Radiation-induced abscopal effect and its enhancement by programmed cell death 1 blockade in the hepatocellular carcinoma: A murine model study
Article information
Abstract
Background/Aims
The abscopal effect, a rare phenomenon induced by radiation, can be reinforced by immunotherapy. Although radiation therapy and immunotherapy are increasingly being utilized for the treatment of hepatocellular carcinoma (HCC), whether immunotherapy could boost the abscopal effect remains unclear. In this study, we aimed to elucidate the immunological mechanisms underlying the abscopal effect induced by the combination of irradiation and immunotherapy in a murine HCC model.
Methods
A syngeneic HCC mouse model was established by transplanting murine Hepa 1–6 HCC cells into both hind legs of immunocompetent C57BL/6 mice. The tumors on the right hind legs were irradiated, and abscopal effects were observed in the non-irradiated tumors on the left hind leg with or without the coadministration of anti-programmed cell death 1 (PD-1) antibodies. Flow cytometric analyses were performed to analyze the distributions of immune cells infiltrating both irradiated and non-irradiated tumors and the tumor-draining lymph nodes (TDLNs).
Results
Administration of 16 Gy in two fractions more effectively inhibited the growth of both irradiated and non-irradiated tumors with higher tumor infiltration of cytotoxic T cells than 8 Gy did in a single fraction. The higher dose also increased activated dendritic cells in TDLNs, which had higher expression of the programmed cell death ligand 1. Coadministration of anti-PD-1 antibodies significantly enhanced the abscopal effect and increased infiltration of activated cytotoxic T cells in both irradiated and non-irradiated tumors.
Conclusions
Our findings show that adding anti-PD-1 therapy to radiation enhanced the abscopal effect in a syngeneic murine model of HCC.
Graphical Abstract
INTRODUCTION
The abscopal effect, which refers to the regression of tumors outside the radiation field during or after radiation therapy (RT), has been reported in various cancers such as melanoma, renal cell carcinoma, and breast cancer for over 60 years [1]. Although its mechanism has not yet been fully elucidated, the abscopal effect is likely mediated by the radiation-induced enhancement of systemic immune responses [2]. However, the abscopal effect has been rarely observed, possibly because of the immune escape mechanisms of cancer cells, including the activation of the immune checkpoint pathway [3]. Newly developed immune checkpoint inhibitors (ICIs) have revolutionized cancer treatment over the last decade [4]. Following the emergence of ICIs that enhance systemic immune responses against cancer cells, new therapeutic regimens combining ICIs and RT are expected to boost the abscopal effect [5]. The synergistic effects of ICIs and RT are being actively investigated and the potential of this combination to intensify the abscopal effect has been reviewed in various reports [6-8].
Hepatocellular carcinoma (HCC) is the most common type of primary liver cancer and the second leading cause of cancer-related death worldwide [9,10]. Patients in the early stage of HCC treated with curative modalities such as resection, liver transplantation, and ablation show an overall survival rate >60%, whereas those with advanced HCC who undergo chemoembolization or systemic therapy have a dismal prognosis [11,12]. To improve this poor outcome, new regimens have recently been incorporated and suggested in the treatment of HCC, including considering the use of ICIs. Therefore, the feasibility and safety of various ICIs, including programmed cell death 1 (PD-1) inhibitors, PD ligand 1 (PD-L1) inhibitors, and cytotoxic T lymphocyte-associated protein-4 (CTLA-4) inhibitors, are under investigation in patients with HCC [13-17]. However, the reported objective response rates (ORRs) of ICIs were only 15–20%, which are still unsatisfactory [14-16]. Furthermore, the recent KEYNOTE-240 trial evaluating the efficacy and safety of pembrolizumab in patients with advanced HCC failed to show statistical significance in the improvement of overall survival and progression-free survival compared with the placebo [18]. RT has received considerable attention for use in enhancing the efficacy of ICIs for advanced HCC because of the abscopal phenomenon induced by its immunogenicity [19].
The efficacy of cotreatment in controlling primary HCC has been recently tested in a few preclinical experiments and clinical trials [20-22]. However, these previous studies did not use metastatic HCC models but rather used orthotopic models, which only enable the observation of the combined effect of RT and ICIs on primary tumors but not the abscopal effect. Therefore, in this study, we aimed to investigate the effect and immunological mechanisms of action of radiation on the abscopal phenomenon and the effect of an anti-PD-1 antibody on the abscopal effect using a bilateral murine syngeneic HCC model.
MATERIALS AND METHODS
Cell culture
The murine Hepa 1–6 HCC cell line was purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). Hepa 1–6 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Carlsbad, CA, USA) containing 10% fetal bovine serum and 1% antibiotic-antimycotic solution (Thermo Fisher Scientific, Waltham, MA, USA) at 37°C exposed to an atmosphere of 5% CO2.
Animal model
Male C57BL/6 mice (5–6 weeks old) were purchased from Orient Bio (Seongnam, Korea). To establish the syngeneic mouse model of bilateral HCC tumors, Hepa 1–6 cells (1×106 cells/mouse) were injected into the right hind leg and then cells were also injected into the left hind leg 3 days after the first injection. Tumor dimensions were measured every 3 days using calipers and the volume was calculated according to the following formula: volume = W2 × L / 2 (L, length [mm]; W, width [mm]).
When the mean tumor volume reached 300 mm3, the mice were randomized into various treatment groups (n≥5 per group) and after 30 days they were euthanized, followed by tumor collection for further analysis. All animal procedures were conducted in accordance with appropriate regulatory standards under the study protocol (ID: 20181227001, approval date: 2019-03-08), which was reviewed and approved by the Institutional Animal Care and Use Committee of Samsung Biomedical Research Institute.
Drug and radiation treatment
For irradiation, 6 MV photon beams were delivered to the tumor-bearing right hind leg at a dose rate of 3.96 Gy/min using a linear accelerator (Varian Medical System, Palo Alto, CA, USA) as described previously [23,24]. Irradiation was performed at a single 8 Gy or total 16 Gy dose in two fractions. Before irradiation, the mice were anesthetized via intraperitoneal injections of 30 mg/kg zolazepam/tiletamine and 10 mg/kg xylazine. All mice were placed in a prone position and the tumor-bearing right hind legs were placed under a 2-cm-thick water-equivalent bolus with a source-to-surface distance of 100 cm and radiation field size of 30×7 cm. The mice were positioned to minimize the radiation dose administered to the tumor-draining inguinal lymph nodes. Details on the experimental setup are provided in Supplementary Figure 1. Starting on day 14, the mouse anti-PD-1 antibodies and isotype control (BE0146 and BE0089, respectively; Bio X cell, West Lebanon, NH, USA) were administered intraperitoneally once a week at a dose of 2 mg/kg.
Flow cytometry analysis
On day 31 after the first injection of Hepa 1–6 cells, tumors were harvested, a single cell suspension was prepared for flow cytometry analysis, and red blood cells were removed by incubation with Pharm Lyse (BD Biosciences, San Jose, CA, USA) for 30 minutes at 4°C. The cells were fixed with Cytofix (BD Biosciences) for 30 minutes at 4°C and then they were resuspended in staining buffer (BD Biosciences). For T cell analysis, cells were permeabilized using the Fix/Perm buffer kit (eBioscience, San Diego, CA, USA) and then they were stained with anti-forkhead box P3 (Foxp3, BD560408) and anti-interferon (IFN)-γ (BD557724) antibodies for 30 minutes at 4°C. After washing, cells were stained with antibodies specific for CD4 (BD55205), CD8 (BD560469), CD3e (BD560771), and CD25 (BD551071).
For dendritic cell (DC) analysis, inguinal lymph nodes of the irradiated tumor side were collected 3 days after irradiation, and DCs were isolated using the EasySepTM mouse plasmacytoid DC isolation kit (#19764, STEMCELL Technologies, Vancouver, Canada). Furthermore, 1×105 DCs were stained with CD11c (BD553801), CD40 (BD562846), CD80 (BD560016), and PD-L1 (12-5982, eBioscience; Thermo Fisher Scientific). Data were acquired using a fluorescence activated cell sorting verse flow cytometer (BD Biosciences) and were analyzed using FlowJo software (Tree Star Inc., Ashland, OR, USA).
Statistical analysis
All data are presented as the means±standard deviation. Statistical analysis was performed using a one-way analysis of variance with Bonferroni’s correction using Prism 8.0 (GraphPad Software, San Diego, CA, USA). A P<0.05 was considered statistically significant and survival curves were assessed using the Kaplan-Meier analysis with a log-rank test. P values are indicated in the figures as follows: *P<0.05, †P<0.01, and ‡P<0.001.
RESULTS
Localized tumor irradiation evokes the abscopal response in a syngeneic HCC mouse model
A syngeneic bilateral HCC mouse model was established using Hepa 1–6 cells (Fig. 1A). The tumor growth curves showed that irradiation with 16 Gy in two fractions but not 8 Gy in a single fraction more significantly inhibited tumor growth (P<0.001, Fig. 1B) and non-irradiated tumors (P<0.05, Fig. 1C) than the sham control, indicating that the regimen of 16 Gy in two fractions was applicable for evoking abscopal effects in the present HCC model. Tumors harvested on day 31 were imaged (Fig. 1D), and irradiation with 16 Gy in two fractions significantly improved the survival of mice more than the sham control treatment or irradiation with a single fraction of 8 Gy did (P<0.001, Fig. 1E).
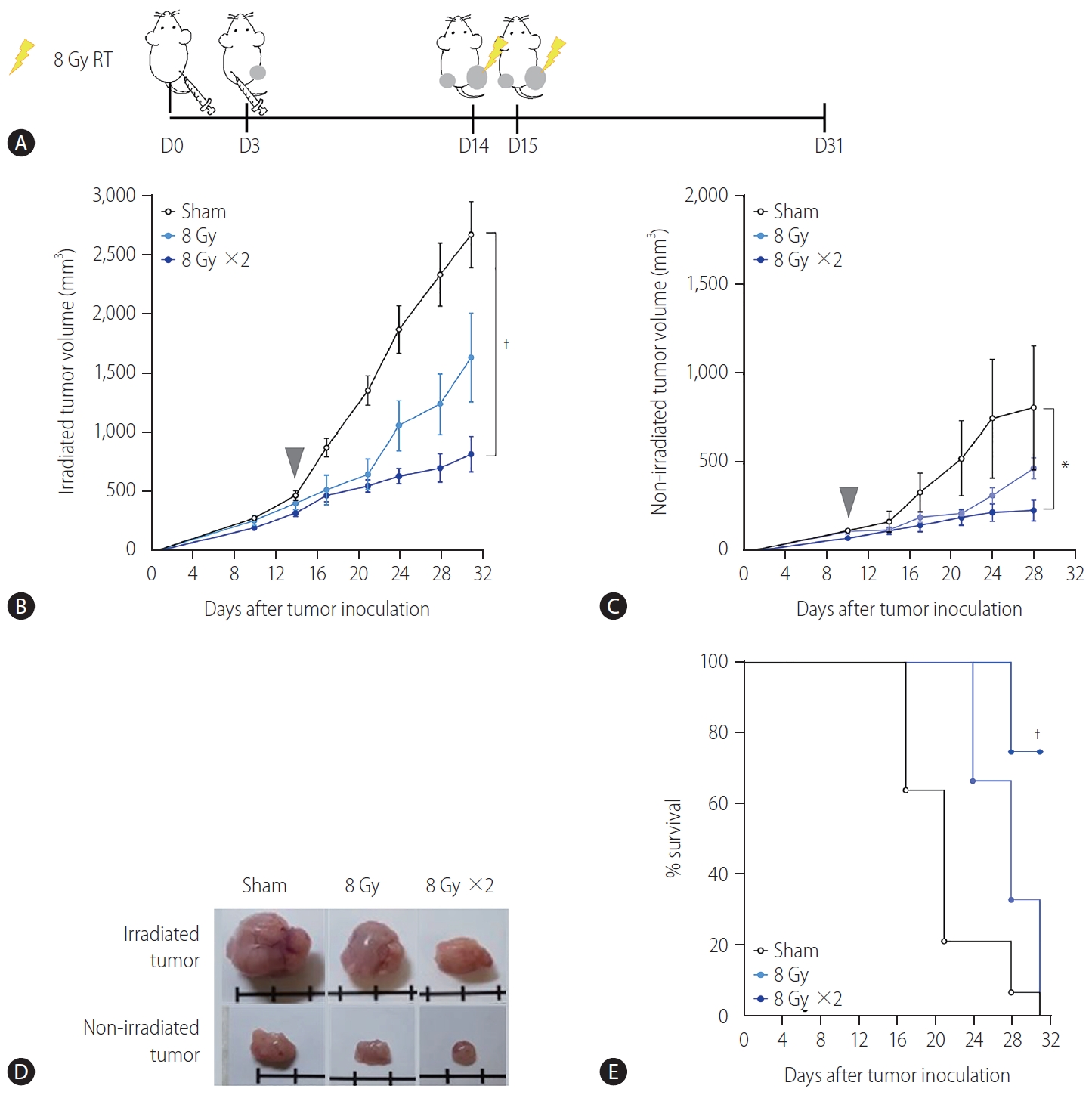
Localized irradiation inhibits the growth of both primary and distant tumors in a syngeneic model of murine hepatocellular carcinoma. (A) Scheme for radiation treatment of a bilateral tumor mouse model established using Hepa 1–6 cells. Growth curves of (B) irradiated tumors and (C) non-irradiated tumors in mice bearing bilateral Hepa 1–6 tumors. Gray arrowheads indicate day 1 of irradiation. (D) Representative images of tumors collected from mice. (E) Survival of mice bearing bilateral Hepa 1–6 tumors for three different treatment groups. RT, radiation therapy. *P<0.05. †P<0.001.
Localized tumor irradiation induces infiltration of activated T cells in both irradiated and non-irradiated tumors
To determine whether the reduction of non-irradiated tumor size by localized irradiation may be associated with immunogenic activation, we performed flow cytometry analysis of tumor infiltrating lymphocytes (Fig. 2A, B). Quantification of the data showed that 16 Gy administered in two fractions but not 8 Gy in a single fraction significantly increased infiltration of CD4+ IFN-γ+ and CD8+ IFN-γ+ T cells in both irradiated (P<0.001 and P<0.05, Fig. 2C) and non-irradiated tumors (P<0.001 and P<0.001, Fig. 2D). Furthermore, 16 Gy administered in two fractions also increased infiltration of regulatory T cells (Treg, CD4+ CD25+ Foxp3+) in irradiated tumors (P<0.05, Fig. 2C).
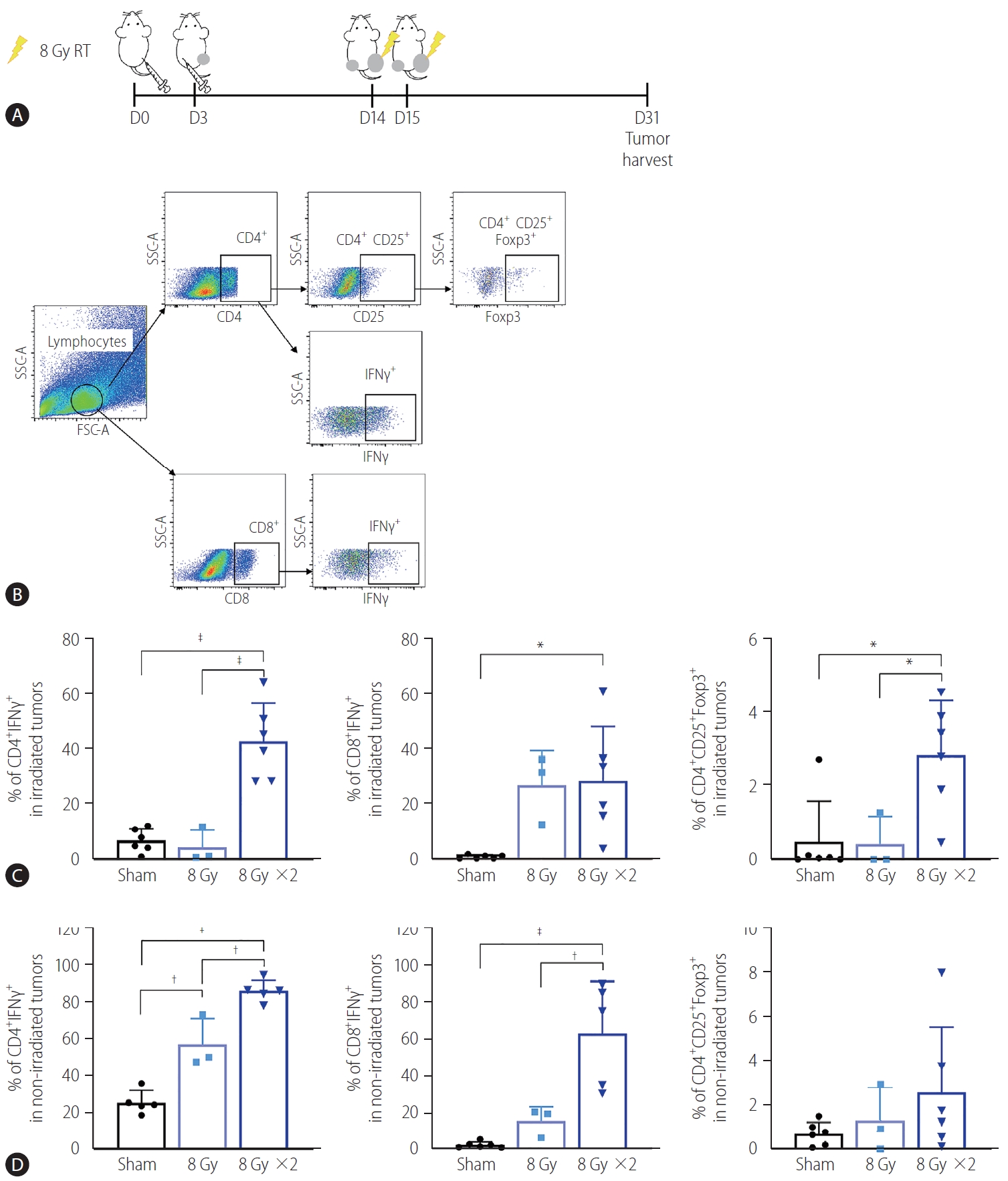
Augmented radiation doses increased infiltration of activated T cells into non-irradiated and irradiated tumors. (A) Scheme for radiation treatment of a bilateral tumor mouse model established using Hepa 1–6 cells. (B) Representative flow cytometry dot plots showing profiles of activated T cell populations and regulatory T cells (Tregs). (C) Quantification data showed that administration of two but not one 8 Gy fraction significantly increased the population of IFNγ expressing CD4+ and CD8+ T cells and Tregs in the irradiated tumors. (D) Two fractions of 8 Gy also significantly increased the population of CD4+ and CD8+ T cells expressing IFNγ cells in non-irradiated tumors. RT, radiation therapy; SSC-A, side scatter analysis; FSC-A, forward scatter analysis; IFN, interferon. *P<0.05. †P<0.01. ‡P<0.001.
Localized tumor irradiation activates DCs in tumordraining lymph nodes (TDLNs)
To understand how localized irradiation reduces non-irradiated tumors, we examined DCs in the inguinal lymph nodes draining from tumors implanted in the right hind legs of the mice using flow cytometry (Fig. 3A, B). Quantification data show that 16 Gy administered in two fractions significantly increased the frequency of activated DCs in the TDLNs, as evidenced by the high expression of CD40 (P<0.001) and CD80 (P<0.01, Fig. 3C). Expression of PD-L1, the co-inhibitory molecule on CD11c+ DCs in the TDLNs, was also induced by irradiation with two fractions of 8 Gy, compared to the sham treatment (P<0.05, Fig. 3C). Moreover, 16 Gy administered in two fractions also activated T cells in the TDLNs, as evidenced by a significant increase in CD4+ IFN-γ+ and CD8+ IFN-γ+ T cells (P<0.001, Fig. 3D). Accumulation of Tregs in the TDLNs was also more evident in the group administered two 8 Gy fractions than in the sham-treated group or the group administered a single 8 Gy fraction (Fig. 3D).
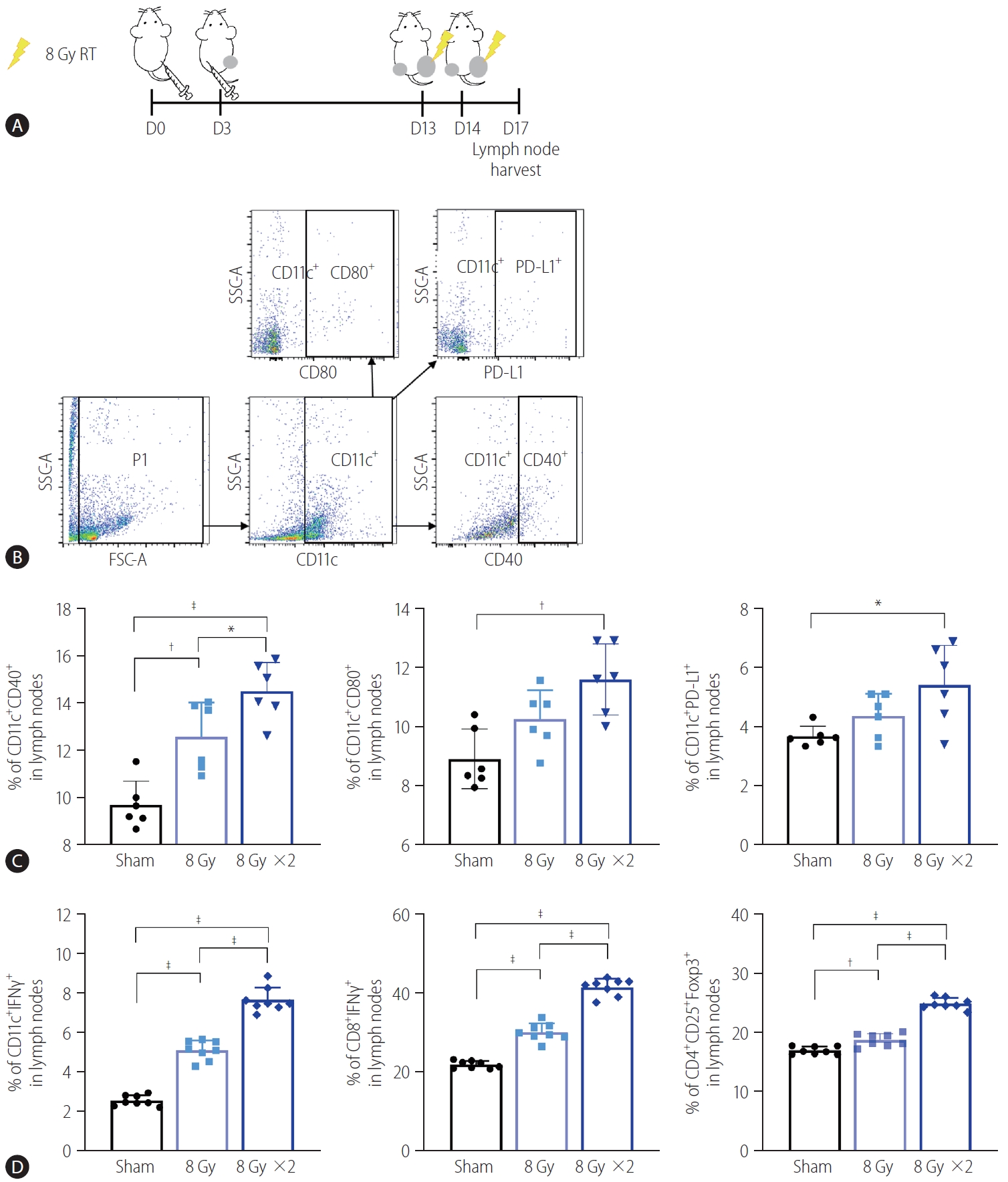
Irradiation of tumor sites increased activation of DCs and T lymphocytes in TDLNs. (A) Scheme for radiation treatment of a bilateral Hepa 1–6 tumor model. (B) Representative flow cytometry dot plots showing profiles of activated DC populations and PD-L1 expressing DCs. (C) Quantification data showed that two fractions of 8 Gy radiation increased activated populations of CD11c+ DCs in TDLNs, as evidenced by CD40 and CD80 expression. Radiation also increased PD-L1 expression of CD11c+ DCs. (D) Both single and two fractions of 8 Gy increased activated T cell populations in TDLNs. RT, radiation therapy; PD-L1, programmed cell death 1 ligand 1; SSC-A, side scatter analysis; FSC-A, forward scatter analysis; IFN, interferon; DCs, dendritic cells; TDLNs, tumor-draining lymph nodes. *P<0.05. †P<0.01. ‡P<0.001.
Cotreatment with radiation and anti-PD-1 antibodies boosts the abscopal effect in the syngeneic HCC mouse model
Based on the result showing that radiation increased the expression of PD-L1 in the TDLN-resident DCs, we determined whether blockade of the PD-1/PD-L1 pathway could further enhance the abscopal effect in the syngeneic, bilateral HCC mouse model (Fig. 4A). Anti-PD-1 antibodies inhibited the growth of bilateral Hepa 1–6 tumors more than the isotype IgG control did (Fig. 4C), but the difference was not statistically significant.
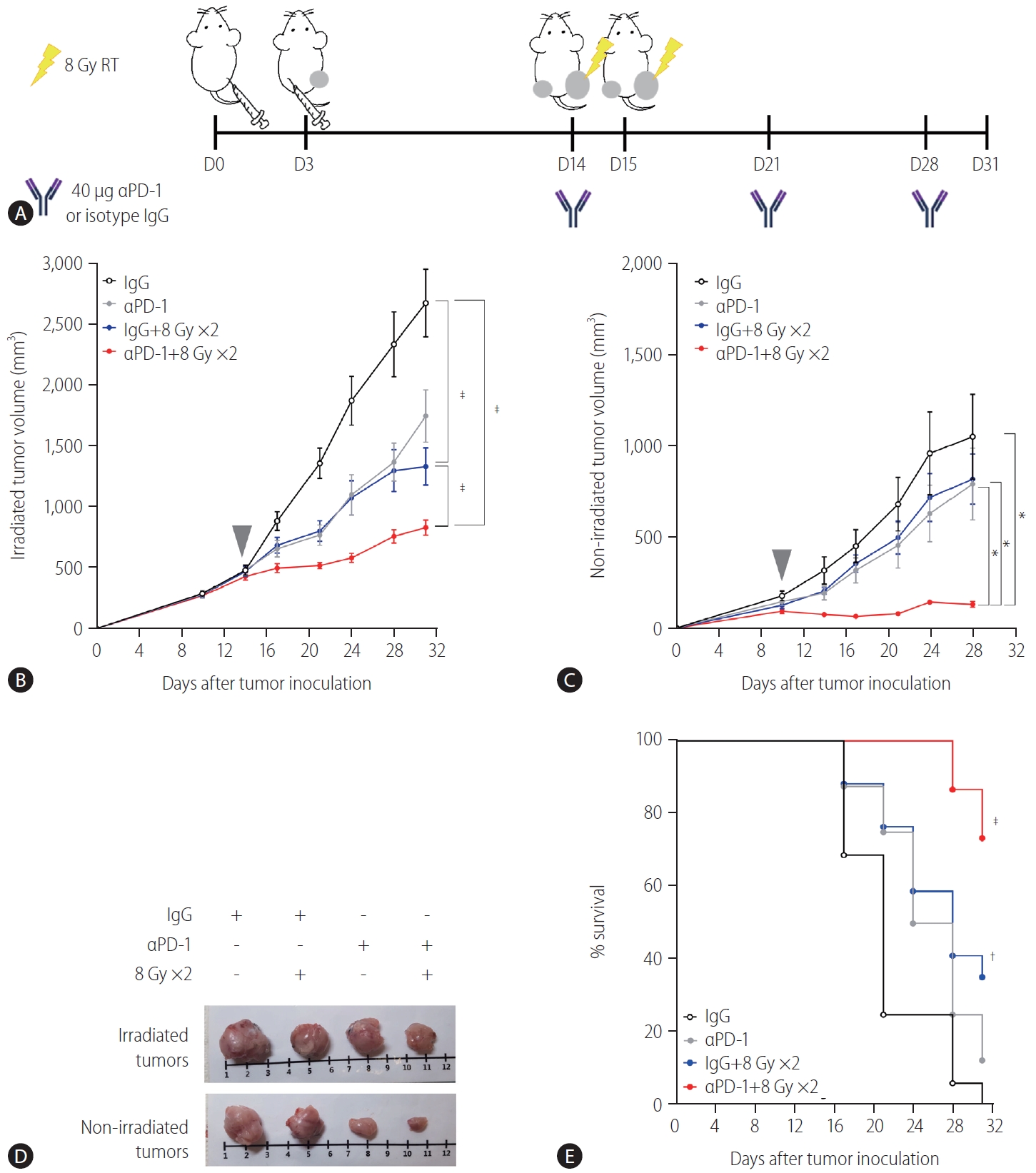
Administration of anti-PD-1 enhances the antitumor effect of radiation on both irradiated and non-irradiated tumors. (A) Scheme for anti-PD-1 and radiation treatment of a bilateral Hepa 1–6 tumor mouse model. Growth curves of (B) irradiated and (C) non-irradiated tumors in mice bearing bilateral Hepa 1–6 tumors. Gray arrowheads indicate day 1 of irradiation. (D) Representative images of tumors collected from mice. (E) Survival of mice bearing bilateral Hepa 1-6 tumors from four different treatment groups. RT, radiation therapy; PD-1, programmed cell death 1; IgG, immunoglobulin G. *P<0.05. †P<0.01. ‡P<0.001.
As expected, 16 Gy administered in two fractions inhibited the growth of both irradiated (P<0.001, Fig 4B) and non-irradiated abscopal tumors (Fig. 4C, not significant). Cotreatment with radiation and anti-PD-1 further suppressed the growth of both irradiated and non-irradiated tumors more than radiation alone or antiPD-1 monotherapy did (Fig. 4B, C). Tumors harvested from mice cotreated with the anti-PD-1 antibody and radiation were clearly smaller than those from mice treated with radiation or anti-PD-1 monotherapy (Fig. 4D). Anti-PD-1 treatment alone did not improve survival but in combination with radiation, it increased survival significantly (P<0.001, Fig. 4E).
Combination of radiation and anti-PD-1 antibodies further enhances T lymphocyte infiltration of abscopal HCC tumors
Immunohistochemistry using antibodies specific to CD4 and CD8 showed that anti-PD-1 antibodies and radiation alone more significantly increased the infiltration of CD4+ and CD8+ cells into irradiated tumors than the IgG control did (P<0.05; Supplementary Fig. 2A, B), along with CD4+ cells into the non-irradiated contralateral tumors (P<0.01 and P<0.001; Supplementary Fig. 2C, D). Cotreatment with the anti-PD-1 antibodies and radiation further enhanced infiltration of CD8+ cells into both tumors, although no additive effect was obvious.
Flow cytometry analysis of tumors harvested on day 31 (Fig. 5A) showed that in the irradiated tumors, 16 Gy administered in two fractions but not anti-PD-1 antibody treatment more significantly increased infiltration of CD4+ and CD4+ IFN-γ+ T cells than the IgG control did (P<0.001, Fig. 5B). Similarly, 18 Gy administered in two fractions increased the infiltration of total CD8+ and CD8+ IFN-γ+ T cells (Fig. 5B). Cotreatment with anti-PD-1 antibodies and radiation further enhanced infiltration of CD4+ and CD8+ T cells more than treatment with the anti-PD1 antibodies alone but not radiation alone (Fig. 5B). Both radiation and the anti-PD-1 antibodies increased CD4+ and CD4+ IFN-γ+ T cells but not CD8+ T cells in the non-irradiated tumors, while their cotreatment increased the total CD8+ and CD8+ IFNγ+ T cells more than either treatment alone (Fig. 5C). Combination of radiation and the antiPD-1 antibodies increased enrichment of CD25+ CD4+ Foxp3+ Tregs in the tumors on both sides, compared to the IgG control treatment (P<0.05 and P<0.01, Fig. 5D), whereas no difference in CD11c+ DCs was observed among the groups (Fig. 5E). Flow cytometric immunophenotyping revealed that the number of CD220+ B cells, F4/80+ macrophages, Ly6G+ CD11b+ neutrophils, Ly6G– CD11b+ Ly6C+ monocytic-myeloid-derived suppressor cells (MDSCs), and Ly6G– CD11b+ Ly6Clow granulocytic-MDSCs was not affected by monotherapy or co-therapy, except for that of CD3+ T cells (Supplementary Fig. 3).
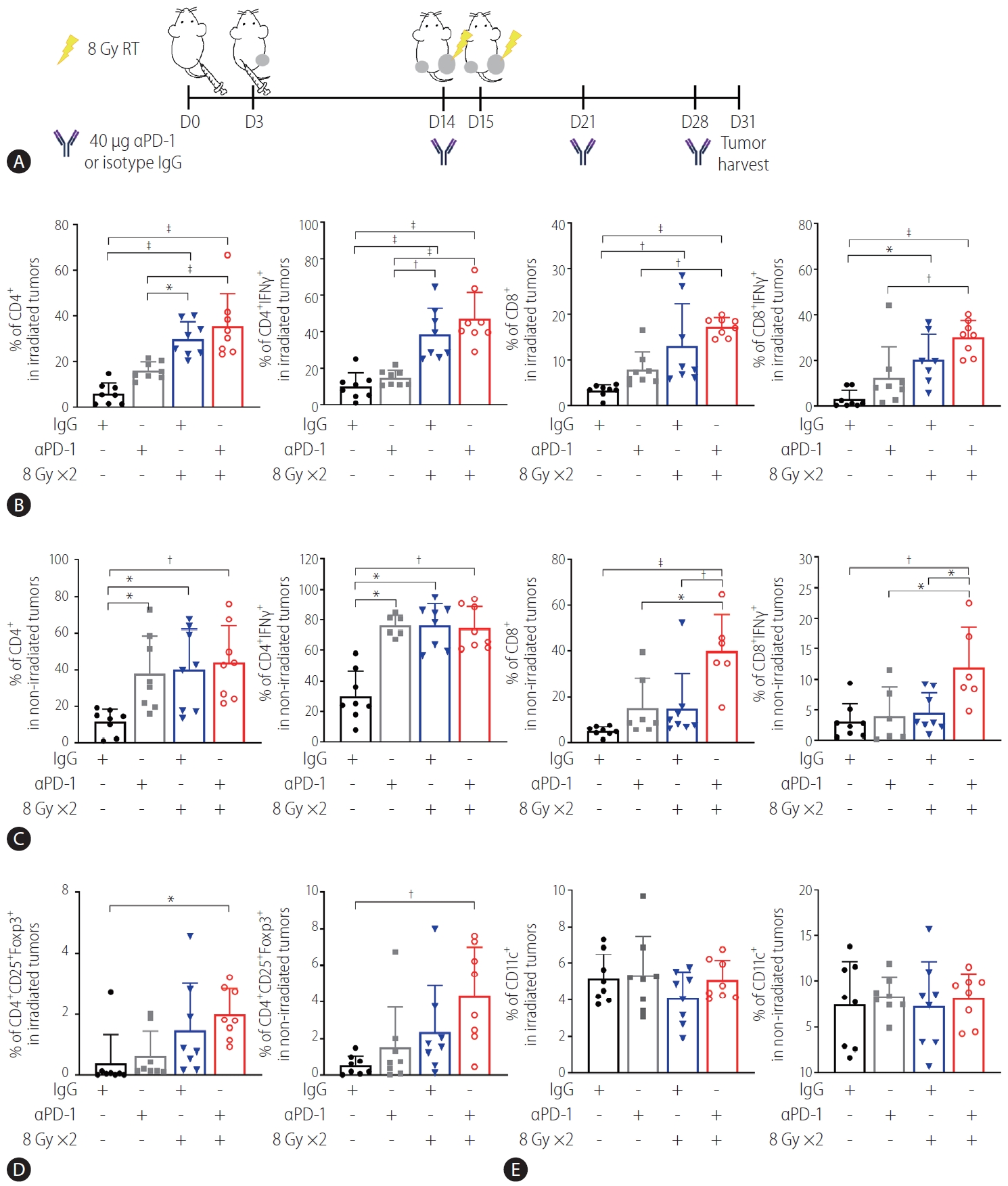
Anti-PD-1 boosts radiation-induced infiltration of activated T cells into both irradiated and non-irradiated tumors. (A) Scheme of anti-PD-1 and radiation treatment of a bilateral Hepa 1–6 tumor mouse model. (B) Flow cytometric analysis revealed that radiation but not anti-PD-1 alone increased infiltration of CD4+ T and CD8+ T cells and expression of IFN-γ in these cells. This was further enhanced by combination with anti-PD-1. (C) Combination of radiation and anti-PD-1 increased population of activated CD4+ and CD8+ T cells in non-irradiated tumors. Population of (D) T regulatory cells (Tregs) but not (E) CD11c+ dendritic cells (DCs) in irradiated and non-irradiated tumors was increased by cotreatment with anti-PD-1 and radiation. RT, radiation therapy; PD-1, programmed cell death 1; IgG, immunoglobulin G; IFN, interferon. *P<0.05. †P<0.01. ‡P<0.001.
DISCUSSION
Following the first published report on the abscopal effect [25], this phenomenon has been investigated in various cancers such as melanoma, renal cell carcinoma, and breast cancer both preclinically and clinically [1]. However, compared with other malignancies, studies on the abscopal effect and its enhancement by immunotherapeutic agents in HCC is lacking, possibly because RT and immunotherapy have not been widely used for HCC management. The few studies that have used HCC models to evaluate the combined effect of RT and ICIs described the effect of ICIs on irradiated primary tumors but not the abscopal effect [20,21]. Friedman et al. [21] reported that in an orthotopic murine HCC model, PD-1 blockade enhanced the response to stereotactic radiation with 30 Gy in three fractions. Another study by Kim et al. [20] demonstrated the synergistic effect of 10 Gy coadministered as a single fraction with an anti-PD-L1 antibody in reducing primary HCC tumor growth. Clinical observation of abscopal effects in HCC is extremely rare [26,27], and a recent study by Chen et al. [28] showed that hypofractionated RT (up to 40 Gy) inhibited non-irradiated tumor growth in a murine HCC model via suppression of MDSCs. To the best of our knowledge, this is the first study evaluating the enhancement of abscopal effects by a combination of radiation and ICIs in a murine syngeneic HCC model.
A suggested mechanism of the abscopal effect is that it starts with the release of tumor antigens and danger-associated molecular patterns from tumor cells damaged by irradiation [19]. The released tumor antigens are presented by antigen-presenting cells including DCs, which are drained into TDLNs. The drained DCs prime T cells, which subsequently become effector cells, migrate to the non-irradiated tumor cells, and induce adaptive immunity [29]. In addition, radiation enhances the expression of type I IFN genes through cyclic guanosine monophosphate-adenosine monophosphate synthase (cGAS) and stimulator of IFN gene (STING) pathways, which are consequentially activated by the accumulation of cytosolic DNA [30]. The increased IFN activates antigen-presenting cells and mediates the recruitment of effector T cells to the target site [31]. Our results showed a significant decrease in the growth of the non-irradiated tumors (Fig. 1C) and increases in the infiltration of activated T cells in the non-irradiated tumors and population of activated DCs in TDLNs (Figs. 2D, 3C) after irradiation with a total of 16 Gy, which is in accordance with the proposed immunologic mechanism of the abscopal effect. Our study demonstrated that 16 Gy delivered in two fractions rather than 8 Gy in a single fraction showed a statistically significant difference in infiltration of activated T cells compared with sham irradiation, implying the potential radiation dose-dependency of the abscopal effect. It is likely that higher doses may further effectively elevate levels of cytosolic DNA, triggering the cGAS/STING pathway [32,33] However, radiation doses >12–18 Gy administered in a single fraction suppressed the abscopal effect by upregulating 3′ repair exonuclease (TREX1) that degrades cytosolic DNA [30]. Further investigation would be required to determine the optimal dose-fraction required to maximize the abscopal effect.
The current study clearly showed that the anti-PD-1 antibodies enhanced the RT-induced abscopal effect (Fig. 4C) and the relevant immunologic phenomenon (Fig. 5C) in murine HCC models. In particular, the infiltration of CD8+ T cells into the non-irradiated tumor was significantly higher when anti-PD-1 antibodies were co-administered with RT than when RT was administered alone. PD-1 is an immunoinhibitory receptor mainly expressed by mature T cells, B cells, and natural killer cells [19]. It specifically binds to PD-L1 whose expression in tumor cells is mainly regulated by IFN-γ and, thus, the interaction of PD-1 with PD-L1 results in T cell exhaustion [34]. Blockade of PD-1/PD-L1 signaling restores effector T cell function to eradicate tumor cells. The antitumor activity of effector T cells can potentially be enhanced by the immunogenic effect of radiation following their coadministration, which was demonstrated by our results.
In contrast to the antitumor immunity, 16 Gy radiation also induced expansion of immunosuppressive cells, including PD-L1-expressing DCs in TDLNs. DC PD-L1 suppresses the activation of CD8+ T cells via the PD-1/PD-L1 signaling axis [35,36]. Thus, treatment with anti-PD-1 antibodies may allow DCs to reinvigorate T cells by blocking PD-1/PD-L1 interaction. Radiation also increased the infiltration of Tregs, another important immunosuppressive cell population, in both irradiated and non-irradiated tumors, which is consistent with the results of previous studies [37,38]. An increased level of Tregs is associated with an unfavorable prognosis in various cancers including ovarian, breast, and gastric cancer and HCC [39-41]. Furthermore, Tregs suppress cytotoxic T cell function with constitutive expression of CTLA-4, another immune checkpoint protein. Therefore, dual blockade of CTLA-4 and PD-1 may amplify the abscopal reaction triggered by RT in HCC.
Recent prospective clinical trials of PD-1 inhibitors such as nivolumab and pembrolizumab in HCC patients have reported overall ORRs of 17–20%, leading to their approval by the US Food and Drug Administration as second-line treatment for patients who do not respond to sorafenib [13,14]. The ORRs with PD-1 inhibitors were higher than those obtained with the standard of care, sorafenib, but they are still unsatisfactory. Furthermore, the KEYNOTE-240 phase III trial, which investigated the benefit of pembrolizumab as a second-line therapy in patients with advanced HCC, failed to show the statistically significant superiority of pembrolizumab to the best supportive care with or without placebo in overall and progression-free survival [18]. However, the overall survival of the pembrolizumab-treated group was satisfactory [18]. Therefore, there is an urgent need to intensify the treatment efficacy, and the present study suggests that combining RT and ICIs may potentially improve the unsatisfactory outcomes. Unfortunately, the relevant clinical data on RT and ICI cotreatment for advanced HCC is currently very limited. Recently, Yu et al. [42] reported that previous or concurrent application of RT or both in the course of nivolumab treatment was related to prolonged progression-free or overall survival in advanced HCC patients. Despite the limitations of the previous study, including its retrospective design and small sample size, the results are meaningful and imply the clinical significance of the RT and immunotherapy combination. Currently, there are several ongoing clinical trials investigating combinations of various ICIs and RT techniques including external beam RT and selective internal RT for advanced HCC [19]. However, trials focusing on metastatic HCC are still limited. Our findings may be extended to the design of a clinical study to evaluate the improvement of systemic disease control by mutual intensification of immunologic activation, which could contribute to developing strategies for overcoming resistance to PD-1 inhibitors in HCC.
There are some limitations in the present study. This study used a single murine HCC Hepa 1–6 cell line and, therefore, further validation is required in different HCC models. In addition, other factors associated with the tumor microenvironment, such as intrinsic tumor factors, could also be associated with the abscopal effect. There was a discrepancy between abscopal effects on the non-irradiated tumors induced by 16 Gy irradiation in addition to the size of the sham control tumors as shown in Figures 1 and 4. We speculate that the size of non-irradiated tumors at the time of irradiation may affect the abscopal effect, which needs further investigation. Although the effectiveness of cotreatment in boosting the abscopal phenomenon was established in the present study, further studies with meticulous controls would be necessary.
In conclusion, using a syngeneic murine bilateral HCC model and flow cytometric profiling of intratumor immune cells, we showed that delivering 16 Gy in two fractions induced an abscopal effect, which was boosted by cotreatment with anti-PD-1 antibodies. Based on our preclinical data, future clinical trials investigating the effect of RT and ICIs in the metastatic setting are warranted.
Notes
Authors’ contribution
WG Ahn and SY Kim performed the experiments. GS Yoo, W Kang and C Choi analyzed the data and prepared the manuscript. C Choi and HC Park designed and supervised the study. GS Yoo, C Choi and HC Park wrote the manuscript. All authors read and approved the final manuscript for publication.
Conflicts of Interest: The authors have no conflicts of interests to disclose.
Acknowledgements
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (NRF-2018R1A2B2002835).
SUPPLEMENTAL MATERIAL
Supplementary material is available at Clinical and Molecular Hepatology website (http://www.e-cmh.org).
Setup for irradiation of Hepa 1–6 tumors implanted in C57BL/6 mice. (A) A treatment plan for making 30×7 cm field size. (B) Cross-sectional dose distribution. (C) Photo image showing that tumor-bearing right hind legs were located within the irradiation field. (D) Schematic diagram illustrating position of two tumors and a tumor-draining inguinal lymph node. A primary tumor implanted into a right leg was exposed to X-ray irradiation, but the left leg harboring secondary tumor and the inguinal lymph node were not included in the radiation field.
Both radiation and anti-PD-1 increase T cell infiltration in both primary and abscopal Hepa 1–6 tumors. Representative images of IHC for CD4 and CD8 in the irradiated tumors (A) and non-irradiated tumors (C). Tumors were harvested on day 31 after tumor injection. Formalin-fixed, paraffin-embedded tumor tissues were analyzed for IHC as described in MATERIALS AND METHODS. Quantification data for CD4 and CD8 immunostaining in the irradiated tumors (B) and non-irradiated tumors (D). IgG, immunoglobulin G; PD-1, programmed cell death 1; IHC, immunohistochemistry. *P<0.05. †P<0.01. ‡P<0.001.
Combination of anti-PD-1 and radiation increases increases the infiltration of total CD3+ T cells but no other immune cells into both irradiated and non-irradiated tumors. Flow cytometric analysis revealed that radiation alone or in combination with anti-PD-1 significantly increased the infiltration of CD3+ T cells into irradiated (A) and non-irradiated tumors (B). Other immune cells including B cells, macrophages, M-MDSC, and G-MDSC were not changed. On day 31 after tumor injection, the mice bearing Hepa 1–6 tumors were euthanized, and the population of immune cells in the tumors was determined by flow cytometry using antibodies recognizing markers for specific immune cell populations, as described in MATERIALS AND METHODS. B cell, CD220+; T cells, CD3+; macrophage, F4/80+; neutrophil, Ly6G+CD11b+; M-MDSC, Ly6G–CD11b+Ly6C+; G-MDSC, Ly6G+CD11b+Ly6Clow. IgG, immunoglobulin G; PD-1, programmed cell death 1; M-MDSC, monocytic-myeloid-derived suppressor cells; G-MDSC, granulocytic-myeloid-derived suppressor cells. *P<0.05. †P<0.01.
Abbreviations
cGAS
cyclic guanosine monophosphate-adenosine monophosphate synthase
CTLA-4
cytotoxic T lymphocyte-associated protein-4
DC
dendritic cell
HCC
hepatocellular carcinoma
ICI
immune checkpoint inhibitor
IFN
interferon
MDSCs
myeloid-derived suppressor cells
ORRs
objective response rates
PD-1
programmed cell death 1
PD-L1
programmed cell death ligand 1
RT
radiation therapy
STING
stimulator of interferon gene
TDLNs
tumor-draining lymph nodes
References
Article information Continued
Notes
Study Highlights
In this study, a syngeneic HCC mouse model was established by transplanting murine Hepa 1–6 HCC cells into both hind legs of immunocompetent C57BL/6 mice. The tumors on the right hind legs were irradiated, and abscopal effects were observed in the non-irradiated tumors on the left hind leg with or without the coadministration of anti-PD-1 antibodies. We observed that the coadministration of anti-PD-1 antibodies significantly enhanced the abscopal effect and increased infiltration of activated cytotoxic T cells in both irradiated and non-irradiated tumors.