Predictors of steroid non-response and new approaches in severe alcoholic hepatitis
Article information
Abstract
Severe alcoholic hepatitis (SAH) remains a disease with high mortality. Steroid is the main stay and has been shown to give modest 28-day survival benefit in carefully selected patients, but no 90-day survival benefit. Since non-responders have high incidence of infections and increased mortality, it would be worthwhile to identify them before starting steroid therapy. A high and rising bilirubin, urinary acetyl carnitine >2,500 ng/mL, high asiloglycoprotein positive microparticles, and specific features in liver biopsy could predict steroid non-response at baseline. There is an ever-growing need to find new and effective therapies for SAH patients. Besides aggressive nutrition, granulocyte colony stimulating factor, fecal microbiota transplantation, and plasma exchange appear promising therapies and provide a hope for steroid ineligible or steroid non-responsive patients. Suppression of hepatic inflammation, preventing new bacterial or fungal infections, and enhancing liver regeneration will remain the key targets for next generation therapies.
INTRODUCTION
The burden of alcohol-related liver diseases (ALD) is rapidly growing and is likely to increase in the coming years. With the availability of better treatment options for managing hepatitis B and C, alcoholic hepatitis (AH) related hospital admissions are adding substantial load on healthcare costs and utilization [1,2]. The spectrum of ALD varies from fatty liver, steatohepatitis, severe AH, alcoholic fibrosis, compensated/decompensated cirrhosis, to hepatocellular carcinoma. ALD now contribute to 47.9% of all liver cirrhosis-related deaths globally [3,4]. AH is the most florid manifestation of ALD with substantial morbidity, mortality, and financial burden.
AH is an acute form of alcohol-induced liver injury, ranges from mild to severe, and usually presents on the background of chronic liver disease. It is clinically defined as recent onset or worsening of jaundice in a patient with chronic heavy alcohol use until at least 6 weeks prior to presentation, elevated liver enzymes with an aspartate aminotransferase to alanine aminotransferase ratio of >1.5:1, with absolute values of these enzymes not exceeding 500 IU/L and exclusion of other liver diseases [5]. Severe AH (SAH) is often a progressive disease with a 28-day mortality of 20–50% [6,7], hence a definite diagnosis is helpful. For a definite diagnosis of AH, a histological evidence of the disease is required besides clinical and biochemical criteria, as 10–20% of AH patients may have other liver diseases.
Predominant histological lesions that characterize AH include the coexistence of steatosis, hepatocyte ballooning, and inflammatory infiltrates, mainly with neutrophils, called satellitosis [8]. Liver biopsy also helps in providing clues to the natural history and outcome of the disease [5]. Presence of megamitochondria, polymorphonuclear leucocytic infiltration, and absence of fibrosis is associated with good prognosis, whereas presence of ballooning hepatocytes and bilirubinostasis is suggestive of poor prognosis. However, majority of the SAH patients have coagulopathy or ascites which are contraindications for percutaneous liver biopsy and necessitate transjugular route for obtaining liver tissue. However, due to invasive nature of the procedure and associated costs, often, the patients are started on treatment on the basis of ‘probable’ diagnosis of AH. This can pose challenges in some situations, especially in patients with obesity, diabetes, and associated drug-induced liver injury.
ASSESSMENT OF SEVERITY OF AH
An important part of therapy for any disease requires assessment of the severity of the disease and the appropriate time for initiation of the therapy. Assessing severity of AH can be done by using a combination of clinical and biochemical parameters. Maddrey’s discriminant function (MDF) [9] is one of the first bedside tool, which is still used across the world, and a score of >32 categorizes the disease as SAH. The MDF however, has not been validated for assessing the treatment response. Model for end-stage liver disease (MELD) score, commonly used for organ allocation for transplant, has also been used. A MELD score of >18 indicates SAH and mandates specific therapy. Alternates like Glasgow alcoholic hepatitis and sequential organ failure assessment (SOFA) scores are dependent on baseline clinical and biochemical features [10].
THERAPEUTIC OPTIONS FOR SEVERE AH
In mild or moderate AH, abstinence does help to some extent. For severe AH, specific and effective therapy is required to suppress the ongoing inflammation and hepatic necrosis. Unfortunately, there are very limited options [11]. Corticosteroids currently remain the first and probably the only accepted medical therapy for SAH patients.
CORTICOSTEROIDS FOR AH
Since AH is considered as a severe form of inflammatory disease of the liver, prednisolone with its anti-inflammatory action, has been used for over 40 years for such patients [9]. Prednisolone however, provides only a modest short-term benefit. Lille score [12] is used to assess the efficacy of steroid therapy and is calculated (based on age, baseline creatinine, albumin, prothrombin time, total bilirubin and repeat total bilirubin) at day 7. Those with Lille score >0.45 indicate poor response to corticosteroids and under 25% survival rate at 6 months. In a meta-analysis [13] to predict treatment response to steroids, patients were classified as complete responders (Lille score ≤0.16), partial responders (Lille score 0.16–0.56), and null-responders (Lille score >0.56). Significant differences in survival (91% vs. 79% vs. 53%, P<0.0001, respectively) were observed. It is necessary to remember that steroids should be continued only in complete responders.
In a recent study of Shasthry et al. [6] have reported that of the 430 hospitalized patients with SAH, only 132 (26.8%) were eligible to receive steroids. Of these, 99 patients (75%) responded to steroids; making a total of 331 of 430 (77%) as steroid non-responder. It has been shown that only 1–2% of severe AH patients actually undergo the definitive therapy, the liver transplant [14]. This draws attention to the fact that nearly four out of five patients with SAH are deprived of an effective therapy.
Furthermore, infections are not uncommon after initiation of steroid therapy. Steroid non-response is associated with a high risk of infection and poor outcomes [15]. In a prospective study [16], around 25% of patients receiving steroids developed infections. Median MELD and MDF were higher in these patients compared to those who were non-infected on steroids, suggesting that sicker group of patients are not suitable for steroids.
There is an unmet need to treat these large cohort of patients with severe AH with newer therapeutic strategies. Furthermore, even for the steroid-eligible patients, it would be highly desirable, if predictors of non-response to steroids could be defined prior to initiation of steroid therapy.
IDENTIFICATION OF STEROID NON-RESPONDERS PRIOR TO THERAPY
If steroid non-responders can be identified prior to starting steroids, several adverse effects like development of new infections, gastrointestinal hemorrhage, hyperglycemia, etc. could be prevented.
Biochemical predictors
Persistently high and rising bilirubin is very suggestive of an unremitting inflammation, likely to be unresponsive to steroids.
Day 4 Lille score
Whether assessment of the same parameters which form the Lille score can reliably predict non-response at day 4 rather than day 7, was evaluated. The data showed that Lille score on day 4 was as good as day 7 to predict 3-month mortality and reduces unnecessary steroid exposure [17]. However, these results have not been validated by other workers. In our own large experience (unpublished data), day 4 is not a very reliable indicator of response to steroids, and legitimacy to continue therapy for 7 days.
Baseline liver biopsy
A pre-therapy liver biopsy helps in stratification of SAH patients not only to assess the severity of liver disease, but also to predict response to therapy. Liver biopsy showing ballooning hepatocytes and bilirubinostasis, indicates a poor response to steroids. In one study, non-responders had higher ballooning degeneration (BD) (mean, 3.87 [standard deviation (SD), -0.91] vs. 2.92 [SD, -1.33], P=0.013) with increased density of Mallory-Denk bodies (MD) (mean, 2.27 [SD, -0.79] vs. 1.69 [SD, -0.97], P=0.028). A score derived using BD and MD (range, 0–8) had high sensitivity and specificity (81.2% and 64%, respectively), and negative predictive value (91.4%) to identify non-responders to steroids at baseline if the score is >5 [18].
Metabolic markers for early detection of steroid response
Patients with SAH have a unique metabolic phenotype, one with severe inflammatory milieu and an impaired immune status. Whether baseline metabolic phenotype could identify non-responders and those with unfavorable outcome was investigated [3]. Baseline urine metabolome of SAH patients was subjected to ultra-high performance liquid chromatography and high-resolution mass spectrometry. Nine urinary metabolites linked to mitochondrial functions significantly discriminated non-responders, most importantly the increased acetyl-L-carnitine (12-fold) levels. A baseline urinary acetyl-L-carnitine level predicted non-response with a receiver operating characteristic of 0.96 (95% confidence interval [CI], 0.85–0.99) and a hazard ratio of 3.5 (95% CI, 1.5–8.3) for the prediction of mortality in patients with SAH. Acetyl-L-carnitine levels of >2,500 ng/mL reliably segregated survivors from non-survivors (P<0.01, log-rank test) in the study cohort [3]. This low cost test, if validated in other studies, could be of help in selection of patients for steroid therapy.
Specific microvesicle signatures
Microvesicles (MVs) reflect the cellular stress and the disease conditions. In one study, pre-therapy peripheral plasma MV levels of haematopoietic stem-cells (CD45+ CD34+; 116.8 vs. 13.4 MV/μL; P=0.0001) and hepatocytes (ASGPR+; 470 vs. 361 MV/μL; P=0.01) were higher in steroid non-responders, compared to responders (Fig. 1). In fact, the two together could predict steroid non-response in 94% patients at baseline in peripheral plasma. The basis for these observations lies in the fact that the MVs from SAH patients, trigger more (P=0.04) reactive oxygen species (ROS) generation, tumor necrosis factor-α production (P=0.04) and upregulate pro-inflammatory cytokine related genes in neutrophils in vitro [19]. The source of these MVs was also validated with higher levels in hepatic vein plasma of non-responders. The MVs from hematopoietic cells suggests that the bone marrow cells do migrate to liver, but probably, a fair proportion is unable to induce effective regeneration due to an unfavorable milieu and, hence possibly perish as assessed by high MV levels in hepatic vein in non-responders [19]. More such biomarkers are needed for proper patient selection for steroid therapy.
Baseline hepatic gene expression
Whether variation to steroid response in SAH patients has a genetic basis, is largely unknown. This was explored by investigating the hepatic transcriptome in patients originating in different countries [20]. Though at presentation the patients were similar, the responders and non-responders had distinct patterns of gene expression. There were >1,100 genes that were overexpressed (>2-fold, P<0.05) in non-responders in comparison to responders. Importantly, the glucocorticoid receptor (NR3C1) expressed a significantly reduced amount of the alpha isoform in non-responders. Additionally, in one population the BCL2 associated athanogene-1 (BAG1) was significantly increased in non-responders. BAG1 is known to cleave the glucocorticosteroid receptor (Fig. 2) [21]. This data shows BAG1 as a promising candidate to be explored in a larger population to segregate steroid non-responders at baseline.
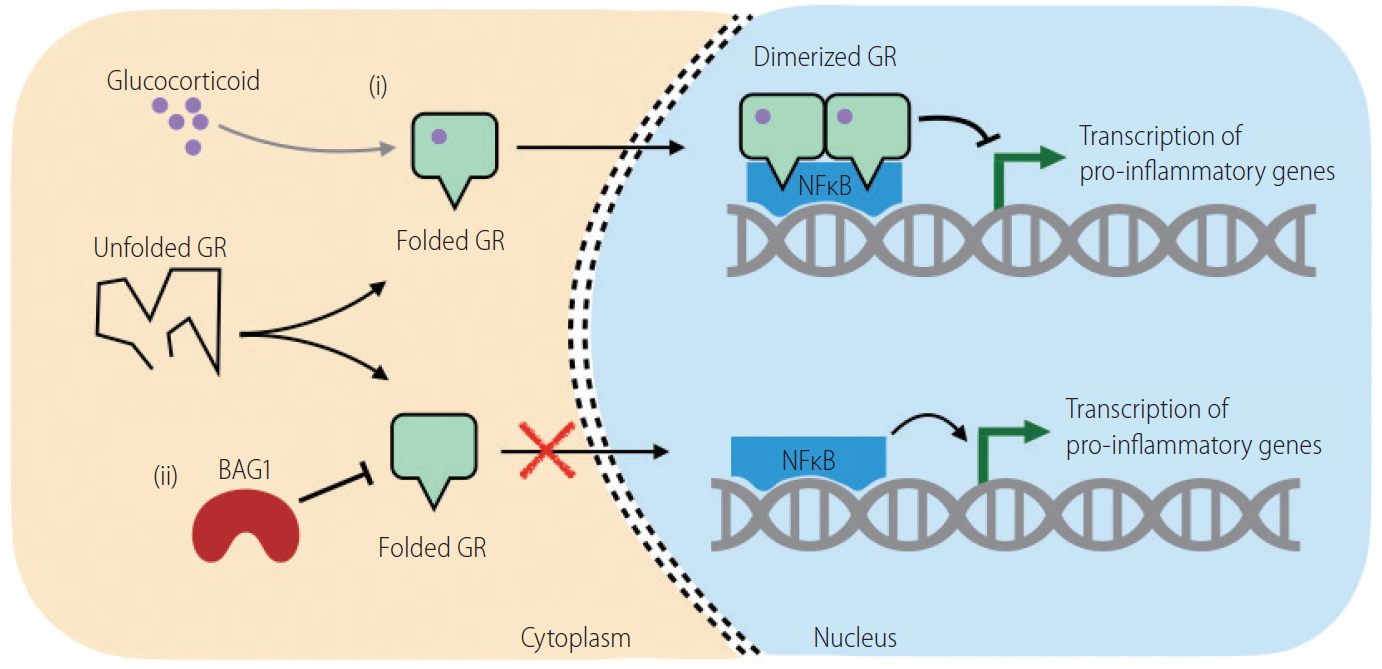
Conformationally folded GR binds to glucocorticoid (i) and shifts to nucleus, where the receptor dimerizes and binds to co-factors (e.g., NFkB) to inhibit proinflammatory gene transcription. BAG1 cleaves GR (ii) in cytoplasm and prevents its downstream suppression leaving pro-inflammatory gene transcription active. GR, glucocorticoid receptor; BAG1, BCl2-associated athanogene 1.
Oxidized albumin forms
Circulatory albumin is a potent ROS scavenger. However, structural modifications in albumin may modulate its antioxidant and immune‐regulatory properties. Such alterations in circulating albumin in SAH patients along with their contribution to neutrophil activation, intracellular stress, and alteration in associated molecular pathways have been assessed [22]. The 3 isoforms of circulating albumin - human mercaptalbumin (HMA) and human non-mercaptalbumin (HNA1 and 2) were measured in SAH patients. Albumin isoforms showed that total HNA1 was significantly (P<0.05) higher in SAH (44.5%) patients compared to healthy controls (28.6%). Additional modifications like glycosylated HMA was also higher in SAH than controls (17.4% vs. 10.6%, P<0.05, respectively).
NEW APPROACHES TO TREATMENT OF AH
Conceptually, the approach to manage SAH includes attempts to abrogate the severe inflammation and necrosis due to alcohol-induced injury, removal of the toxic metabolites produced due to severe inflammation in AH, stimulate liver regeneration despite a toxic milieu of a failing liver using endogenous or exogenous growth factors and modulation of gut bacteria to availability of appropriate nutrition and immune modulation. (Fig. 3). Unfortunately, at present, there are limited therapeutic options for patients, besides steroids. Moreover, for those who do not respond to steroids, the choices are meager. In the past decade, some progress has been made in this direction (Fig. 4).
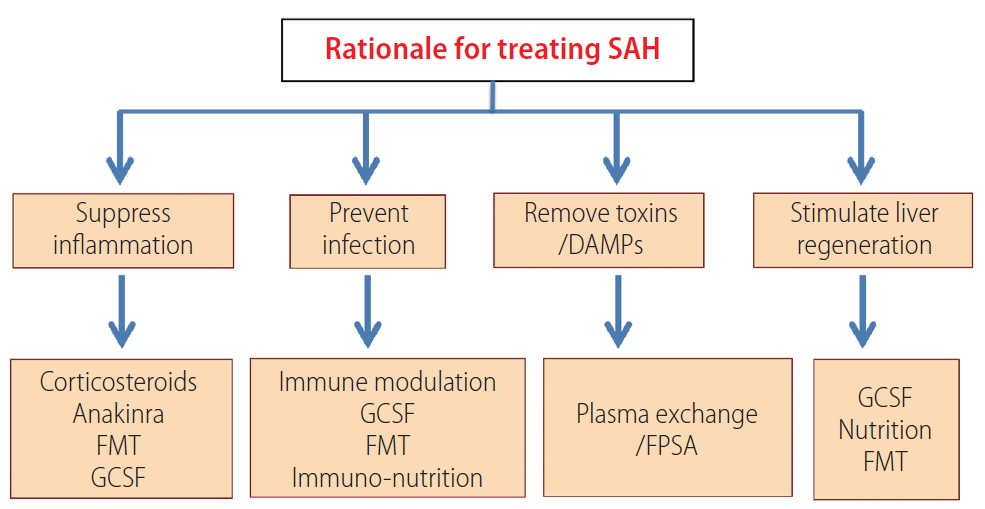
Rationale approaches for treating severe alcoholic hepatitis. SAH, severe alcoholic hepatitis; DAMPs, damage-associated molecular patterns; FMT, fecal microbiota transplantation; G-CSF, granulocyte colony stimulating factor; FPSA, fractional plasma separation and adsorption.
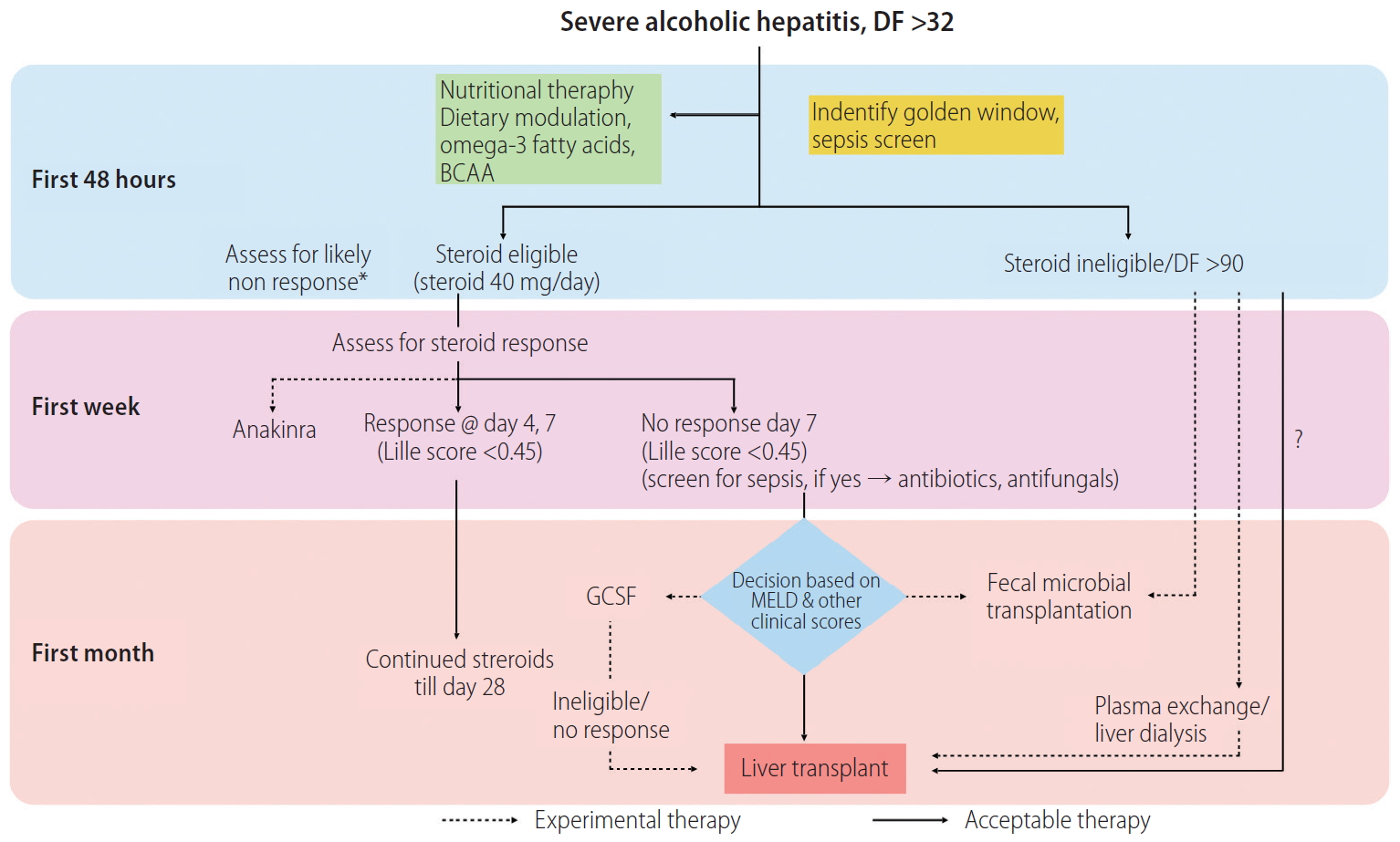
Proposed algorithm for treatment of patients with severe alcoholic hepatitis; steroid non-responders or ineligible patients could be counseled to try experimental therapies. DF, discriminent factor; G-CSF, granulocyte colony stimulating factor. *A baseline assessment of likely steroid non-response could be performed.
Granulocyte colony stimulating factor (G-CSF)
G-CSF (also known as CSF3) is a colony stimulating factor which stimulates bone-marrow precursor cells to produce granulocytes and stem cells, and release them into the blood [23].
Inflammation and impaired liver repair and regeneration are the major factors responsible for liver failure in patients with AH. A state of hyper-inflammatory response leading to progressive hepatocyte damage is accompanied by skewed and deficient immune system in SAH patients. Agents that can ameliorate gut-derived infection and inflammation, and enhance native liver regeneration including bone marrow response are needed. The maximal level of CD34+ cells are released on day 5 after daily G-CSF administration, and the levels fall rapidly on subsequent days despite a continued rise in white blood cell counts [24].
G-CSF regulates innate immune cell response by modulating the production, maturation, migration, and function of neutrophils, monocytes and dendritic cells (DCs). Excessive alcohol consumption impairs this granulopoiesis and the functionality of granulocytes resulting in defects in immune defense and susceptibility to serious infections [25]. Despite, peripheral neutrophilia, AH patients are susceptible to infections, due to neutrophil dysfunction in the form of neutrophil resting oxidative burst greater than or equal to 55% and reduced phagocytic capacity of <42% due to persistent endotoxemia [26]. This is partly due to the defects in the interleukin (IL)-33/suppression of tumorigenicity-2 pathway which is involved in sepsis control and is associated with decreased C-X-C motif chemokine receptor 2 expression on the cell surface and lower neutrophil migration capacity [27]. It is therefore important to identify patients in the golden window [28], the time between the inflammatory response and the onset of sepsis, as the ideal time for therapeutic interventions for SAH. It is probably the most suitable time to introduce the G-CSF therapy, to prevent infections and enhance liver regeneration.
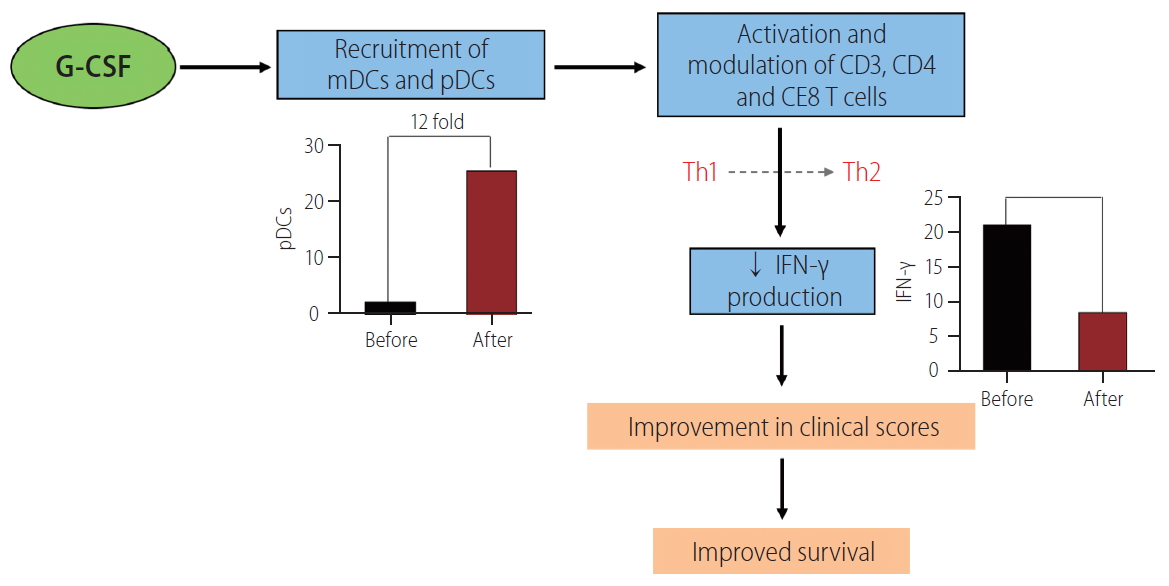
Locally produced G-CSF within the injured tissue also affects the function of recruited neutrophils and DCs. The frequencies of intrahepatic myeloid DCs and plasmacytoid DCs are lower in non-survivors compared to survivors. Intravenously given G-CSF has been shown to enhance the recruitment of plasmacytoid DCs, nearly 12-folds, resulting in reduction in the intrahepatic gamma interferon production and amelioration of hepatic injury (Fig. 5) [29].
G-CSF mobilizes bone marrow hematopoietic stem cells into the circulation by reduced C-X-C motif chemokine ligand 12 levels and adhesive action and facilitates their release into the circulation. G-CSF also stimulates the peripheral sympathetic nervous system, increasing catecholamine concentrations [30]. G-CSF facilitates liver regeneration by migration of bone marrow-derived progenitors to the liver and by enhancing hepatic progenitor cell proliferation by its chemoattractant and mitogenic effects [31].
Clinical trials exploring G-CSF therapy in AH
In a randomized trial of patients with acute-on-chronic liver failure, mostly due to alcohol, G-CSF was able to reduce the incidence of new infections, hepatorenal syndrome and hepatic encephalopathy with an improved 28-day survival compared to placebo (69% vs. 29%, P<0.001) [32]. Also, CD34+ hematopoietic cells were increased in liver biopsies with post G-CSF therapy. The drug was given as 5 mg/kg/day subcutaneously for 5 days and then once on alternate day for a period of 28 days (total 12 doses). It was well tolerated and no major side effects were reported.
G-CSF (given as 5 mg/kg, twice daily for 5 days) in another study on 46 SAH patients with a mean MDF of 85, was found to improve CD34+ cells in peripheral blood at the end of therapy along with improved 90-day survival (78.3% vs. 30.4%, P<0.001) [33].
These reports are mainly from Asian countries. In one study [34] on 58 decompensated alcohol-related cirrhotics, isolated bone marrow derived mononuclear cells including CD34+ hematopoietic cell infusion in the hepatic artery did not show survival benefit or better regeneration on repeat liver biopsy. Similarly, when given in compensated cirrhotics, G-CSF therapy for 5 days followed by leukapheresis and intravenous infusion of CD133-positive hematopoietic stem cells on day 5, 30, and 60 did not show benefit. An important aspect to be noted is that the studies from Europe, had included entirely different set of patients, not those with active AH, and with many having had sepsis. A recent meta-analysis has highlighted the differences in various studies [35]. Based on multiple randomized control trials (RCTs), it can be concluded that in carefully selected patients of AH, G-CSF therapy can help in reducing inflammation, incidence of infection and sepsis, reducing liver severity scores and possibly improve the survival (Fig. 6).
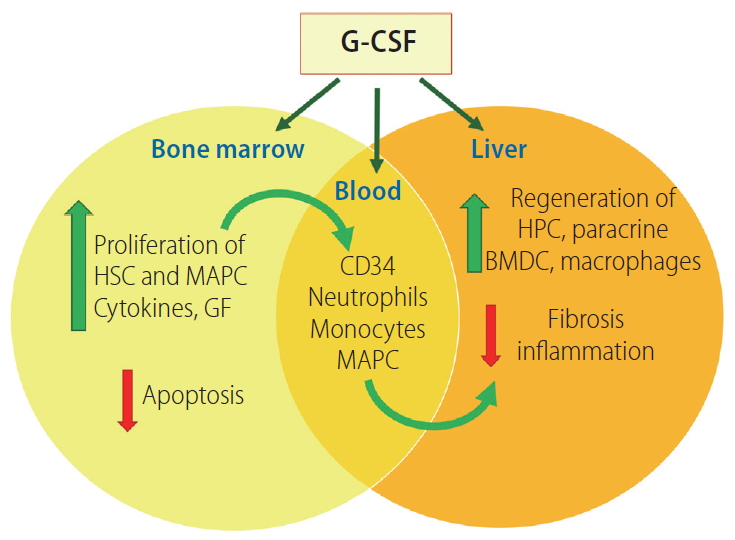
G-CSF has multifaceted applicability in severe alcoholic hepatitis patients; reduction of inflammation, prevention of infection and hepatic regeneration. Adapted from Sarin and Choudhury [67]. G-CSF, granulocyte colony stimulating factor; HSC, hematopoietic stem cells; MAPC, multipotent adult progenitor cells; GF, growth factor; HPC, hepatic progenitor cells; BMDC, bone marrow derived cells.
For steroid non-responders, there are few choices. In the only available study, G-CSF was evaluated in steroid non-responsive SAH patients. Shasthry et al. [6] in a group of 32 steroid non-responders, showed that G-CSF administration reduced the MDF and MELD scores and in-turn the mortality, compared to the placebo (35.7% vs. 71.4%, P=0.04).
Patient selection for G-CSF therapy
Patients with severe AH, not amenable to steroids treatment or steroid non-responders can be considered for G-CSF administration. However, as noted in most of the RCTs, patients with MDF >90, hemoglobin <8 g/dL, high ferritin levels, macrophage activation syndrome (MAS), evidence of iron overload, hypersplenism, ascites, total leucocyte count of >40,000/mm3, infections, culture positive sepsis, acute kidney injury [36], hemodynamic instability, human immunodeficiency virus seropositivity, high adenomatous colorectal polyps or suspicion of malignancy, should be excluded from G-CSF therapy [6,8]. In fact, it is safer and the response is more likely, if the MELD is <15 [37]. A good bone marrow is very helpful in stimulating the hepatic regeneration. Patients with severe sarcopenia, osteoporosis and anemia are less likely to have a responsive bonemarrow [38]. Also, a careful screening for the presence of infections should be undertaken. The efficacy of G-CSF is mainly in prevention of infection, rather than in the management of sepsis in SAH patients, where systemic inflammatory response syndrome (SIRS) can get exaggerated.
Modulation of gut microbiota
The gut microbiome (GM) plays a major role in liver disease. A chronically altered and unhealthy microbiome contributes to the development and perpetuation of liver disease, and this is probably true for SAH. The GM digests the ingested alcohol, with an increase in acetaldehyde concentrations in the gut lumen, and disruption of the mucosal barrier, permitting translocation of viable bacteria and their products, into portal circulation. Locally in the gut, the overgrowth of pathogenic species changes the bile acid composition, enteral metabolites, and entero-hepatic circulation [39]. Resultant immune dysregulation makes the host susceptible to infections and continued hepatic injury [40,41].
Patients with AH, have a pathobiont of their own [40]. This alcohol pathobiont represents altered bacteria, their metabolites, and imbalanced cytokine milieu. Transferring intestinal microbiota from a patient with severe AH into mice, can lead to the development of severe liver inflammation in the animal [42].
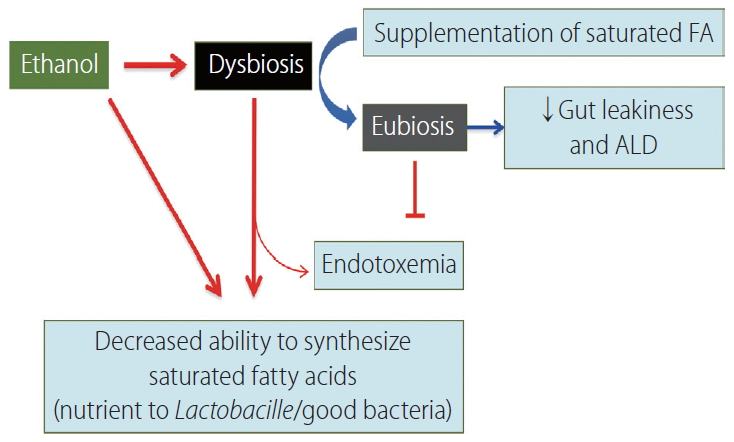
Quantitative and qualitative variations in fecal lipid metabolites like the short-chain fatty acids butyrate and propionate have been reported in rats following chronic ethanol administration [43]. Lactobacillus species use saturated long chain-fatty acids (LCFAs) as a source of energy, which are consequently reduced, resulting in tight junction barrier disruption. In mice models, saturated LCFA supplementation has been shown to restore eubiosis, stabilize the intestinal gut barrier, and reduce ethanol-mediated liver disease (Fig. 7). Ethanol exerts a direct effect on the saturated fatty acid (FA) biosynthetic gene in intestinal bacteria, independently of the host [44]. Hepatocyte synthesized bile acids are secreted via bile duct into the duodenum facilitating digestion, and absorption of lipids, and lipid soluble vitamins. The bile acids modulate the gut microbiota through direct and indirect mechanisms [45]. The gut microbiota then metabolizes primary bile acids into secondary bile acids [44]. More than 95% bile acids actively taken up by enterocytes in the terminal ileum are exported through the basolateral membrane into the portal vein. Bile acids bind to the farnesoid X nuclear receptor and induce its target gene, the fibroblast growth factor (FGF)-19 in humans (or in mice, FGF-15). Both, FGF19/15 are released into the portal circulation, inhibiting hepatic bile acid synthesis and exerting beneficial effects on hepatic lipid metabolism [46]. A significant increase of serum FGF19, total and conjugated bile acids is observed AH patients.
AH-related dysbiosis is associated with an increase in Bifidobacteria, Streptococci, Enterobacteria with concomitant decrease in anti-inflammatory Clostridium leptum or Faecalibacterium prausnitzii. The population density of these protective strains inversely correlates with clinical parameters such as bilirubin [42]. Fecal microbiota transplantation (FMT) studies showed that there is a distinct microbiota signature associated with severe AH, and that a specific alcohol ‘pathobiont’ possibly exists [47]. Identification of these microbial species would identify new targets and therapies.
Fecal microbiota transplantation
Alteration in the GM of a patient can be achieved by using probiotics, FMT or using genetically altered bacteria [36]. While the later has not reached the stage of clinical application, the first two approaches have been evaluated in small sets of patients.
Probiotics have been shown in a small randomized trial to be of benefit in cirrhotic patients, including those with hepatic encephalopathy [48]. No large clinical studies are available of the use of probiotics in SAH patients.
FMT has been used in multiple clinical conditions in gastroenterology. Its use was evaluated in a pilot study of eight steroidineligible SAH patients, and was compared to anecdotal controls. FMT showed significant survival benefit at day 90 with the donor microbiota co-existing in the recipients till 12 months [47].
It is known that subjects in the same household, living and eating the same food, generally share common bacterial taxa. We therefore preferred using FMT material from a related healthy individual living in the same household, with a presumption that the bacteria could adapt readily in the patient.
Recently, concerns have been raised about the safety of FMT therapy as fecal instillate may serve as a medium to carry pathogens [49]. Infection with pathogenic Escherichia coli and development of adverse events in immunocompromised and immunocompetent patients indicates need for additional caution [50]. However, risk of bacterial and fungal infections is high after steroid therapy in SAH patients, and may add to mortality [47].
We have completed a large study on 112 SAH patients, comparing the safety and efficacy in 90 day-survival in those receiving steroids with healthy donor FMT. We serially assessed the recipients for the improvement in clinical and biochemical parameters, changes in their GM, occurrence of adverse events, new complications, and infections. Since Lille score has been validated only for steroid therapy, new scores for assessing the therapeutic response to emerging therapies have also been developed. The initial results of the FMT in SAH patients are quite encouraging. Attempts have been made to inhibit the effects of pro-inflammatory IL-1 by using antagonist to its receptor, anakinra. In a clinical trial comparing anakinra efficacy in combination with pentoxyfylline and zinc against methylprednisolone, the results suggest that long term survival was similar between the two groups (66.8%, anakinra group vs. 52.8%, prednisolone group, P=0.26) [51]. Although the inflammation was shown to be reduced, and gut barrier function was improved. Targeted studies are required to identify the patients suitable for such inhibitors and combinations thereof.
Others
Nutrition therapy and Immuno-nutrition
The goals of nutrition therapy are briefly summarized in the Table 1. A detailed discussion is beyond the scope of this review.
There is also a growing realization of the benefits of immunonutrition in critical illnesses. Administration of omega-3 FAs, contained in fish oil has been shown to reduce morbidity and mortality in both, postoperative and critically ill patients, without undesired side effects. Sepsis is common in SAH patients and can develop within the first few days of the illness [52].
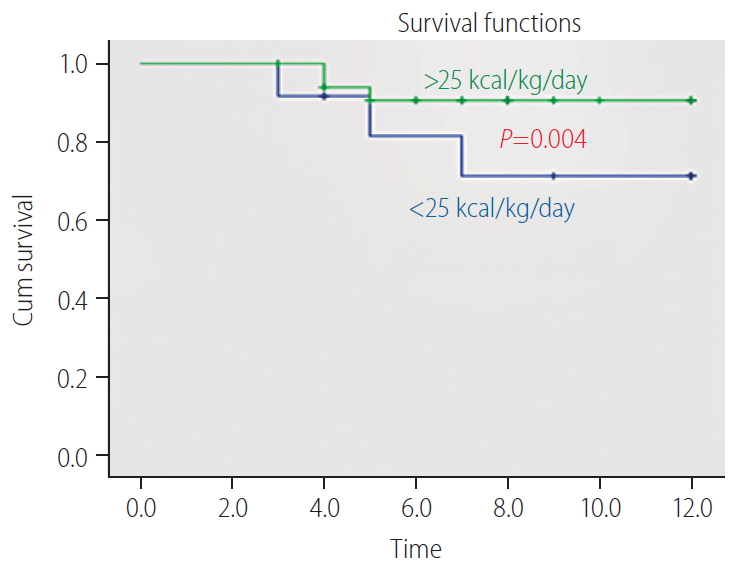
Patients with SAH have insulin resistance fulfilling three dominant criteria: low high-density lipoprotein cholesterol, high triglycerides, and high fasting blood sugar. The study [53] has shown in a recent study that omega-3 polyunsaturated FAs given intravenously for 5 days reduced the incidence and delayed the development of sepsis by improving the immunity. In fact, it was helpful in reducing the serum endotoxin levels (P<0.001) and the severity of illness. Omega-3 polyunsaturated FAs should be explored as an economical choice [54] for the prevention of sepsis and as a source of energy. Supplementation with of energy >25 kcal (and protein >0.8 g/kg/day) has shown significant improvement in 12 month-survival in patients with alcoholic cirrhosis (Fig. 8) [55].
Extracorporeal liver support
Extracorporeal liver support procedures, with potential to remove toxic and highly oxidative metabolites and circulating molecules may be of help in patients with SAH. However, artificial liver support, using different liver dialysis devices have shown limited benefits [56,57].
Plasma exchange
Plasma exchange has been shown to clear damage-associated molecular patterns, improve SIRS and survival in patients with acute liver failure in a large multi-centric RCT [58]. Liver failure-related deaths were averted by plasma-exchange, both at 30 and 90 days and the therapy was superior to standard medical therapy and fractional plasma separation and adsorption in preventing organ failure and improving survival compared to the matched cohort. Apart from this, as expected plasma exchange also improved other clinical parameters and reduced the MELD scores. There is emerging data that plasma exchange, can serve as a bridge to liver transplantation in SAH patients [59]. Though, the risk of fluid overload and infections has to be carefully weighed before taking patients for this therapy.
Anti-oxidants
Although oxidative stress has been implicated in the pathogenesis of AH [4]. Several studies have refuted benefit of N-acetylcysteine (NAC) in comparison to steroids in AH treatment [60,61]. A few studies have shown short-term benefit of steroid plus NAC combination therapy with increased 1-month survival in patients with severe AH, but no increase in 6-month survival [61]. A Cochrane review showed that the use of S-adenosyl-L-methionine is not of any help during treatment of AH [62]. Detailed studies are required to establish the definitive role of NAC in management of severe AH. Together with oxidative stresses generated by gut microbiota investigations analyzing multiple pathological factors are required to improve our understanding, and bring in a paradigm shift in the treatment approaches for such patients.
Granulocytapheresis
This technique involves removal of up to 60% of activated granulocytes and monocytes from circulating blood. It has been found to be well tolerated and of some benefit in patients responding to steroids [63,64]. To further elaborate the role of granulocytapheresis high quality studies are required in addition to existing case series.
Anakinra
Besides being compared for its anti-inflammatory potential against steroid, anakinra has also been tested for treatment of MAS, a common presentation of very sick SAH patients [65]. The IL-1 receptor antagonist has been shown to reduce 28-day mortality in MAS patients with sepsis [66]. Elaborate studies are required to confirm whether anakinra could be used as in combination with other anti-inflammatory drugs in steroid non-responsive patients.
CONCLUSION
Severe AH remains to be a disease with a high mortality, and there is an ever-growing need to find effective therapies to treat the patients early or delay mortality. From the many therapeutic options discussed above, it is unlikely that a single therapy would be an all-effective option. Given the complex molecular mechanisms varying with different stages of the disease, a combination of therapies is likely to improve efficiency. Importantly, addressing suppression of bacterial or viral infections, and hepatic inflammation, in conjunction with liver regeneration will remain the key strategy for next generation therapies.
Notes
Authors’ contributions
SKS and SS, both contributed to compilation of cited works, writing of review and development of figures. SKS conceptualized the manuscript.
Conflicts of Interest
The authors have no conflicts to disclose.
Abbreviations
AH
alcoholic hepatitis
ALD
alcohol-associated liver diseases
BAG1
BCL2-associated athanogene1
BD
ballooning degeneration
CI
confidence interval
DCs
dendritic cells
FA
fatty acid
FGF
fibroblast growth factor
FMT
fecal microbiota transplantation
G-CSF
granulocyte colony stimulating factor
GM
gut microbiome
HMA
human mercaptalbumin
HNA
human non-mercaptalbumin
IL
interleukin
LCFA
long chain-fatty acid
MAS
macrophage activation syndrome
MD
Mallory-Denk bodies
MDF
Maddrey’s discriminant function
MELD
model for end-stage liver disease
MV
microvesicle
NAC
N-acetylcysteine
RCT
randomized control trial
ROS
reactive oxygen species
SAH
severe alcoholic hepatitis
SD
standard deviation
SIRS
systemic inflammatory response syndrome
SOFA
sequential organ failure assessment