The role of the gut microbiome and diet in the pathogenesis of non-alcoholic fatty liver disease
Article information
Abstract
Non-alcoholic fatty liver disease (NAFLD) is the leading cause of chronic liver disease, with a prevalence that is increasing in parallel with the global rise in obesity and type 2 diabetes mellitus. The pathogenesis of NAFLD is complex and multifactorial, involving environmental, genetic and metabolic factors. The role of the diet and the gut microbiome is gaining interest as a significant factor in NAFLD pathogenesis. Dietary factors induce alterations in the composition of the gut microbiome (dysbiosis), commonly reflected by a reduction of the beneficial species and an increase in pathogenic microbiota. Due to the close relationship between the gut and liver, altering the gut microbiome can affect liver functions; promoting hepatic steatosis and inflammation. This review summarises the current evidence supporting an association between NAFLD and the gut microbiome and dietary factors. The review also explores potential underlying mechanisms underpinning these associations and whether manipulation of the gut microbiome is a potential therapeutic strategy to prevent or treat NAFLD.
INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD) is a major cause of chronic liver disease worldwide [1,2]. Recently it has become evident that NAFLD not only increases the risk of chronic liver disease and primary liver cancer, but NAFLD has important implications for the development of diseases beyond the liver, such as type 2 diabetes mellitus (T2DM), cardiovascular disease and chronic kidney disease [3-5]. The prevalence of NAFLD is increasing in parallel with the global rise in obesity and T2DM [6]. NAFLD represents a spectrum of liver disease severity, beginning with the accumulation of triacylglycerols in the liver (steatosis). Almost a quarter of individuals with steatosis develop liver inflammation and progress to non-alcoholic steatohepatitis (NASH). NASH is a potentially progressive liver condition and with ongoing liver injury and cell death can result in fibrosis and cirrhosis.
The pathogenesis of NAFLD is complex and multifactorial, involving environmental, genetic and metabolic factors. Given the well-known association between obesity and NAFLD, in recent years there has been a growing interest in the role of diet and the gut microbiome in the pathogenesis of NAFLD. The gut-liver axis is defined by the strong anatomical and functional interactions between the gastrointestinal tract and the liver. An important element of this axis is the gut microbiome, which is involved in host nutrient metabolism, maintenance of structural integrity of the gut mucosal barrier and immunomodulation [7]. Disturbance of the gut microbiome has been implicated in many disease processes, particularly those in which the gut-liver axis plays an important role, such as NAFLD. Similarly, dietary antigens have a major influence on the gut-liver axis, including modification of the gut microbiome, and therefore are implicated in the pathogenesis of NAFLD. This review summarises the current evidence supporting an association between NAFLD and the gut microbiome and dietary factors. The review also explores potential underlying mechanisms underpinning these associations and whether manipulation of the gut microbiome is a potential therapeutic strategy to prevent or treat NAFLD.
GUT MICROBIOME
The intestinal lumen is naturally colonized by trillions of microorganisms from more than 1,000 species including bacteria, protozoa, archaea, fungi, and viruses [7]. Advances in culture‐independent microbiologic technology over the last decade have facilitated the characterisation of the composition and diversity of the bacterial component of the gut microbiome. Although the profound variability between individuals’ gut microbiome complicates attempts to define what is ‘normal’ microbiota, the most common bacterial phyla found in the faeces of healthy subjects are; Bacteroidetes (65.2%), Firmicutes (29.6%), Proteobacteria (2.9%), and Actinobacteria (0.5%) [8]. Early life plays an important role in establishing the gut microbiome, with both vaginal delivery (vs. caesarean section) and breastmilk (vs. formula milk) demonstrating beneficial effects on the gut microbiome composition [9,10]. Early microbiota perturbation can lead to long-term deranged metabolic phenotypes, including NAFLD and obesity [10,11]. Later in life, the gut microbiome composition is dependent on many factors including; genetics, age, diet and medications [12]. Ageing is associated with alterations in the gut microbiome composition and reduced phylogenetic diversity, which has been postulated to partly underlie the pathogenesis and progression of various metabolic diseases that are prevalent in old people such as adiposity, insulin resistance, and NAFLD [13]. Figure 1 shows the proportions of the phylum, class and genus of bacteria commonly found in the health gut [8,12,14-16].
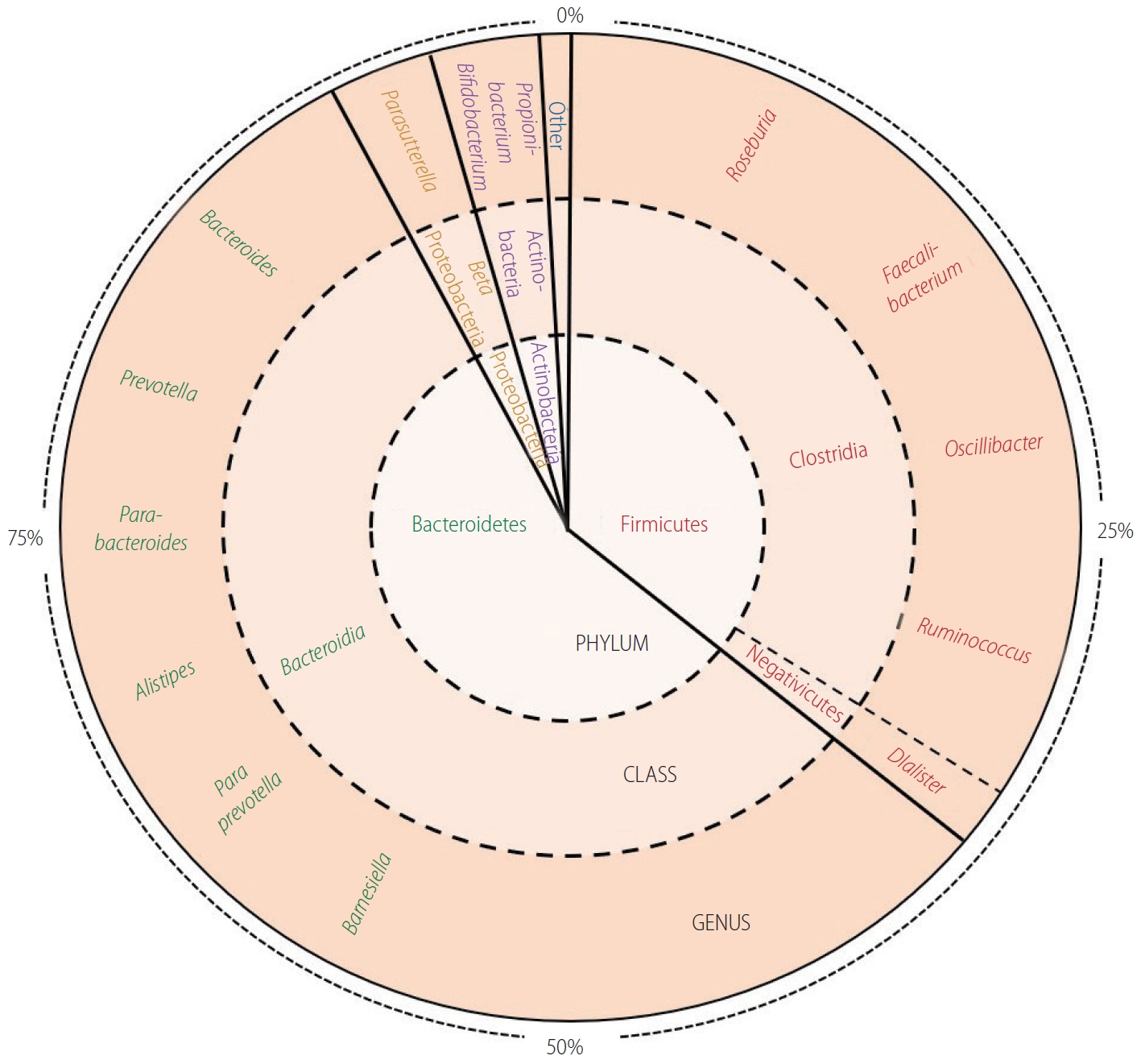
Represents the proportions of the phylum, class and genus of bacteria commonly found in the health gut [8]. Bacteroidetes comprise the majority phylum of the gut microbiome, of which the majority genus is Bacteroides. Firmicutes consists of predominant genera such as Faecalibacterium, Roseburia, and Oscillibacter. The Proteobacteria phylum is proportionally less abundant and mainly represented by the Parasutterella genus. Actinobacteria, such as Propionibacterium and Bifidobacterium, are found in small numbers in the healthy gut.
EVIDENCE FOR A ROLE OF DYSBIOSIS IN THE PATHOGENESIS OF NAFLD
The term dysbiosis refers to disruption of the normal gut microbiome that is associated with pathology within the host. Dysbiosis has been linked to several aspects of the metabolic syndrome, including NAFLD [17,18]. In NAFLD, early evidence linking gut dysbiosis with liver injury came from human studies showing an association between NASH and small intestinal bacterial overgrowth [19]. More recent evidence, from both animal and human studies, indicates that microbial populations are altered in patients with NAFLD.
Several animal studies have demonstrated that dysbiosis is associated with more severe hepatic steatosis and hepatic inflammation; these findings are summarised in Table 1 [20-23]. Even cohousing non-dysbiotic mice with dysbiotic mice can lead to transfer of gut microbes and a subsequent exacerbation of NAFLD [21]. Although dysbiosis is harmful, the presence of a healthy gut microbiome in animals has been shown to be protective against the development of NAFLD. For example, Cano et al. [23] demonstrated that inducing beneficial changes to the gut microbiome with the probiotic Bifidobacterium pseudocatenulatum, reduced the risk of developing NAFLD in mice and Mazagova et al. [24] found that germ-free mice developed more severe experimental liver fibrosis compared to conventional mice, demonstrating that the presence of commensal gut microbes is hepatoprotective.

The design and main findings of animal studies investigating the role of the gut microbiome in the pathogenesis of NAFLD
Several studies have explored the composition of the gut microbiome among cohorts of human subjects with varying stages of NAFLD [25-34]. Table 2 summarises the overall characteristics of these studies and provides details regarding the specific microbiologic results. Despite the variability in study design, methods, and clinical endpoints, these human studies demonstrate measurable differences in the gut microbiome between healthy controls and individuals with hepatic steatosis and NASH. The gut microbiome composition has also be found to vary according to the severity and stage of NAFLD [30,35,36]. Loomba et al. [30] found distinct changes in the gut microbiome composition between Individuals with advanced fibrosis (stage 3 or 4) and those with mild fibrosis (stage 1 or 2). The authors went on to suggest that a faecal-microbiome-derived metagenomic signature could be used as an adjunct tool to current invasive approaches to determine the stage of liver disease in NAFLD.
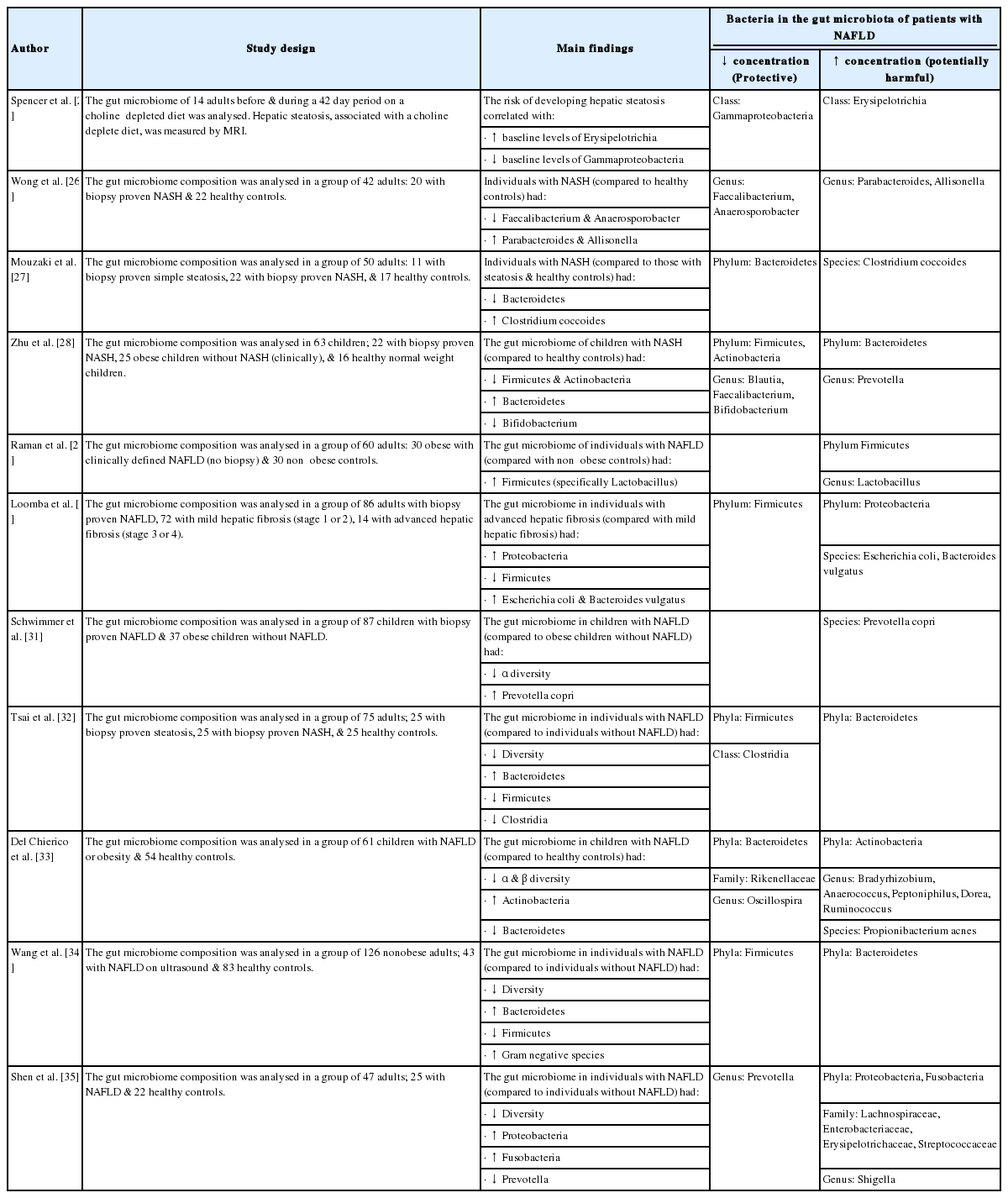
The design and main findings of human studies investigating the role of the gut microbiome in the pathogenesis of NAFLD
The findings in both animal and human studies are inconsistent and sometimes conflicting, and it is still unclear which specific microorganisms are harmful or protective. This may in part be due to the heterogeneous cohorts used across studies in addition to the varying gut microbiome sequencing technologies that were used. More work is needed, particularly longitudinal human studies, to delve further into the significance of gut microbiome alterations in NAFLD, especially if analyses of the gut microbiome is to become part of clinical practice.
POTENTIAL MECHANISMS LINKING DIET, DYSBIOSIS AND NAFLD
Role of short chain fatty acids (SCFAs) in metabolic and inflammatory pathways
SCFAs, such as acetic, propionic and butyric acid, are generated through the fermentation of polysaccharides by gut microbes in the large bowel. The diet and composition of the gut microbiome impacts the quantity and type of SCFAs synthesized in the gut. Diets high in fibre, particularly from plant-based foods or a Mediterranean diet, are associated with increased levels of faecal SCFAs [37,38]. Altering the gut microbiome with prebiotics and probiotics that promote the growth of beneficial microbiota can induce changes in SCFA production [39,40]. SCFAs have a role in inflammation, lipid and glucose metabolism and regulation of energy harvested from the diet [41]. As the effects of SCFAs are so diverse and widespread, elucidating the overall impact has been difficult. While animal studies have shown diets high in SCFAs to have favorable metabolic affects (reduced hepatic cholesterol and fatty acid synthesis and increased lipid oxidation), human studies have not been so clear [42,43].
SCFAs are a major source of energy, although most SCFAs are utilized in the gut, some are transported through the portal vein and channelled into the tricarboxylic acid cycle or utilised for hepatic gluconeogenesis or lipogenesis. Thus, changes in the gut microbiome that favor SCFA production can increase energy delivery to the liver and reduce faecal energy loss. The first evidence to support this was provided by Turnbaugh et al. [44] who found that, compared to their lean littermates, obese mice had more carbohydrate metabolising genes in their gut microbiome, increased concentration of SCFAs in their caecum, and less energy in their stool. Furthermore, faecal microbial transplant (FMT) from obese mice to germ free mice caused greater fat gain than FMT from lean animals. In humans, increased production of SCFAs by the gut microbiome was also observed in obese people, compared to lean subjects [45].
SCFAs can alter lipid and glucose metabolic pathways through activation of G-coupled receptors. Kimura et al. [46] showed that through activation of G-protein receptor-43, SCFAs suppress insulin signalling in adipocytes, which inhibits fat accumulation in adipose tissue and promotes the metabolism of unincorporated lipids and glucose in the liver and other tissues. The intestinotrophic effects of SCFA were first proposed by Koruda et al. [47] who found that SCFA supplementation to rats having parenteral nutrition prevented associated mucosal atrophy. Beneficial effects of SCFA on the intestinal mucosa are thought to be mediated through glucagon-like-peptide-2 (GLP-2), as SCFA supplementation induces the expression of ileal proglucagon mRNA and plasma GLP-2 [48]. In mice, increasing GLP-2 levels, by microbial intervention or subcutaneous GLP-2 administration, reduces intestinal permeability, which leads to lower plasma lipopolysaccharide (LPS) and cytokines levels, and consequently reduced hepatic oxidative stress and inflammation [49].
SCFA supplementation has shown beneficial effects on several inflammatory conditions including asthma, arthritis and colitis [50,51]. The anti-inflammatory effects of SCFA are thought to be mediated through activation of G-protein coupled receptor-43. Maslowski et al. [50] induced colitis in mice and demonstrated that inflammation is more severe in germ-free mice than in those conventionally raised. The colitis was ameliorated by acetate supplementation, a finding that was absent in G-protein coupled receptor-43 knock out mice [50]. By suppressing colitis, SCFAs can improve gut permeability and therefore reduce hepatic delivery of harmful microbial cell components and metabolites. Conversely, Rau et al. [52] found that patients with NAFLD had higher concentrations of intestinal acetate and propionate, which correlated with peripheral levels of pro-inflammatory T-cells. Therefore the role on SCFAs in inflammatory pathways within NAFLD is still controversial.
Bacteria-derived ethanol
Histologically, NAFLD and alcohol-induced liver injury are remarkably similar, and are therefore likely to share common pathogenic pathways [53]. Some microbiota harbour genes that can ferment dietary sugars into ethanol. The amount of alcohol produced depends on the availability of carbohydrates from the diet and the composition of the gut microbiome, particularly the presence of Proteobacteria (especially Klebsiella pneumoniae and Escherichia coli) [28,54]. Animal studies have demonstrated that obese mice with NASH have higher early-morning breath alcohol content compared with lean mice without NASH; which is eliminated by neomycin treatment [55]. In human studies, patients with NAFLD, particularly children, have been shown to have increased blood ethanol levels [28,56,57]. Yuan et al. [54] found high-alcohol-producing Klebsiella pneumoniae in the gut microbiome of up to 60% of individuals with NAFLD in a Chinese cohort. The study went on to show that transfer of high-alcohol-producing Klebsiella pneumoniae, by oral gavage or FMT, into healthy mice induced NAFLD [54]. Gut-derived ethanol is not only directly hepatotoxic but it may alter the gut-liver axis, by increasing intestinal permeability and endotoxemia, to compound hepatic damage [58].
Role of choline deficiency in lipid metabolism and inflammation
Choline is an essential nutrient sourced from foods such as meat and eggs. Choline deficiency induces many features of NAFLD and is often utilized in studies to create animal models of NAFLD. Choline deficiency leads to impaired synthesis of phosphatidylcholine resulting in diminished very low density lipoprotein assembly and secretion and consequently reduced hepatic triglyceride clearance [59]. In addition to hepatic steatosis, choline deficient mice also develop hepatic inflammation and fibrosis, which is thought to be caused by impaired mitochondrial β-oxidation and increased oxidative stress [60,61].
The gut microbiome plays an important role in choline metabolism and therefore regulates the concentration that is delivered to the liver via the portal circulation. Dysbiosis, and in particular an excess of the class Erysipelotrichia, has been associated with choline depletion in both animal and human studies [25,62,63]. Choline is metabolised by the gut microbiome to generate methylamines such as trimethylamine (TMA), which is converted in the liver to TMA-N-oxide (TMAO) by flavin-containing monooxygenase-3 (FMO3). TMAO has adverse effects on glucose homeostasis and is implicated in atherosclerosis [62]. NAFLD is associated with lower levels of choline and higher levels of TMA in the blood, implicating the role of gut microbiota in the imbalance of choline metabolism [63]. Around 10–15% of bacterial species require choline to synthesize phosphatidylcholine, a component of their membrane; therefore in the context of bacterial overgrowth, the demand for choline may increase and contribute to choline deficiency [64,65].
Lipopolysaccharide-induced hepatic inflammation
LPS, a constituent of gram negative bacteria, are found in the systemic circulation of individuals with NAFLD, and correlate with the severity of steatohepatitis in both human and animal studies [66-69]. The concentration of LPS in the gut microbiome and plasma is increased by the consumption of a western diet, high in saturated fat and low in fibre [70,71]. It has been hypothesised that a high-fat diet may facilitate LPS uptake through elevated chylomicron production in intestinal epithelial cells [72]. In mice on a standard diet, continuous subcutaneous infusion of low-dose LPS results in hepatic steatosis and hepatic insulin resistance [73,74]. LPS interact with toll-like receptors (TLRs), particularly TLR-4, on hepatic Kupffer cells and stellate cells to stimulate pro-inflammatory and profibrotic pathways via a range of cytokines, including interleukin (IL)-1, IL-6, and tumour necrosis factor (TNF) [75-78]. TLR-4‐deficient mice display decreased liver injury, inflammation, and lipid accumulation in comparison with wild‐type mice in NAFLD models [79,80]. TLR signalling in the mucosa also lead to the production of inflammasomes (cytosolic multiprotein oligomers of the innate immune system) which initiate a variety of pathways to produce proinflammatory and pro-fibrotic mediators such as caspase-1, IL-1β, and IL-18 [81,82]. In support of a role for inflammasomes in the development of more severe liver disease in NAFLD, Wree et al. [83] found significantly higher levels of the inflammasome NLRP3 (NOD-, LRR- and pyrin domain-containing protein 3) in the livers of individuals with NASH compared to those with simple steatosis. Conversely, other studies have shown that the absence of inflammasomes is associated with more aggressive disease. Pierantonelli et al. [84] demonstrated that, in a Western lifestyle model, the combination between high-fat and high-carbohydrate diet and the lack of the NLRP3-inflammasome increased the degree of liver injury and was associated with an abundance of gram-negative Proteobacteria and Verrucomicrobia (mucus-degrading bacteria that promote bacterial translocation), higher intestinal bacterial translocation, increased TLR activation and a more severe degree of liver injury.
The role of bile acids (Bas) in lipid metabolism and gut microbiome modulation
BAs have been implicated in the pathogenesis of NAFLD through their role in lipid metabolism and as signalling molecules via the farnesoid X receptor (FXR). By binding to the FXR, BAs can increase insulin sensitivity and decrease hepatic gluconeogenesis and circulating triglyceride concentrations [85]. Obeticholic acid, an FXR agonist, has been shown to improve hepatic histology in ‘The Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT)’ trial [86]. However, results with this FXR agonist were less promising in a planned interim analysis of a further large trial in patients with NASH, where treatment with obeticholic acid failed to produce resolution of NASH (compared to placebo), although treatment with obeticholic acid 25 mg/day significantly improved liver fibrosis [87].
BAs and the gut microbiome have a bi-directional relationship and are both highly influenced by the diet. Dietary fat content regulates BA synthesis; in particular a high-fat diet increases production, while a low-fat diet reduces production [88,89]. The gut microbiome regulates BA homeostasis through a number of mechanisms including dihydroxylation of primary BAs to secondary BAs. BAs reciprocally regulate the gut microbiome, both directly via antibacterial activity (particularly deoxycholic acid) and indirectly via FXR induced antimicrobial peptides. The gut microbiome and BAs indirectly interact through TMAO, a product of gut microbiome choline metabolism. TMAO inhibits two key enzymes involved in BA metabolism: CYP7A1 and CYP27A1, therefore reducing the overall BA pool size [90]. Furthermore, FXR activation can regulate FMO3 activity and therefore TMAO production. Bennet et al. [91] demonstrated that FXR ligands administered to wild-type mice could induce FMO3 expression and increase TMAO levels, but this effect was abrogated in FXR knock out mice.
Several studies have demonstrated that the interaction of BAs with the gut microbiome plays an important role in NAFLD pathogenesis. Parséus et al. [92] fed germ-free and conventionally raised wild-type and FXR knock-out mice a high-fat diet for 10 weeks. The gut microbiome promoted weight gain and hepatic steatosis in an FXR-dependent manner, and the BA profiles and gut microbiome composition differed between FXR knock-out and wild-type mice [92]. These findings were supported by Jiang et al. [93] who found that improvements in hepatic steatosis related to antibiotic treatment were dependent on FXR signalling.
Intestinal permeability induced hepatic inflammation
The portal vein exposes the liver to potentially harmful substances derived from the gut, including translocated bacteria and toxic bacterial products such as LPS. Dysbiosis can disrupt the integrity of the mucosal wall, increasing mucosal permeability. Dietary factors have an important role in the maintenance of the intestinal mucosal barrier, and not just through their role in altering the gut microbiome composition. Dietary depletion of glutamine, tryptophan and zinc or excess ingestion of fat, alcohol and food additives, have been directly associated with increased intestinal permeability [94-98].
Animal studies have shown convincing evidence that increased intestinal permeability leads to translocation of bacteria and bacterial toxins, such as LPS, that worsen the severity of NASH [66,73]. Miele et al. [99] found that patients with NAFLD had significantly increased gut permeability (as measured by urinary excretion of 51 chromium-radiolabeled ethylenediaminetetraacetic acid) compared to healthy volunteers. Furthermore, in patients with NAFLD both gut permeability and the prevalence of small intestinal bacterial overgrowth correlated with severity of steatosis, although not with steatohepatitis [99]. Verdam et al. [100] found plasma immunoglobulin G levels against endotoxin were increased in biopsyproven human NASH and positively correlated with the severity of inflammation. Conversely, Yuan et al. [101] found that in paediatric patients with NASH, serum endotoxin levels were not correlated with disease severity, however peripheral endotoxin levels may not represent the concentration in the portal system, and the pathogenesis of NAFLD in children may be different to adults.
Adipose tissue metabolism and inflammation
Adipose tissue expansion, dysfunction, and inflammation are hallmarks of obesity and play a critical role in the development of NAFLD. Through several mechanisms the composition of the gut microbiome is thought to alter the metabolism and function of adipose tissue. LPS and other gut microbiome derived TLR ligands have been shown to contribute to adipose tissue inflammation. Caesar et al. [102] showed that mice fed a lard diet had increased TLR signalling and white adipose tissue inflammation compared with mice fed an isocaloric fish oil diet. In addition, mice genetically deficient in various components of the TLR signalling pathway were protected against white adipose tissue inflammation. de Groot et al. [103] demonstrated that FMT from a donor after Rouxen-Y bariatric surgery, to a host with metabolic syndrome led to a reduction in the adipocyte inflammatory marker chemokine ligand-2 gene expression in adipose tissue and circulating levels in plasma.
SCFAs, in particular propionate, have been demonstrated to regulate adipose tissue metabolism, both directly through modulation of the transcription factor peroxisome proliferator-activated receptor-γ, and indirectly through activation of the sympathetic nervous system [43,104]. The gut microbiome composition alters adipose tissue thermogenesis, including brown adipose tissue activity and browning of white adipose tissue. Schugar et al. [105] demonstrated that in mice the genetic deletion of the TMAO-producing enzyme FMO3 protected against high-fat diet induced obesity, in part by stimulating the beiging and enhanced thermogenesis of white adipose tissue.
DIET AND NAFLD
Dietary factors can impact the development of NAFLD via their critical role in the gut-liver axis. A consistent finding from studies that have examined the link between diet and NAFLD is that a hypercaloric diet, regardless of whether the excess calories have been provided either as fat, sugar, or both, increases liver fat content [106]. Figure 2 summarises the effects of dietary factors on the gut microbiome and their effects on hepatic pathways leading to the development of hepatic steatosis, inflammation and fibrosis.

A summary of the effect of dietary factors on the gut microbiome and their effects on hepatic pathways leading to the development of hepatic steatosis, inflammation and fibrosis. Green tea, caffeine, coffee, a Mediterranean diet and some polyunsaturated fatty acids, such as omega-3, have favorable effects on the composition of the gut microbiome. Consumption of saturated fatty acids, fructose and advanced glycated end products cause harmful changes to the gut microbiome composition. Dysbiosis is associated with altered production of SCFA, altered choline and bile acid metabolism, higher abundance of LPS containing bacteria, increased bacterial derived ethanol, increased intestinal permeability and upregulation of inflammatory processes. The harmful consequences of dysbiosis affect normal liver physiology, particularly given the close relationship between the gut and liver. Hepatic lipogenesis and triglyceride storage are upregulated whilst lipid oxidation is reduced, leading to hepatic steatosis. Activation of hepatic TLR (e.g., TLR-4) and the generation of ROS drives hepatic inflammation and fibrosis. LPS, lower plasma lipopolysaccharide; SCFA, short chain fatty acid; TMA, trimethylamine; TG, triglycerides; TLR, toll-like receptor; ROS, reactive oxygen species.
Fat
Increased dietary fat intake, particularly saturated fat, is associated with the development of hepatic steatosis [107-109]. A high fat diet can alter the gut microbiome rapidly, decreasing the microbial diversity and favoring gut bacteria associated with the development of NAFLD [110]. Changes associated with a high fat diet include reduced levels of the Bifidobacterium genus and an increase in the ratio of Firmicutes to Bacteroidetes phyla [73]. Ingestion of long-chained saturated fats, compared to short-chained unsaturated fats, are associated with a more pronounced reduction in phylogenetic diversity and beneficial bacteria [102]. The mechanisms by which dietary fatty acids affect the gut microbiome are not well defined. Only a minority of fatty acids will pass through the gastrointestinal tract and directly modulate gut microbiome composition. Fatty acids have a broad spectrum of antibacterial activity including lysis of bacterial cell membranes and inhibition of bacterial adenosine triphosphate (ATP) production [111,112], and the antibacterial action of fatty acids is affected by carbon chain length, saturation and double bond position [113].
Changes in the gut microbiome caused by a high fat diet are associated with; increased energy harvest from the gut, upregulation of genes related to lipid metabolism in the distal small bowel and the production of SCFAs favoring the development of NASH [73,102]. High fat diets in animals, particularly those involving long-chained saturated fatty acids, are accompanied by increased intestinal permeability, resulting in bacterial translocation and elevated LPS levels [73]. Inflammatory processes are also upregulated, with increased hepatic TLR-4 activation and a rise in inflammatory mediators including TNF-α, IL-1, and plasminogen activator inhibitor-1 [73,102].
Despite the association between a high fat diet and the development of NAFLD and dysbiosis, some fatty acids have demonstrated favorable effects on the gut microbiome and the development of hepatic steatosis. In a randomized controlled trial (RCT), Scorletti et al. [114] demonstrated that consumption of the omega-3 polyunsaturated fatty acid, docosahexaenoic acid, was independently associated with a decrease in liver fat percentage in patients with NAFLD. The ingestion of omega-3 polyunsaturated fatty acids and oleic acid have been shown to protect against high-fat diet induced dysbiosis and improve the composition of the gut microbiome; including increased abundance of butyrateproducing bacteria and the Bifidobacterium genus [115-117].
Fructose
Dietary fructose has been strongly implicated in the pathogenesis of NAFLD. A large association study demonstrated an increased risk of NAFLD in those consuming regular sugary drinks, especially if overweight [118]. Fructose is both a substrate and an inducer of hepatic de novo lipogenesis. In individuals with NAFLD, 26% of hepatic triglycerides are produced by de novo lipogenesis using fructose and other dietary sugars as substrates [119]. Fructose increases hepatic lipogenesis by activating several key transcription factors such as sterol response element-binding protein-1c and carbohydrate-responsive element-binding protein [120]. Dietary fructose can induce rapid and harmful changes in the gut microbiome composition, including a reduction in the phylogenetic diversity and a lower concentration of Bifidobacterium genus [121]. Fructose induced dysbiosis has been demonstrated to increase intestinal macrophage counts and lower tight junction occludin protein expression, associated with worse endotoxaemia, more bacterial translocation and increased hepatic TLR expression [122].
During hepatic fructose metabolism by fructokinase, ATP is rapidly consumed, which results in the breakdown of adenosine monophosphate (AMP) to inosine monophosphate and the generation of uric acid. Uric acid therefore increases in the plasma and liver with fructose consumption, and may mediate some of the adverse effects associated with fructose [123]. Uric acid can exacerbate hepatic insulin resistance through activation of retinol binding protein-4 [123], impair hepatic fatty acid oxidation through inhibition of AMP-activated protein kinase, and induce hepatic oxidative stress through NADPH oxidase activation [124]. Xanthine oxidase inhibitors that block uric acid generation, have been shown to inhibit fructose induced hepatic steatosis [125], and serum uric acid concentrations and a high dietary fructose consumption are both independently associated with NASH in children [126].
Advanced glycated end products (AGEs)
AGEs are formed in food when reducing sugars react non-enzymatically with the amino groups on proteins. The concentration of AGEs is high in western diets and contributes to tissue injury via activation of receptor for AGEs (RAGEs) and generation of reactive oxygen species. RAGEs have been found on hepatic stellate cells and stimulation has been shown to exacerbated liver inflammation and increase hepatic proliferation and expression of collagen in animal models of NAFLD [127]. The absorption of dietary AGEs is limited, the majority pass through the gastrointestinal tract to the colon, where they can act as substrates for the gut microbiome [128]. Several animal and human studies have shown that the consumption of AGEs is associated with a compositional change in the gut microbiome and altered production of SCFAs, however there is a lack of agreement between studies on the specific microbial changes, which may be due to the different glycated substrates used [128].
Mediterranean diet
A Mediterranean diet is characterised by increased consumption of vegetables, legumes, fruits, nuts, olive oil and fish and low consumption of red meat, dairy products and saturated fats. A weight neutral Mediterranean diet can reduce liver steatosis and may improve insulin sensitivity [129,130]. A Mediterranean diet contains a high concentration of monounsaturated fatty acids and a balanced polyunsaturated fatty acid omega-6 to omega-3 ratio, which has favorable effects on hepatic lipid metabolism [131]. The high levels of polyphenols, carotenoids, vitamin E and vitamin D are protective against the development of NAFLD through their antioxidant and immunomodulatory properties [132-135]. Some of the hepatoprotective effects of a Mediterranean diet may be mediated through beneficial changes in the gut microbiome composition, including; increased levels of the Bifidobacterium genus, reduced levels of gram-negative LPS containing bacteria, altered SCFA production and reduced toxic metabolites such as TMAO [37,136].
Noncaloric artificial sweeteners (NCS)
NCS are widely used by patients with the metabolic syndrome; however their safety and side effect profile remains a topic of controversy. The use of NCS have been linked to the pathogenesis of obesity, T2DM and NAFLD through a number of postulated mechanisms. In an animal study the use of NCS weakened the ability of sweet taste to predict energy and evoke autonomic and endocrine learned responses, such as the cephalic response, that prepares the digestive tract to optimally deal with ingested food [137]. Other animal studies have shown that NCS interact with sweet taste receptors expressed in enteroendocrine cells and increase intestinal glucose absorption (through sodium-dependent glucose transporter isoform 1 and GLUT-2) leading to obesity, hyperinsulinemia and insulin resistance [138,139].
Many NCS are associated with changes in the composition of the gut microbiome, while others have shown no effect [140]. Suez et al. [141] found that saccharin intake in mice was associated with reductions in intestinal Akkermansia muciniphila, a commensal bacterium that exhibits probiotic properties. These mice developed impaired glucose tolerance, an effect that was abrogated by antibiotic treatment, and fully transferrable by FMT. Trocho et al. [142] found that aspartame accumulates in the liver of both healthy and cirrhotic rats and might increase the risk of NAFLD via mitochondrial dysfunction and ATP depletion in the liver. Conversely, other animal studies have shown that NCS are not associated with hepatic steatosis, and some, such as xylooligosaccaride, may actually be protective [143].
Green tea
Green tea and its polyphenols, such as epigallocatechin gallate, have demonstrated protective effects against the development of NAFLD. The consumption of green tea in mouse models of NAFLD has led to reductions in; hepatic steatosis, hepatic inflammation, hepatic fibrosis and insulin resistance [144-147]. There are various mechanisms by which green tea may exert these beneficial effects. Consumption of green tea has been found to restore the changes in gut microbiome composition, such as the Firmicutes to Bacteroidetes ratio, which are associated with the development of obesity, hepatic steatosis and insulin resistance [148]. Several studies have also demonstrated that green tea has anti-inflammatory effects, possibly through interaction with the 67-kDa laminin receptor [149]. Green tea can also act as an anti-oxidant, directly by scavenging reactive oxygen and nitrogen species [150], and indirectly by upregulating the transcription of genes related to the cellular antioxidant defence [151]. In a placebo-controlled RCT, green tea consumption was associated with significantly reduced alanine transaminase and aspartate transaminase levels after 12 weeks, in individuals with NAFLD [152]. In contrast, a cross-sectional study of 1,024 Japanese men did not find an association between green tea consumption (≥3 cups of green tea a day) and hepatic steatosis diagnosed by ultrasonography [153].
Caffeine and coffee
There is evidence suggesting that caffeine and coffee may be protective against the development of several elements of the metabolic syndrome, including NAFLD [154,155]. A large meta-analysis found both caffeinated and decaffeinated coffee consumption to be inversely associated with the risk of T2DM in a dose-response manner [156]. The mechanism of action is thought to be largely mediated by an ester of caffeic acid named chlorogenic acid, which improves glucose metabolism by inhibiting gut absorption of glucose and hepatic gluconeogenesis [157]. Although chlorogenic acid is not specific to coffee and occurs in other food stuffs another meta-analysis found a significantly decreased risk of both developing NAFLD and it progressing to fibrosis in individuals who drank coffee on a regular basis [158]. Caffeine is thought to have antifibrotic properties, through antagonism of adenosine receptor A2a, which inhibits hepatic stellate cells [159]. Caffeine also leads to more rapid gut transit and decreased energy harvest from the diet, in part through reducing the expression of aquaportin-8, a water channel protein expressed in the intestinal mucosa [160]. Consumption of coffee can alter the gut microbiome composition, in healthy volunteers and consumption of three cups of instant coffee per day was associated with an increase in the quantity and metabolic activity of Bifidobacterium, a genus of bacteria thought to be protective against the development of NALFD [161].
LINKING SEX DIFFERENCES IN NAFLD TO THE GUT MICROBIOME
The prevalence and severity of NAFLD is higher in men than in women during the reproductive age. After the menopause, NAFLD occurs at a higher rate in women, suggesting that oestrogen is protective [162-166]. Several possible mechanisms for sexual dimorphism in NAFLD have been proposed, including; altered distribution of fat, differences in mitochondria functioning and variation of gene expression in key organs that determine insulin sensitivity [167,168]. Variation in several aspects of the gut-liver axis between men and women may also be responsible. The physiological differences in the gut microbiome composition between sexes are modest, but some researchers have found variations in gut microbiome metabolic activity and in several of the pathways by which the gut microbiome is thought to influence NAFLD pathogenesis.
Oestrogen has an influence on the composition of the gut microbiome. Dietary administration of oestrogen-like compounds can promote the proliferation and growth of certain types of gut bacteria, for example the consumption of soy phytoestrogen can increase the concentration of Bifidobacterium [169,170]. Some bacteria can metabolise oestrogen-like compounds to produce more biologically active forms that have high affinity for human oestrogen receptors [171]. For example, some gut bacteria can metabolize phytoestrogens into O-desmethylangolensin (ODMA) and equol, which are structurally similar to mammalian oestrogen [172]. These metabolites have beneficial metabolic effects; ODMA producing individuals are leaner, and Equol supplementation improves glycaemic control and lowers low-density lipoprotein cholesterol [170,172,173].
Sex hormones strongly affect BA profiles and significant gender-specific differences become more prominent in response to a high-fat high-sugar diet [174]. Jena et al. [175] demonstrated that male mice fed a high-fat western diet, develop more severe hepatic inflammation, hepatic steatosis and insulin resistance compared to female mice, in an FXR dependent manner. Xie et al. [176] investigated the mechanistic link between the gut microbiome and hepatocellular carcinoma (HCC) in NAFLD using a streptozotocin-high fat diet (STZ-HFD) induced NASH-HCC murine model and compared results for both sexes. STZ-HFD feeding induced a much higher incidence of HCC in male mice with substantially increased intrahepatic retention of hydrophobic BAs and decreased hepatic expression of tumour-suppressive microRNAs. Metagenomic analysis showed differences in the gut microbiome involved in BA metabolism between normal male and female mice, and such differences were amplified when mice of both sexes were exposed to STZ-HFD. Treating STZ-HFD male mice with 2% cholestyramine led to significant improvement of hepatic BA retention, tumour-suppressive microRNA expressions, microbial gut communities, and prevention of HCC.
THE GUT MICROBIOME AS A THERAPEUTIC TARGET IN NAFLD
Despite the significant rising epidemic of NAFLD, no pharmacological interventions are currently specifically licensed for its treatment. Modifications in lifestyle such as a low-calorie, low-fat, low-glycaemic index diet and increased physical activity, are the only reliable treatment options shown to reverse the early histologic damage caused by NAFLD. Pharmacologic options, such as metformin, vitamin E, omega-3 fatty acids, ursodeoxycholic acid and lipid lowering drugs, have all been studied in patients with various stages of NAFLD, with variable results. Due to poor patient compliance with lifestyle interventions, and given the importance of the gut-liver axis in the pathogenesis of NAFLD, the effect of manipulating the gut microbiome has attracted considerable recent research interest. Through altering the gut microbiome composition, this therapeutic strategy has been postulated to; improve intestinal barrier function, prevent bacterial translocation, decrease the prevalence of LPS containing bacteria, decrease the production of harmful bacterial products, and reduce overall inflammation [177].
Mechanisms for altering the gut microbiome include antibiotics, FMT or probiotics, pre-biotics and synbiotics (a combined pro-and pre-biotic). The use of antibiotics is limited due to their side effects and the emergence and prevalence of bacterial resistance. The therapeutic benefit of FMT has been investigated for several aspects of the metabolic syndrome, with variable results [178]. Two studies reported improved peripheral insulin sensitivity at 6 weeks in patients with the metabolic syndrome receiving FMTs from lean donors, versus patients receiving the placebo control [179,180]. One study went on to show that the improvement in insulin sensitivity was dependent on the baseline composition of the hosts gut microbiome [179]. FMT from lean donors does not appear to reduce the BMI of overweight recipients [181-183]. Very limited human data is currently available regarding the impact of FMT on NAFLD. Craven et al. [184] found that FMT from slim healthy donors to individuals with NAFLD did not affect hepatic steatosis or insulin sensitivity but did reduce gut permeability. More work is needed before the therapeutic role of FMT in NAFLD can be established.
Table 3 lists the RCTs addressing the potential benefit of gut microbiome manipulation with probiotics, prebiotics and synbiotics in patients with NAFLD [185-202]. Most of these clinical trials use probiotics containing bacteria of the genus Lactobacillus, Bifidobacterium, and Streptococcus with or without a prebiotic such as fructo-oligosaccharide or inulin. Although studies differ in their design and intervention, the outcome of treatment appears positive, with many of the studies showing that in patients with NAFLD, probiotics can significantly improve hepatic steatosis and fibrosis as well as other metabolic parameters including glucose tolerance and obesity. It is uncertain from the study design of these trials whether the probiotics acted to favorably change the gut microbiome. Furthermore, other studies have shown that probiotics/prebiotics/synbiotics have no effect on biochemical or radiological end points in NAFLD. Thus, several questions remain unanswered. For example, what is the mechanism by which probiotics improve NAFLD; what is the most effective probiotic-prebiotic combination; what is the optimal duration of treatment and which aspect of the disease process in NAFLD benefits from treatment. Although promising results along with minimal cost and side effects make probiotics an exciting treatment option for NAFLD that could be extensively used as a health-food supplement to treat early disease, further RCTs with larger sample sizes, longer follow-up, and assessments of efficacy based on liver histology (or acceptable alternative diagnostic surrogate markers) [203] are urgently needed.
CONCLUSION
Abundant evidence from animal and human studies show that the diet and the gut microbiome play a role in the pathogenesis of NAFLD. The consumption of long-chained saturated fatty acids, fructose and AGEs, all plentiful within the western diet, can cause dysbiosis and potentially contribute to the development of hepatic steatosis and inflammation. Dysbiosis alters metabolic pathways and inflammatory processes through; altered production of SCFAs, altered choline and BA metabolism, increased production of bacteria-derived ethanol, higher abundance of LPS containing gram negative bacteria and increased intestinal permeability. The clinical significance of specific gut microbial alterations associated with NAFLD still remains unclear and therefore there is currently no diagnostic or therapeutic role for analysing the gut microbiome or modulating its composition in NAFLD. Further work is required to investigate the significance of gut microbiome alterations in NAFLD, and to clarify the therapeutic role of probiotics, prebiotics and synbiotics in the management of patients at different stages of the disease process.
Notes
Authors’ contribution
EEJ and CDB were involved in the writing of the article.
Conflicts of Interest: The authors have no conflicts of interests to disclose.
Acknowledgements
CDB is Principal Investigator for the Investigation of synbiotic treatment in NAFLD (INSYTE) trial (www.clinicaltrials.gov registration number NCT01680640). CDB is supported in part by the Southampton National Institute for Health Research Biomedical Research Centre, UK.
Abbreviations
AGEs
advanced glycated end products
AMP
adenosine monophosphate
ATP
adenosine triphosphate
BAs
bile acids
FMO3
flavin-containing monooxygenase-3
FMT
faecal microbial transplant
FXR
farnesoid X receptor
GLP-2
glucagon-like-peptide-2
HCC
hepatocellular carcinoma
IL
interleukin
LPS
lower plasma lipopolysaccharide
NAFLD
non-alcoholic fatty liver disease
NASH
non-alcoholic steatohepatitis
NCS
noncaloric artificial sweeteners
ODMA
O-desmethylangolensin
RAGE
receptor for advanced glycated end products
RCT
randomized controlled trial
SCFAs
short chain fatty acids
STZ-HFD
streptozotocin-high fat diet
T2DM
type 2 diabetes mellitus
TLRs
toll-like receptors
TMA
trimethylamine
TMAO
TMA-N-oxide
TNF
tumour necrosis factor

