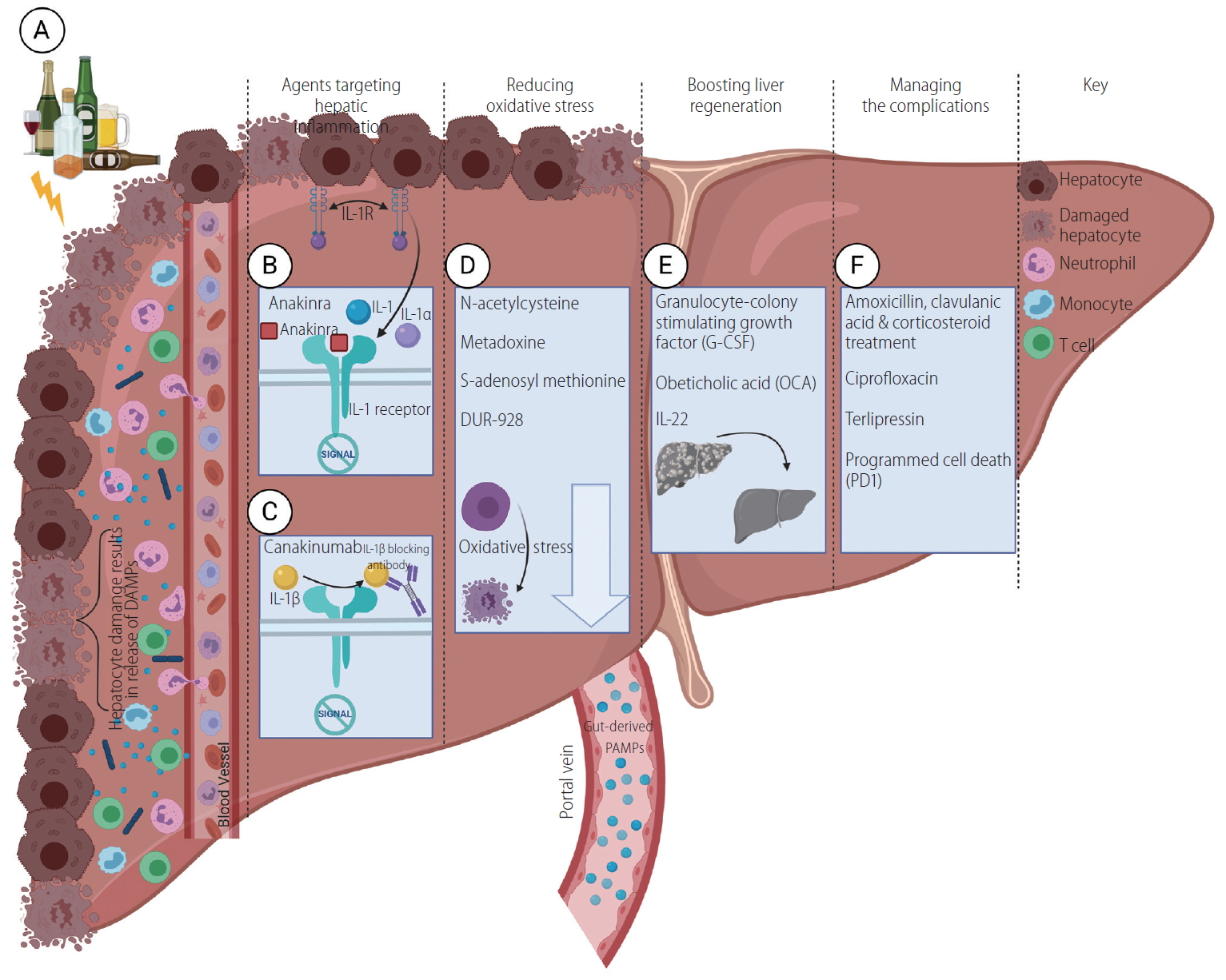
Inflammation plays a pivotal role in the pathogenesis of acute AH. Heavy alcohol and its metabolites damage hepatocytes and result in the release of danger-associated molecular patterns (DAMPs; Fig. 1A). In the gut, alcohol-induced dysbiosis and bacterial translocation lead to the accumulation of pathogen-associated molecular patterns (PAMPs). The combination of DAMPs and PAMPs results in TLR4 and NLRP3 inflammasome-mediated inflammatory responses in the liver [3-5].
Recently, our laboratory demonstrated that interleukin (IL)-1╬▓ is a central molecule in the pathogenesis of ALD and provided evidence that administration of IL-1 receptor antagonist (IL-1Ra) attenuates inflammasome-dependent alcoholic steatohepatitis in mice [6]. IL-1Ra competes with IL-1╬▒ and IL-1╬▓ to bind to the IL-1 receptor, but does not initiate downstream signaling pathways. Anakinra, a recombinant IL-1Ra, has been tested in the USA, a multicenter, double-blind, randomized clinical trial in severe (model for end stage liver disease [MELD] score Ōēź20) AH patients in combination with pentoxifylline and zinc-sulfate compared to prednisolone treatment (NCT01809132; Fig. 1B). A subsequent trial based on these preliminary results is already recruiting patients for a larger AH cohort (NCT04072822). Notably, related studies in patients with rheumatic arthritis suggest anti-IL-1╬▓ treatment with anakinra does not increase susceptibility to associated infections [7].
Treatment with canakinumab, a recombinant human monoclonal antibody against IL-1╬▓, utilizes the same concept, although specifically targets IL-1╬▓ and does not interfere with IL-1╬▒ signaling that acts on the same receptor (Fig. 1C) [8]. A multicenter, double-blind, randomized, placebo-controlled clinical trial with canakinumab is enrolling in the United Kingdom. This study in severe AH patients excludes individuals with MELD scores Ōēź27 and evaluates histological improvement after 28 days (NCT03775109). Finally, the previous ŌĆ£CANTOSŌĆØ (Canakinumab Anti-Inflammatory Thrombosis Outcomes Study) study involved 10,061 patients with a history of myocardial infarction and generally showed that canakinumab was effective in preventing cardiac events. However, the authors found higher infection rates in canakinumab treated individuals compared to the placebo group, raising a caution in its use [9,10].
Alcohol exposure results in several inflammatory processes including caspase-dependent activation of inflammasomes, apoptosis, and the release of extracellular vesicles with inflammatory properties [6,11,12]. Caspase inhibitors may decrease apoptosis and inflammation, and could therefore aid in the treatment of a variety of liver diseases. Emricasan is a pan-caspase inhibitor that appears to decrease aminotransferase activity and subsequently be beneficial to non-cirrhotic hepatitis C patients [13]. A phase 2, randomized, open-label clinical trial in cirrhotic patients showed that emricasan treatment resulted in significantly improved MELD and Child-Pugh scores, bilirubin levels, and international normalized ratios (INRs) after 3 months in patients with baseline MELD Ōēź15 (but not in the overall population) compared to placebo [14]. In contrast, emricasan showed no benefit in non-alcoholic steatohepatitis in a multicenter, randomized, double-blind, placebo-controlled clinical trial [15]. In AH patients, a placebo-controlled, multicenter, double-blind, randomized clinical trial of emricasan was terminated after recruiting five patients (NCT01912404) due to excessively high blood levels of the caspase inhibitor and lack of safe dosing in severe AH patients.
Alcohol metabolism, apoptosis and necrosis, inflammation and mitochondrial dysfunction are all part of AH pathology accompanied by the production of reactive oxygen species (ROS) that further increase cellular damage [16,17]. Protection against oxidative stress has been the focus of several therapeutic attempts (Fig. 1D). Increased production of ROS is associated with reduction in cellular glutathione levels in AH. N-acetylcysteine (NAC) is an antioxidant that can restore glutathione, the main protective agent against oxidative stress in hepatocytes and other cells, and protects the liver in acetaminophen-induced liver injury [18]. However, when administered alone, it showed no survival benefits in AH patients compared to placebo [19,20] or corticosteroids [21]. When NAC was used in conjunction with prednisolone in a France-based randomized, double-blind clinical trial, it increased one-month survival by reducing both infection and subsequent hepatorenal syndrome development although long-term benefits were not observed [22]. Another small, randomized open-label trial currently underway in the United Kingdom aims to clarify whether the early survival benefit of NAC is due to an improvement in the monocyte oxidative burst that leads to decreased infection rate (NCT03069300).
Metadoxine is another antioxidant drug that increases the level of glutathione and adenosine triphosphate levels in hepatocytes, reduces free fatty acid accumulation and inhibits tumor necrosis factor-╬▒ (TNF-╬▒) secretion [23]. In a small randomization-controlled clinical trial, metadoxine accelerated both alcohol elimination from the circulation and recovery from alcoholic detoxification [24]. Two Mexico-based randomization-controlled clinical trials of AH patients evaluated metadoxine or placebo paired with corticosteroid treatment. Notably, 30- and 90-day survival significantly improved with metadoxine treatment, and the development of encephalopathy and hepatorenal syndrome slowed in patients who received the combination therapy. Lower Lille scores in the metadoxine group were also observed, suggesting an overall better response to steroids. However, no difference was observed between groups in development or progression of variceal hemorrhage or infection [25]. A subsequent study with 4 arms (prednisolone and pentoxifylline with or without metadoxine) had similar conclusions including improvement of 3- and 6-month survival [26]. However, it is important to note that these were small studies with 35 or fewer patients; therefore results should be validated in larger, multicentered cohorts.
S-adenosyl methionine is a direct precursor of glutathione. Despite promising preclinical studies, large-scale studies in human ALD are still forthcoming [27]. Two randomization-controlled clinical trials in AH patients started in 2009 (NCT00851981) and 2013 (NCT02024295), and results have not yet been published.
Sulfated oxysterol (DUR-928) is a newly discovered, endogenous, small regulatory molecule that modulates various nuclear receptors that play an important regulatory role in lipid homeostasis, inflammation, cell survival and tissue regeneration. In mouse models of non-alcoholic steatohepatitis, DUR-928 administration reduced hepatic transcripts of TNF-╬▒ and monocyte chemoattractant protein-1, and led to decreased inflammation and fibrosis [28]. A phase-2, open-label, dose-escalation study was recently completed (NCT03432260), and demonstrated significant reductions of bilirubin, MELD and Lille scores [29]. Another study looking at the efficacy of DUR-928 was recently started in AH patients (NCT03917407).
The liver possesses high regenerative capacity. This highly complex process involves more than one hundred different genes, molecules and signaling pathways as well as several cell types, although involvement of certain cell types may depend on the nature of injury [30,31]. The complex process of liver regeneration involves interplay between oval cells, bone marrow-derived pluripotent cells as well as recruited mature immune cells like monocytes and macrophages in the liver with participation of a wide range of growth factors and cytokines (Fig. 1E) [30].
Granulocyte-colony stimulating growth factor (G-CSF) facilitates the mobilization of immune cells from bone marrow that may aid liver regeneration [32]. Several small clinical trials highlighted the importance of G-CSF in patients with cirrhosis and ACLF, and reported increased survival rates and liver-related scores, and decreased rates of infection [33-35]. A pilot clinical trial with AH patients showed similar benefits [36]. Furthermore, a single center, randomized, double-blind clinical trial in India investigated the efficacy of G-CSF treatment compared to placebo in steroid-nonresponsive AH patients. This study demonstrated improved survival rates, decreased infection rates with reduced MELD scores, and decreased MaddreyŌĆÖs discriminant function after 90 days in G-CSF treatment group [37]. Several other trials are currently underway exploring G-CSF (NCT02442180, NCT03703674, NCT04066179).
IL-22 is a pluripotent cytokine and member of the IL-10 family. It is produced by immunocytes, but affects only parenchymal cells. Through the Signal Transducer and Activator of Transcription 3 pathway, it displays anti-apoptotic, anti-oxidative, anti-lipogenic and proliferative effects in hepatocytes, and promotes the production of antimicrobial proteins [38]. A small, phase 2, open-label clinical trial was recently completed in the USA including 24 AH patients (MELD score, 11ŌĆō28) to test F652, a recombinant fusion protein of IL-22 and human immunoglobulin G2. The primary aim of this study was to assess safety with a dose-escalation procedure and evaluate the clinical response (NCT02655510). While the results have not yet been published, a larger randomized placebocontrolled clinical trial has been proposed (NCT01918462).
Bile acids are the physiologic ligands of farnesoid X receptor (FXR), a nuclear hormone receptor and master regulator of bile acid homeostasis. Obeticholic acid (OCA) is a selective FXR agonist that is 100-fold more potent than the endogenous ligands of the receptor [39]. By regulating bile acid synthesis and transport, FXR activation decreases cholestasis and toxic bile acid accumulation [40]. Through CYP2E1 and endothelial nitric oxide synthase, it reduces oxidative stress and portal hypertension, respectively [41,42]. OCA also modulates carbohydrate and lipid metabolism and plays a role in liver regeneration after injury. The hepatoprotective and antifibrotic effects of FXR agonist therapy were recently demonstrated in rodent models [41,43,44]. It is worth noting that in 2018 the Food and Drug Administration issued a ŌĆ£black box warningŌĆØ instructing patients with Child-Pugh class B/C stadium or with prior hepatic decompensation to take OCA weekly instead of daily, since there have been reported safety issues with higher dosages in more severe patients [45]. Indeed, a phase 2, placebo-controlled, randomized clinical trial was terminated after recruiting 19 moderate AH patients (MELD score, 12ŌĆō19) (NCT02039219).
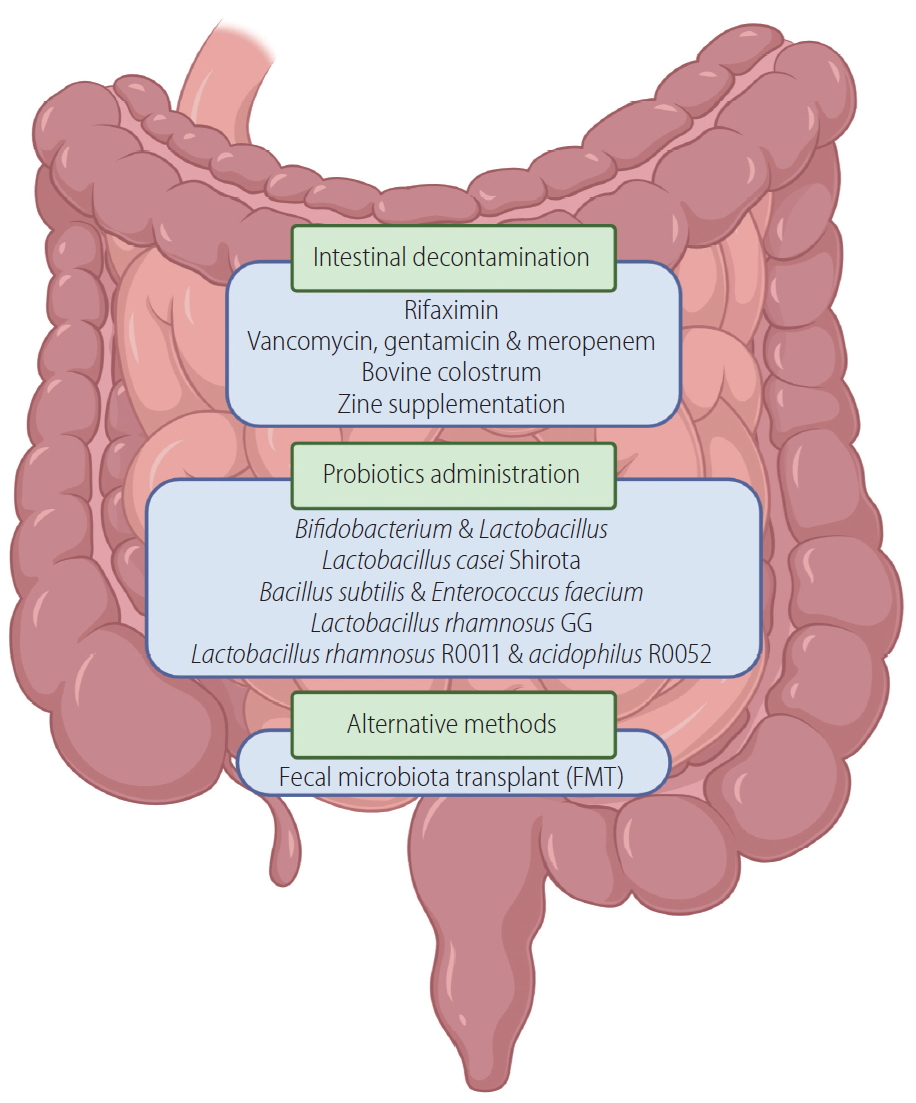
Excessive alcohol consumption leads to bacterial overgrowth and changes in the composition of the intestinal microbiome in the small and large intestine [46,47]. The impaired gut permeability results in bacterial translocation and increased levels of bacteria and bacterial products in systemic circulation [48]. Several researchers have demonstrated that susceptibility to alcohol-induced liver injury was transferrable from AH patients to mice with intestinal microbiome transplants [49]. Hence, intestinal microbiota regulation appears critical in the pathogenesis of AH.
Recently, several researchers have tried to reverse these processes via intestinal decontamination with antibiotics (Fig. 2). Rifaximin is effective against a broad spectrum of bacteria and absorbs poorly from the gut, which ensures that it acts locally [50]. A randomization-controlled, open-label trial from Denmark that included 30 AH patients found improved levels of endotoxin, cytokine, cell activation markers and metabolites as measured periodically during rifaximin administration with 90 days of follow-up (EudraCT 2014-002264-33). The status of two currently active studies based in Spain and South Korea are unknown (NCT02116556, NCT02485106).
Vancomycin, Gentamicin, and Meropenem combinatory therapy were used in another completed open-label trial in Denmark. Notably, these drugs have poor absorption when taken orally. The study involved 15 AH patients and compared them to a historical AH cohort. The endpoints were macrophage activation measured by sCD163 as well as endotoxin and cytokine levels (NCT03157388).
A different approach to reduce bacterial translocation driven inflammation is the administration of bovine colostrum, which is abundant in different immunoglobulins and contains immunoglobulin G antibodies against lipopolysaccharide (LPS). Bovine colostrum was found effective in reducing translocation of bacterial products and subsequent inflammatory responses in rat models [51,52]. Two clinical trials are currently investigating its usefulness in the treatment of AH. In the USA, a multicenter, double-blind, randomized clinical trial is focusing on IMM-124E, a purified hyper immune bovine colostrum versus placebo in addition to standard of care (i.e., corticosteroid) in the treatment of severe AH patients (MELD score, 20ŌĆō28) (NCT01968382). The primary outcome is a reduction of endotoxin levels in patientsŌĆÖ circulation. Another trial from India will evaluate 90-day survival of severe AH patients (MELD score, Ōēź21) treated with bovine colostrum versus placebo (NCT02473341).
An alternative attempt to protect the intestinal barrier is zinc supplementation. Zinc is an essential element in the human body, and ~10% of our genome codes proteins with zinc binding capacity. Reduced serum zinc levels are associated with chronic alcohol use. Zinc regulates tight junctions between intestinal epithelial cells and controls bacterial translocation [53]. Since malnutrition is common in individuals with alcohol-related disorders, zinc supplementation has been hypothesized to ameliorate leaky gut in these patients. The combination therapy involving zinc-sulfate (with anakinra and pentoxifylline) was discussed above.
Other approaches targeting the microbiome utilize probiotic administration. A study with mice receiving 8 weeks of alcohol feeding demonstrated that Lactobacillus rhamnosus GG administered in the last 2 weeks of the experiment significantly reduced alcohol-induced hepatic inflammation and injury by attenuation of toll-like receptor (TLR) activation and TNF-╬▒ production [54]. In an open-label, randomized, controlled pilot study with mild AH patients, researchers demonstrated that administration of probiotics rich in Bifidobacterium and Lactobacillus for 5 days increased levels of these beneficial bacteria in patientsŌĆÖ feces. Liver-associated enzyme levels were also improved [55].
In another open-label randomized trial in patients with compensated alcoholic cirrhosis, the administration of Lactobacillus casei Shirota for 1 month improved neutrophilic phagocytic function and reduced TLR expression compared to baseline and non-treated patients, although soluble TNF-receptor and IL-10 levels remained unchanged [56].
In a multicenter, randomized, controlled clinical trial, 117 patients with AH received Bacillus subtilis and Enterococcus faecium containing probiotic treatment or placebo for 7 days. Escherichia coli quantity in stool and endotoxemia were reduced while liver function improved [57].
Two ongoing randomized clinical trials are investigating the impact of probiotics on AH patients. One, based in the USA, is a phase 2 study examining the efficacy and safety of Lactobacillus rhamnosus GG and includes 130 moderate AH patients (MELD scores, 11ŌĆō20). The primary endpoint is change in MELD scores after 30 days (NCT01922895). The other study, based in South Korea, evaluates the effect of Lactobacillus rhamnosus R0011 and acidophilus R0052 administration on liver enzymes, endotoxin and cytokine levels after 7 days of treatment in 140 AH patients (NCT02335632).
Fecal microbiota transplant (FMT) may also provide a solution to restore healthy intestinal flora. In an open-label pilot study with eight male, steroid-resistant severe AH patients, fecal transplant from healthy donors demonstrated increased survival, reduced pathogen levels, and increased levels of beneficial bacterial strains 1 year after treatment [58]. These outcomes were compared to a historical cohort of 18 AH patients who received standard of care. Another open-label clinical trial compared 90-day survival in corticosteroids, pentoxifylline, nutritional therapy or FMT-treated male AH patients. They found 38%, 30%, 29%, and 75%, respectively significantly better survival in the FMT treatment group and favorable changes in the composition of intestinal microbiota with improved functionality [59]. Another small open-label trial in 2016 also investigated the efficacy of FMT versus pentoxifylline in steroid-ineligible severe AH patients by reversing dysbiosis (NCT02458079). Two other open-label trials are enrolling in India to evaluate overall survival and improvement in liver-related scores at 3 months in FMT versus steroid-treated or nutrition-supplemented severe AH patients (NCT03091010, NCT03827772).
Duan et al. [60] recently identified cytolysin-secreting Enterococcus faecalis (E. faecalis) strains as critical factors contributing to the mortality of severe AH patients through exacerbated hepatocyte injury and death. The authors found that patients with AH have highly increased E. faecalis levels in their stool compared to non-alcoholic individuals or alcohol-use disorder patients. Interestingly, the total number of E. faecalis seems critical to the severity of liver disease and subsequent mortality rather than the mere presence of cytolysin-positive strains. By using humanized mice colonized with bacteria from feces of AH patients, the researchers demonstrated that certain bacteriophages can specifically target cytolytic E. faecalis and decrease cytolysin in the liver and abolish ethanolinduced liver disease. Importantly, this approach provides a method for precisely editing the intestinal microbiota [60]. A clinical trial with a larger cohort would be required to validate these findings.
End-stage liver disease patients, particularly those with severe AH, are characterized by increased immune activity and inflammation that manifest as exhaustion of the immune system and decreased ability to fight against bacterial infections [61]. Indeed, AH patients are highly susceptible to bacterial infections, which ultimately culminate in organ failure and death [62]. Therefore, management of these complications and the underlying processes is a high priority of research (Fig. 1F).
A large, phase 3, multicenter, double-blind, randomized, controlled clinical trial in France is currently evaluating the benefit of amoxicillin and clavulanic acid in addition to corticosteroids in patients with severe AH (MELD score, Ōēź21). The primary endpoint of this study is mortality at 2 months. Secondary endpoints are reduction of infection, hepatorenal syndrome and ratio of patients with MELD score <17 (NCT02281929).
Ciprofloxacin is another antibiotic that is under investigation as an adjuvant of steroid therapy. A multicenter, randomized, open-label clinical trial was recently completed in Finland with a goal of improving survival at 1-, 3- and 6-months after diagnosis (NCT02326103).
Terlipressin, a vasopressin analogue that can reduce vasodilatation in the splanchnic area, triggers compensatory mechanisms leading to acute kidney injury and hepatorenal syndrome. Encouraging results were recently published of patients with type I hepatorenal syndrome [63-65]. Unfortunately, the trial that was designed to investigate the benefits of this treatment in severe AH patients was prematurely ceased due to difficulties with patient recruitment and the retirement of the associated primary investigator (EudraCT 2006-002837-19).
Recently, immune checkpoint receptors have been gaining attention as potential targets for restoring healthy immune responses. These inhibitory receptors regulate the balance between protective immunity and host immune-mediated damage. In an ex vivo study, Markwick et al. [66] found that programmed cell death 1 (PD-1) and T cell immunoglobulin and mucin domain-containing protein 3 (TIM-3) expression were significantly increased in T cells from AH patients compared to healthy controls and patients with stable alcoholic cirrhosis. AH patients had fewer interferon-╬│ and more IL-10 producing T cells when stimulated with LPS compared to T cells of other groups. Blockade of these receptors with inhibitory antibodies restored normal cytokine production of T cells, and increased neutrophil antimicrobial activities. The researchers hypothesized that reactive immunosuppression rather than the initial pro-inflammatory cytokine storm might be the driving force for morbidity and mortality in AH patients, as it makes them particularly susceptible to septic episodes [66]. The clinical relevance of these results requires in vivo validation.
Extracorporeal liver assist device (ELAD) is an extracorporeal cellular therapy utilizing hepatoblastoma-derived C3A cells that express anti-inflammatory proteins and growth factors to support the liver. ELAD was examined in a randomized open-label trial including 203 patients who received standard of care with or without ELAD. The study did not show any survival benefits in the entire severe AH population with MELD scores ranging from 18ŌĆō35. However, in a sub-group analysis of patients with MELD <28, ELAD was associated with a non-significant trend (P=0.08) of higher overall survival at 3 months. The authors identified elevated INR and creatinine as predictors of a negative response to this therapy. Therefore, a new clinical trial was initiated to study the potential benefit of ELAD in subjects with less severe renal function and coagulation abnormalities [67]. However, this study failed its primary endpoint (NCT02612428), and all trials were terminated.
Severe AH is increasingly recognized as an acute inflammatory syndrome with complex pathology that contributes to the complex clinical picture and often life-threatening severity of disease. At the time of this review, treatment options for AH patients are still limited to supportive care, corticosteroids, or in limited cases, liver transplantation. Numerous preclinical studies and early phase clinical trials are underway to explore new therapeutic modalities for AH.
In addition to studies discussed in this review, early data promises approaches to inhibit inflammasome activation by decreasing uric acid levels [68], inhibition or stimulation of harmful or protective miRNAs, respectively [69-71], blockade of triggering receptors expressed on myeloid cells-1 [72] or C-C chemokine receptors type 2 and 5 [73].
The recent enthusiasm of AH researchers involved in both translational and clinical studies and our understanding of the disease pathology is expanding and supporting optimism for new pharmacological therapies of severe AH in the near future.
ACKNOWLEDGMENTS
The authors greatly appreciate the support of Olivia Potvin with the critical review and preparation of the manuscript. Figures were created with BioRender.com.
This review was supported by NIH/NIAAA grants U01AA026977, U01AA026933 and R01AA011576 to GS. The funders had no role in the preparation of this review.
FOOTNOTES
Conflicts of Interest
Dr. Szabo consults for Allergan, Alnylam, Arrow, Durect Corporation, Generon, Glympse Bio, Terra Firma, Quest Diagnostics, Pandion Therapeutics, Surrozen, and Zomagen. She has received grants from Gilead, Genfit, Intercept, Novartis, SignaBlok, and Shire. She holds intellectual property rights with UpToDate. The co-author declares no conflict of interest.
Figure┬Ā1.
Alcoholic hepatitis: a disease without an efficient cure. (A) Agents targeting hepatic inflammation. (B) Anakinra, a recombinant form of IL-1Ra, is currently under investigation as a therapeutic agent for alcoholic hepatitis patients, as is (C) canakinumab, a recombinant human monoclonal antibody against IL-1╬▓. (D) Protection against oxidative stress has been the target of several therapeutic attempts. (E) Boosting liver regeneration may be a key element of therapeutic interventions. (F) Therapeutic agents aimed at managing the complications of end stage liver disease patients; specifically targeting increased immune activity and inflammation that manifest as exhaustion of the immune system and decreased ability to fight against bacterial infections. IL, interleukin.
Figure┬Ā2.
Targeting the gut-liver axis. Therapeutic agents aimed at reducing intestinal inflammation-related damage and gut permeability.
Table┬Ā1.
Failed therapeutic interventions in studies for alcoholic hepatitis
Abbreviations
ACLF
acute-on-chronic liver failure
AH
alcoholic hepatitis
ALD
alcohol-related liver disease
DAMPs
danger-associated molecular patterns
DUR-928
sulfated oxysterol
ELAD
extracorporeal liver assist device
E. faecalis
Enterococcus faecalis
FMT
fecal microbiota transplant
FXR
farnesoid X receptor
G-CSF
granulocyte-colony stimulating growth factor
IL-1Ra
interleukin-1 receptor antagonist
IL
interleukin
INR
international normalized ratio
LPS
lipopolysaccharide
MELD
model for end stage liver disease
NAC
N-acetylcysteine
OCA
obeticholic acid
PAMPs
pathogen-associated molecular patterns
PD-1
programmed cell death 1
ROS
reactive oxygen species
TIM-3
T-cell immunoglobulin and mucin domain-containing protein 3
TLR
toll-like receptor
TNF-╬▒
tumor necrosis factor-╬▒
REFERENCES
1. Szabo G, Kamath PS, Shah VH, Thursz M, Mathurin P; EASL-AASLD Joint Meeting. Alcohol-related liver disease: areas of consensus, unmet needs and opportunities for further study. Hepatology 2019;69:2271-2283.


4. Szabo G, Petrasek J. Gut-liver axis and sterile signals in the development of alcoholic liver disease. Alcohol Alcohol 2017;52:414-424.




5. Saha B, Tornai D, Kodys K, Adejumo A, Lowe P, McClain C, et al. Biomarkers of macrophage activation and immune danger signals predict clinical outcomes in alcoholic hepatitis. Hepatology 2019;70:1134-1149.



6. Petrasek J, Bala S, Csak T, Lippai D, Kodys K, Menashy V, et al. IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice. J Clin Invest 2012;122:3476-3489.



7. Bresnihan B, Alvaro-Gracia JM, Cobby M, Doherty M, Domljan Z, Emery P, et al. Treatment of rheumatoid arthritis with recombinant human interleukin-1 receptor antagonist. Arthritis Rheum 1998;41:2196-2204.


8. Alten R, Gram H, Joosten LA, van den Berg WB, Sieper J, Wassenberg S, et al. The human anti-IL-1 beta monoclonal antibody ACZ885 is effective in joint inflammation models in mice and in a proof-of-concept study in patients with rheumatoid arthritis. Arthritis Res Ther 2008;10:R67.



9. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017;377:1119-1131.


10. Lachmann HJ, Kone-Paut I, Kuemmerle-Deschner JB, Leslie KS, Hachulla E, Quartier P, et al. Use of canakinumab in the cryopyrin-associated periodic syndrome. N Engl J Med 2009;360:2416-2425.


12. Verma VK, Li H, Wang R, Hirsova P, Mushref M, Liu Y, et al. Alcohol stimulates macrophage activation through caspase-dependent hepatocyte derived release of CD40L containing extracellular vesicles. J Hepatol 2016;64:651-660.



13. Pockros PJ, Schiff ER, Shiffman ML, McHutchison JG, Gish RG, Afdhal NH, et al. Oral IDN-6556, an antiapoptotic caspase inhibitor, may lower aminotransferase activity in patients with chronic hepatitis C. Hepatology 2007;46:324-329.


14. Frenette CT, Morelli G, Shiffman ML, Frederick RT, Rubin RA, Fallon MB, et al. Emricasan improves liver function in patients with cirrhosis and high model for end-stage liver disease scores compared with placebo. Clin Gastroenterol Hepatol 2019;17:774-783 e4.


15. Harrison SA, Goodman Z, Jabbar A, Vemulapalli R, Younes ZH, Freilich B, et al. A randomized, placebo-controlled trial of emricasan in patients with NASH and F1-F3 fibrosis. J Hepatol 2020;72:816-827.


16. Wu D, Cederbaum AI. Alcohol, oxidative stress, and free radical damage. Alcohol Res Health 2003;27:277-284.


18. Smilkstein MJ, Knapp GL, Kulig KW, Rumack BH. Efficacy of oral N-acetylcysteine in the treatment of acetaminophen overdose. Analysis of the national multicenter study (1976 to 1985). N Engl J Med 1988;319:1557-1562.


19. Moreno C, Langlet P, Hittelet A, Lasser L, Degr├® D, Evrard S, et al. Enteral nutrition with or without N-acetylcysteine in the treatment of severe acute alcoholic hepatitis: a randomized multicenter controlled trial. J Hepatol 2010;53:1117-1122.


20. Stewart S, Prince M, Bassendine M, Hudson M, James O, Jones D, et al. A randomized trial of antioxidant therapy alone or with corticosteroids in acute alcoholic hepatitis. J Hepatol 2007;47:277-283.


21. Phillips M, Curtis H, Portmann B, Donaldson N, Bomford A, OŌĆÖGrady J. Antioxidants versus corticosteroids in the treatment of severe alcoholic hepatitis--a randomised clinical trial. J Hepatol 2006;44:784-790.


22. Nguyen-Khac E, Thevenot T, Piquet MA, Benferhat S, Goria O, Chatelain D, et al. Glucocorticoids plus N-acetylcysteine in severe alcoholic hepatitis. N Engl J Med 2011;365:1781-1789.


23. Addolorato G, Ancona C, Capristo E, Gasbarrini G. Metadoxine in the treatment of acute and chronic alcoholism: a review. Int J Immunopathol Pharmacol 2003;16:207-214.


24. Shpilenya LS, Muzychenko AP, Gasbarrini G, Addolorato G. Metadoxine in acute alcohol intoxication: a double-blind, randomized, placebo-controlled study. Alcohol Clin Exp Res 2002;26:340-346.


25. Higuera-de la Tijera F, Serv├Łn-Caama├▒o AI, Cruz-Herrera J, SerraldeZ├║├▒iga AE, Abdo-Francis JM, Guti├®rrez-Reyes G, et al. Treatment with metadoxine and its impact on early mortality in patients with severe alcoholic hepatitis. Ann Hepatol 2014;13:343-352.


26. Higuera-de la Tijera F, Serv├Łn-Caama├▒o AI, Serralde-Z├║├▒iga AE, Cruz-Herrera J, P├®rez-Torres E, Abdo-Francis JM, et al. Metadoxine improves the three- and six-month survival rates in patients with severe alcoholic hepatitis. World J Gastroenterol 2015;21:4975-4985.



27. Anstee QM, Day CP. S-adenosylmethionine (SAMe) therapy in liver disease: a review of current evidence and clinical utility. J Hepatol 2012;57:1097-1109.


28. Kim MJ, Lin WQ. DUR-928, an endogenous regulatory molecule, exhibits anti-inflammatory and antifibrotic activity in a mouse model of NASH. Emerging Trends Conference: Emerging Trends in Non alcoholic Fatty Liver Disease. 2017.
29. Hassanein T, Stein LL, Flamm SL, Martin P, Cave MC, Blevins C, et al. Safety and efficacy of DUR-928: a potential new therapy for acute alcoholic hepatitis. Late-breaking oral presentation at AASLDŌĆÖs The Liver Meeting. 2019.
31. Lin S, Nascimento EM, Gajera CR, Chen L, Neuh├Čfer P, Garbuzov A, et al. Distributed hepatocytes expressing telomerase repopulate the liver in homeostasis and injury. Nature 2018;556:244-248.




32. Piscaglia AC, Shupe TD, Oh SH, Gasbarrini A, Petersen BE. Granulocyte-colony stimulating factor promotes liver repair and induces oval cell migration and proliferation in rats. Gastroenterology 2007;133:619-631.



33. Spahr L, Lambert JF, Rubbia-Brandt L, Chalandon Y, Frossard JL, Giostra E, et al. Granulocyte-colony stimulating factor induces proliferation of hepatic progenitors in alcoholic steatohepatitis: a randomized trial. Hepatology 2008;48:221-229.


34. Garg V, Garg H, Khan A, Trehanpati N, Kumar A, Sharma BC, et al. Granulocyte colony-stimulating factor mobilizes CD34(+) cells and improves survival of patients with acute-on-chronic liver failure. Gastroenterology 2012;142:505-512 e1.


35. Kedarisetty CK, Anand L, Bhardwaj A, Bhadoria AS, Kumar G, Vyas AK, et al. Combination of granulocyte colony-stimulating factor and erythropoietin improves outcomes of patients with decompensated cirrhosis. Gastroenterology 2015;148:1362-1370 e7.


36. Singh V, Sharma AK, Narasimhan RL, Bhalla A, Sharma N, Sharma R. Granulocyte colony-stimulating factor in severe alcoholic hepatitis: a randomized pilot study. Am J Gastroenterol 2014;109:1417-1423.



37. Shasthry SM, Sharma MK, Shasthry V, Pande A, Sarin SK. Efficacy of granulocyte colony-stimulating factor in the management of steroidnonresponsive severe alcoholic hepatitis: a double-blind randomized controlled trial. Hepatology 2019;70:802-811.


38. Khawar MB, Azam F, Sheikh N, Abdul Mujeeb K. How does interleukin-22 mediate liver regeneration and prevent injury and fibrosis? J Immunol Res 2016;2016:2148129.




39. Pellicciari R, Fiorucci S, Camaioni E, Clerici C, Costantino G, Maloney PR, et al. 6alpha-ethyl-chenodeoxycholic acid (6-ECDCA), a potent and selective FXR agonist endowed with anticholestatic activity. J Med Chem 2002;45:3569-3572.


40. Xu JY, Li ZP, Zhang L, Ji G. Recent insights into farnesoid X receptor in non-alcoholic fatty liver disease. World J Gastroenterol 2014;20:13493-13500.



41. Wu W, Zhu B, Peng X, Zhou M, Jia D, Gu J. Activation of farnesoid X receptor attenuates hepatic injury in a murine model of alcoholic liver disease. Biochem Biophys Res Commun 2014;443:68-73.


42. Verbeke L, Farre R, Trebicka J, Komuta M, Roskams T, Klein S, et al. Obeticholic acid, a farnesoid X receptor agonist, improves portal hypertension by two distinct pathways in cirrhotic rats. Hepatology 2014;59:2286-2298.


43. Fiorucci S, Antonelli E, Rizzo G, Renga B, Mencarelli A, Riccardi L, et al. The nuclear receptor SHP mediates inhibition of hepatic stellate cells by FXR and protects against liver fibrosis. Gastroenterology 2004;127:1497-1512.


44. Fiorucci S, Rizzo G, Antonelli E, Renga B, Mencarelli A, Riccardi L, et al. Cross-talk between farnesoid-X-receptor (FXR) and peroxisome proliferator-activated receptor gamma contributes to the antifibrotic activity of FXR ligands in rodent models of liver cirrhosis. J Pharmacol Exp Ther 2005;315:58-68.


45. Peeraphatdit TB, Simonetto DA, Shah VH. Exploring new treatment paradigms for alcoholic hepatitis by extrapolating from NASH and cholestasis. J Hepatol 2018;69:275-277.



46. Bode JC, Bode C, Heidelbach R, D├╝rr HK, Martini GA. Jejunal microflora in patients with chronic alcohol abuse. Hepatogastroenterology 1984;31:30-34.

47. Yan AW, Fouts DE, Brandl J, St├żrkel P, Torralba M, Schott E, et al. Enteric dysbiosis associated with a mouse model of alcoholic liver disease. Hepatology 2011;53:96-105.



48. Yan AW, Schnabl B. Bacterial translocation and changes in the intestinal microbiome associated with alcoholic liver disease. World J Hepatol 2012;4:110-118.



49. Llopis M, Cassard AM, Wrzosek L, Boschat L, Bruneau A, Ferrere G, et al. Intestinal microbiota contributes to individual susceptibility to alcoholic liver disease. Gut 2016;65:830-839.


50. Flamm SL. Rifaximin treatment for reduction of risk of overt hepatic encephalopathy recurrence. Therap Adv Gastroenterol 2011;4:199-206.



51. D├Čhler JR, Nebermann L. Bovine colostrum in oral treatment of enterogenic endotoxaemia in rats. Crit Care 2002;6:536-539.



52. Choi HS, Jung KH, Lee SC, Yim SV, Chung JH, Kim YW, et al. Bovine colostrum prevents bacterial translocation in an intestinal ischemia/reperfusion-injured rat model. J Med Food 2009;12:37-46.


53. Ohashi W, Fukada T. Contribution of zinc and zinc transporters in the pathogenesis of inflammatory bowel diseases. J Immunol Res 2019;2019:8396878.




54. Wang Y, Liu Y, Kirpich I, Ma Z, Wang C, Zhang M, et al. Lactobacillus rhamnosus GG reduces hepatic TNF╬▒ production and inflammation in chronic alcohol-induced liver injury. J Nutr Biochem 2013;24:1609-1615.



55. Kirpich IA, Solovieva NV, Leikhter SN, Shidakova NA, Lebedeva OV, Sidorov PI, et al. Probiotics restore bowel flora and improve liver enzymes in human alcohol-induced liver injury: a pilot study. Alcohol 2008;42:675-682.



56. Stadlbauer V, Mookerjee RP, Hodges S, Wright GA, Davies NA, Jalan R. Effect of probiotic treatment on deranged neutrophil function and cytokine responses in patients with compensated alcoholic cirrhosis. J Hepatol 2008;48:945-951.


57. Han SH, Suk KT, Kim DJ, Kim MY, Baik SK, Kim YD, et al. Effects of probiotics (cultured Lactobacillus subtilis/Streptococcus faecium) in the treatment of alcoholic hepatitis: randomized-controlled multicenter study. Eur J Gastroenterol Hepatol 2015;27:1300-1306.


58. Philips CA, Pande A, Shasthry SM, Jamwal KD, Khillan V, Chandel SS, et al. Healthy donor fecal microbiota transplantation in steroid-ineligible severe alcoholic hepatitis: a pilot study. Clin Gastroenterol Hepatol 2017;15:600-602.


59. Philips CA, Phadke N, Ganesan K, Ranade S, Augustine P. Corticosteroids, nutrition, pentoxifylline, or fecal microbiota transplantation for severe alcoholic hepatitis. Indian J Gastroenterol 2018;37:215-225.



60. Duan Y, Llorente C, Lang S, Brandl K, Chu H, Jiang L, et al. Bacteriophage targeting of gut bacterium attenuates alcoholic liver disease. Nature 2019;575:505-511.




61. Sipeki N, Antal-Szalmas P, Lakatos PL, Papp M. Immune dysfunction in cirrhosis. World J Gastroenterol 2014;20:2564-2577.



62. Louvet A, Wartel F, Castel H, Dharancy S, Hollebecque A, CanvaDelcambre V, et al. Infection in patients with severe alcoholic hepatitis treated with steroids: early response to therapy is the key factor. Gastroenterology 2009;137:541-548.


63. Sanyal AJ, Boyer T, Garcia-Tsao G, Regenstein F, Rossaro L, Appenrodt B, et al. A randomized, prospective, double-blind, placebocontrolled trial of terlipressin for type 1 hepatorenal syndrome. Gastroenterology 2008;134:1360-1368.



64. Mart├Łn-Llah├Ł M, P├®pin MN, Guevara M, D├Łaz F, Torre A, Monescillo A, et al. Terlipressin and albumin vs albumin in patients with cirrhosis and hepatorenal syndrome: a randomized study. Gastroenterology 2008;134:1352-1359.


65. Neri S, Pulvirenti D, Malaguarnera M, Cosimo BM, Bertino G, Ignaccolo L, et al. Terlipressin and albumin in patients with cirrhosis and type I hepatorenal syndrome. Dig Dis Sci 2008;53:830-835.



66. Markwick LJ, Riva A, Ryan JM, Cooksley H, Palma E, Tranah TH, et al. Blockade of PD1 and TIM3 restores innate and adaptive immunity in patients with acute alcoholic hepatitis. Gastroenterology 2015;148:590-602.


67. Thompson J, Jones N, Al-Khafaji A, Malik S, Reich D, Munoz S, et al. Extracorporeal cellular therapy (ELAD) in severe alcoholic hepatitis: a multinational, prospective, controlled, randomized trial. Liver Transpl 2018;24:380-393.



68. Iracheta-Vellve A, Petrasek J, Satishchandran A, Gyongyosi B, Saha B, Kodys K, et al. Inhibition of sterile danger signals, uric acid and ATP, prevents inflammasome activation and protects from alcoholic steatohepatitis in mice. J Hepatol 2015;63:1147-1155.



69. Bala S, Petrasek J, Mundkur S, Catalano D, Levin I, Ward J, et al. Circulating microRNAs in exosomes indicate hepatocyte injury and inflammation in alcoholic, drug-induced, and inflammatory liver diseases. Hepatology 2012;56:1946-1957.



70. Lippai D, Bala S, Catalano D, Kodys K, Szabo G. Micro-RNA-155 deficiency prevents alcohol-induced serum endotoxin increase and small bowel inflammation in mice. Alcohol Clin Exp Res 2014;38:2217-2224.



71. Babuta M, Furi I, Bala S, Bukong TN, Lowe P, Catalano D, et al. Dysregulated autophagy and lysosome function are linked to exosome production by micro-RNA 155 in alcoholic liver disease. Hepatology 2019;70:2123-2141.



72. Tornai D, Furi I, Shen ZT, Sigalov AB, Coban S, Szabo G. Inhibition of triggering receptor expressed on myeloid cells 1 ameliorates inflammation and macrophage and neutrophil activation in alcoholic liver disease in mice. Hepatol Commun 2018;3:99-115.



73. Ambade A, Lowe P, Kodys K, Catalano D, Gyongyosi B, Cho Y, et al. Pharmacological inhibition of CCR2/5 signaling prevents and reverses alcohol-induced liver damage, steatosis, and inflammation in mice. Hepatology 2019;69:1105-1121.



74. Thursz MR, Forrest EH, Ryder S; STOPAH investigators. Prednisolone or pentoxifylline for alcoholic hepatitis. N Engl J Med 2015;373:282-283.


75. Naveau S, Chollet-Martin S, Dharancy S, Mathurin P, Jouet P, Piquet MA, et al. A double-blind randomized controlled trial of infliximab associated with prednisolone in acute alcoholic hepatitis. Hepatology 2004;39:1390-1397.


76. Boetticher NC, Peine CJ, Kwo P, Abrams GA, Patel T, Aqel B, et al. A randomized, double-blinded, placebo-controlled multicenter trial of etanercept in the treatment of alcoholic hepatitis. Gastroenterology 2008;135:1953-1960.



77. Trinchet JC, Beaugrand M, Callard P, Hartmann DJ, Gotheil C, Nusgens BV, et al. Treatment of alcoholic hepatitis with colchicine. Results of a randomized double blind trial. Gastroenterol Clin Biol 1989;13:551-555.

78. Morgan TR, Weiss DG, Nemchausky B, Schiff ER, Anand B, Simon F, et al. Colchicine treatment of alcoholic cirrhosis: a randomized, placebo-controlled clinical trial of patient survival. Gastroenterology 2005;128:882-890.


79. Bird GL, Prach AT, McMahon AD, Forrest JA, Mills PR, Danesh BJ. Randomised controlled double-blind trial of the calcium channel antagonist amlodipine in the treatment of acute alcoholic hepatitis. J Hepatol 1998;28:194-198.


80. Bird G, Lau JY, Koskinas J, Wicks C, Williams R. Insulin and glucagon infusion in acute alcoholic hepatitis: a prospective randomized controlled trial. Hepatology 1991;14:1097-1101.


81. Trinchet JC, Balkau B, Poupon RE, Heintzmann F, Callard P, Gotheil C, et al. Treatment of severe alcoholic hepatitis by infusion of insulin and glucagon: a multicenter sequential trial. Hepatology 1992;15:76-81.


82. Fede G, Germani G, Gluud C, Gurusamy KS, Burroughs AK. Propylthiouracil for alcoholic liver disease. Cochrane Database Syst Rev 2011;2011:CD002800.



83. Oral abstracts (abstracts 1ŌĆō299). Hepatology 2018;68(Suppl 1):1-183.
- TOOLS
-
METRICS

- ORCID iDs
-
Gyongyi Szabo

https://orcid.org/0000-0003-0836-2527 - Related articles
-
Screening strategy for non-alcoholic fatty liver disease2023 February;29(Suppl)
KASL clinical practice guidelines for management of chronic hepatitis B2022 April;28(2)
Current and future strategies for the treatment of chronic hepatitis C2021 April;27(2)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print



