The cut-off value of transient elastography to the value of hepatic venous pressure gradient in alcoholic cirrhosis
Article information
Abstract
Background/Aims
The hepatic venous pressure gradient (HVPG) reflects portal hypertension, but its measurement is invasive. Transient elastography (TE) is a noninvasive method for evaluating liver stiffness (LS). We investigated the correlation between the value of LS, LS to platelet ratio (LPR), LS-spleen diameter-to-platelet ratio score (LSPS) and HVPG according to the etiology of cirrhosis, especially focused on alcoholic cirrhosis.
Methods
Between January 2008 and March 2017, 556 patients who underwent HVPG and TE were consecutively enrolled. We evaluated LS, LPR, and LSPS according to the etiology of cirrhosis and analyzed their correlations with HVPG.
Results
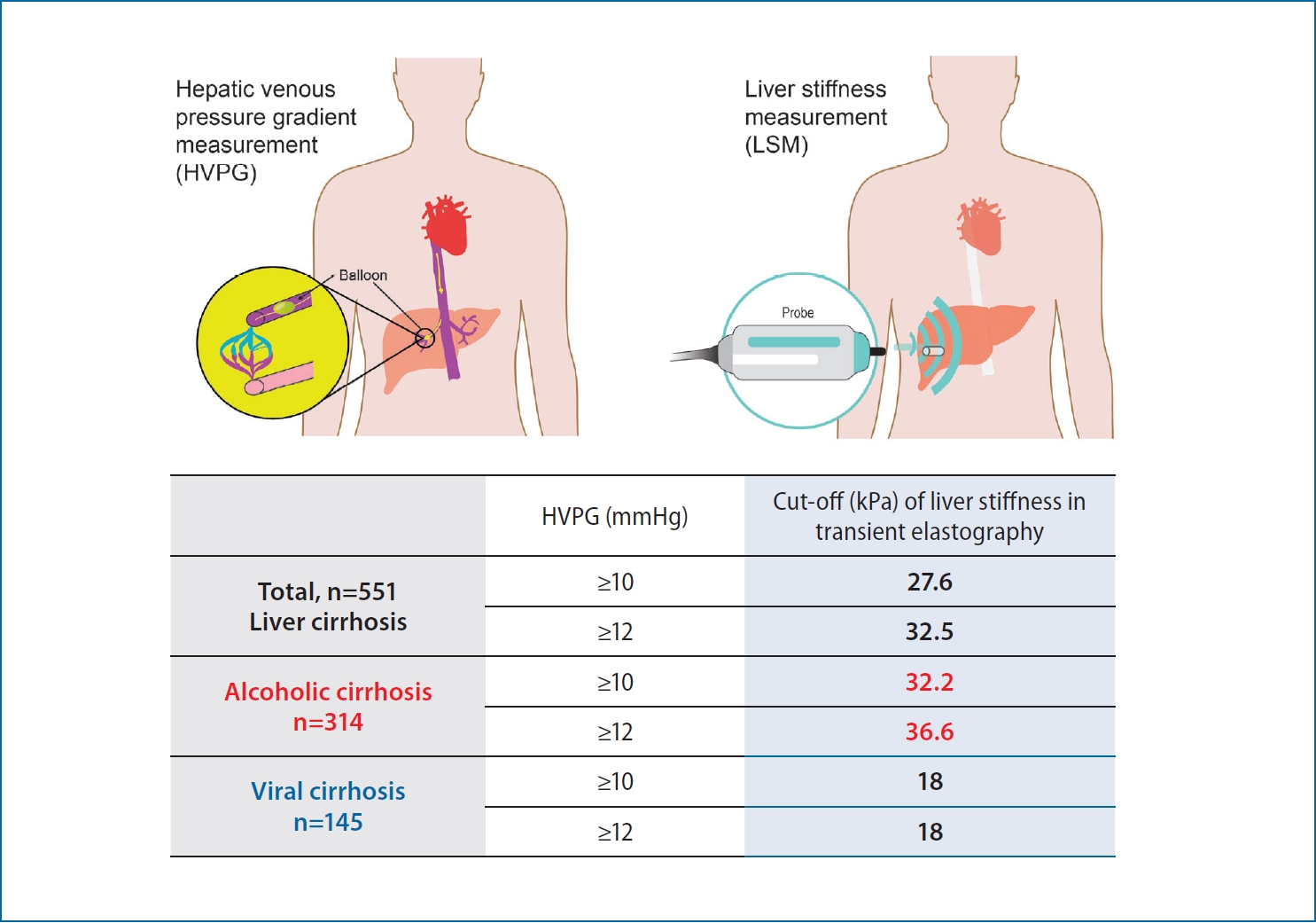
The LS value was higher in patients with alcoholic cirrhosis than viral cirrhosis based on the HVPG (43.5 vs. 32.0 kPa, P<0.001). There were no significant differences in the LPR or LSPS between alcoholic and viral cirrhosis groups, and the areas under the curves for the LPR and LSPS in subgroups according to HVPG levels were not superior to that for LS. In alcoholic cirrhosis, the LS cutoff value for predicting an HVPG ≥10 mmHg was 32.2 kPa with positive predictive value (PPV) of 94.5% and 36.6 kPa for HVPG ≥12 mmHg with PPV of 91.0%.
Conclusions
The LS cutoff value should be determined separately for patients with alcoholic and viral cirrhosis. In alcoholic cirrhosis, the LS cutoff values were 32.2 and 36.6 kPa for predicting an HVPG ≥10 and ≥12 mmHg, respectively. However, there were no significant differences in the LPR or LSPS between alcoholic and viral cirrhosis groups.
Graphical Abstract
INTRODUCTION
In patients with liver cirrhosis, portal hypertension (PTH) may create complications including variceal bleeding, ascites, and hepatic encephalopathy. The hepatic venous pressure gradient (HVPG) is a measure of PTH that predicts liver fibrosis, the prognosis of acute variceal bleeding, the effectiveness of beta-blocker prophylaxis [1-3], and the risk for hepatocellular carcinoma [4]. The HVPG is an important predictor of prognosis in patients with liver cirrhosis; each 1 mmHg increase in the HVPG increases the risk of death by 3% [5]. However, in a small number of cases, it is difficult to measure the HVPG because of contrast hypersensitivity or underlying cardiopulmonary disease or encephalopathy. In addition, although the frequency of complications is <1%, these include local pain, a vagal reaction, and transient cardiac arrhythmia, which are associated with low patient acceptance of undergoing this measurement. Recent studies have suggested that liver stiffness measurements (LSM) using transient elastography (TE) reflect liver stiffness (LS), the HVPG value [6,7], and the presence of esophageal varices [8]. Many studies have explored the utility of noninvasive LSM, seeking cutoffs reflecting the HVPG, but few studies have distinguished patients by the etiology of cirrhosis especially alcoholic origin cirrhosis. In addition, the accuracy of LSM is compromised by the total bilirubin level [9-11], severe obesity [12], and hepatic inflammation [13]. To overcome the problems associated with HVPG measurement, several studies have sought indices that correlate well with fibrosis status and the HVPG value. The LS-to-platelet ratio (LPR) and the LS-spleen diameter-to-platelet ratio score (LSPS) are useful for staging fibrosis [14-17]. In this study, we investigated the differences in LSM, LPR, and LSPS according to the etiology of cirrhosis, and calculated LS cutoff values predicting HVPG in patients with alcoholic cirrhosis.
PATIENTS AND METHODS
Patients
From January 2008 to March 2017, 556 patients who underwent HVPG and transient TE were consecutively enrolled at three Korean tertiary medical centers. All HVPG and TE tests were performed within 1-month intervals. The indications for patients undergoing HPVG procedure are as follows: 1) predicting the prognosis of patients with a history of varix bleeding or high-risk patients, 2) determination of transjugular intrahepatic portosystemic shunt (TIPS) procedure in varix bleeding or refractory ascites, and 3) determining the therapeutic effect after beta blocker treatment. We excluded patients with noncirrhotic PTH, an unreliable LSM, concomitant extrahepatic malignancy, and a condition that might interfere with LSM, such as active inflammation with a total bilirubin level >10 mg/dL and alanine aminotransferase level >200 IU/L. Ultimately, 551 patients were analyzed. We recorded, age, sex, liver cirrhosis etiology, baseline laboratory data, and the LSM and LPR. Baseline laboratory data included spleen size and the levels of albumin, bilirubin, platelets, creatinine, aspartate aminotransferase, and alanine aminotransferase. Model for end-stage liver disease scores were calculated. Patients were classified as having cirrhosis caused by hepatitis B virus (HBV), hepatitis C virus (HCV), alcohol, mixed etiology, or other etiology (e.g., autoimmune, non-alcoholic fatty liver disease, and cryptogenic causes). HBV patients were positive for hepatitis B virus surface antigen and/or HBV DNA; HCV patients were positive for HCV Ab and/or HCV RNA; and those with alcoholic cirrhosis had ingested >60 g alcohol daily (>40 g daily for females) more than three times weekly for >10 years. The study protocol was approved by the Institutional Review Boards of Soonchunhyang University Seoul Hospital. The study protocol conformed to the ethical guidelines of the World Medical Association Declaration of Helsinki and was approved by the Institutional Review Board of Soonchunhyang University Seoul Hospital (Number 2020-09-005).
HVPG
The HVPG was measured by professional interventional radiologists in each hospital. The right jugular vein was the most commonly used access route; free hepatic venous pressure (FHVP) was measured after placing a 6-French balloon catheter in the right hepatic vein. The wedge hepatic venous pressure (WHVP) was measured by inflating the balloon catheter in the right hepatic region. HVPG was calculated by subtracting the FHVP from the WHVP. HVPG values were categorized as ≥6, ≥10, ≥12, and ≥16 mmHg. An HVPG ≥6 mmHg indicates the presence of cirrhosis (21), an HVPG ≥10 mmHg indicates an increasing risk for varices and thus is termed “clinically significant” PTH (CSPH) (21), an HVPG ≥12 mmHg indicates an increased risk for variceal bleeding and thus is termed “severe” PTH (SPH), and an HVPG ≥16 mmHg is an important predictor of poor outcomes, greatly increasing the risk of 1-year mortality (5, 22, 23).
LSM, LPR, and LSPS
TE was performed using Fibroscan® 502 Touch instruments (Echosens, Paris, France); an interquartile range/median ratio <30% and a success rate >60% were considered to reflect goodquality data. The LPR was calculated as LS (kPa) / platelet count (109/L) and LSPS was calculated as LS (kPa) × spleen diameter (cm) / platelet count (109/L).
Statistical analyses
Data were analyzed using the Statistical Package for the Social Sciences (SPSS) ver. 22.0 for Windows (SPSS, Inc., Chicago, IL, USA). Correlations were sought using Spearman’s tests. Receiver operating characteristic (ROC) curves were drawn to explore the utilities of LSM and the LPR in terms of predicting PTH. The optimal LSM and LPR cutoffs were obtained using the Youden index.
RESULTS
Baseline characteristics
We analyzed 551 patients with liver cirrhosis. Alcohol was the most common etiology (334, 60.6%) followed by HBV (127, 23.0%) (Table 1). The causes of cirrhosis were mixed in 32 cases (5.8%) and other in 39 (7.1%). Most patients were male (450, 81.7%), and the mean age was 53.30±9.76 years. The mean platelet count was 111.17±65.53 cells/L. The mean HVPG value and LSM were 13.73±5.32 mmHg and 38.43±22.37 kPa, respectively.
Differences in LSM, LPR, and LSPS values according to the etiology of liver cirrhosis
When we analyzed the LS according to HVPG level (≥6, ≥10, ≥12, and ≥16 mmHg), the values were higher in alcoholic cirrhosis than viral cirrhosis in all HVPG subgroups. However, the LPR and LSPS were not significantly different between alcoholic and viral cirrhosis in any of the HVPG subgroups (P=0.689 and 0.494 at HVPG ≥6 mmHg; P=0.647 and 0.125 at HVPG ≥10 mmHg; P=0.454 and 0.097 at HVPG ≥12 mmHg; and P=0.877 and 0.331 at HVPG ≥16 mmHg for LPR and LSPS, respectively) (Table 2).
LSM, LPR, and LSPS correlations and diagnostic accuracies for HVPG
In all patients, the LSM, LPR, and LSPS exhibited good positive correlations with the HVPG (Fig. 1). The correlation coefficients were 0.523 (P<0.001) for LSM, 0.410 for the LPR (P<0.001), and 0.390 for the LSPS (P<0.001). The diagnostic accuracies of LSM, LPR, and LSPS for HVPG were assessed using ROC curves (Fig. 2). For LSM, the areas under the curves (AUCs) were 0.736 (95% confidence interval [CI], 0.523–0.884; P<0.01), 0.785 (95% CI, 0.716–0.866; P<0.01), 0.755 (95% CI, 0.686–0.842; P<0.01), and 0.747 (95% CI, 0.680–0.827; P<0.01) for HVPG ≥6, ≥10, ≥12, and ≥16 mmHg. For LPR, the AUCs were 0.733 (95% CI, 0.585–0.864; P<0.01), 0.811 (95% CI, 0.744–0.861; P<0.01), 0.786 (95% CI, 0.689–0.844; P<0.01), and 0.765 (95% CI, 0.685–0.845; P<0.01) for HVPG values ≥6, ≥10, ≥12, and ≥16 mmHg. For LSPS, the AUCs were 0.737 (95% CI, 0.577–0.894; P<0.01) for HVPG ≥6 mmHg, 0.811 (95% CI, 0.734–0.880; P<0.001) for HVPG ≥10 mmHg, 0.784 (95% CI, 0.707–0.855; P<0.01) for HVPG ≥12 mmHg, and 0.759 (95% CI, 0.680–0.841; P<0.01) for HVPG ≥16 mmHg. The ROC curves for LSM, LPR, and LSPS had high AUCs but with no significant differences among the three indexes for predicting HVPG ≥6 mmHg; the LPR and LSPS showed no greater value in predicting HVPG than that of the LSM (Supplementary Table 1). Overall, the AUCs for LSM, LPR, and LSPS decreased with increasing HVPG levels but with no significant differences among the three measures. When subgroup analyses were performed according to the etiology of cirrhosis, patients with alcoholic cirrhosis had same result as above (Supplementary Fig. 1). However, in patients with viral cirrhosis, the AUC tended to be higher for LPR and LSPS than for LS at HVPG ≥10 and ≥12 mmHg (LSM, LPR, and LSPS: 0.785, 0.811, and 0.811 for HVPG ≥10 mmHg; 0.755, 0.786, and 0.784 for HVPG ≥12 mmHg; Supplementary Fig. 2).

Correlations of the HVPG with LSM, LPR, and LSPS in cirrhosis patients. (A) The correlation between the HVPG and LSM (r=0.542, P<0.001). (B) The correlation between the HVPG and LPR (r=0.537, P<0.001). (C) The correlation between the HVPG and LSPS (r=0.522, P<0.001). LSM, liver stiffness measurements; HVPG, hepatic venous pressure gradient; LPR, liver stiffness to platelet ratio; LSPS, liver stiffness-spleen diameter-to-platelet ratio score.
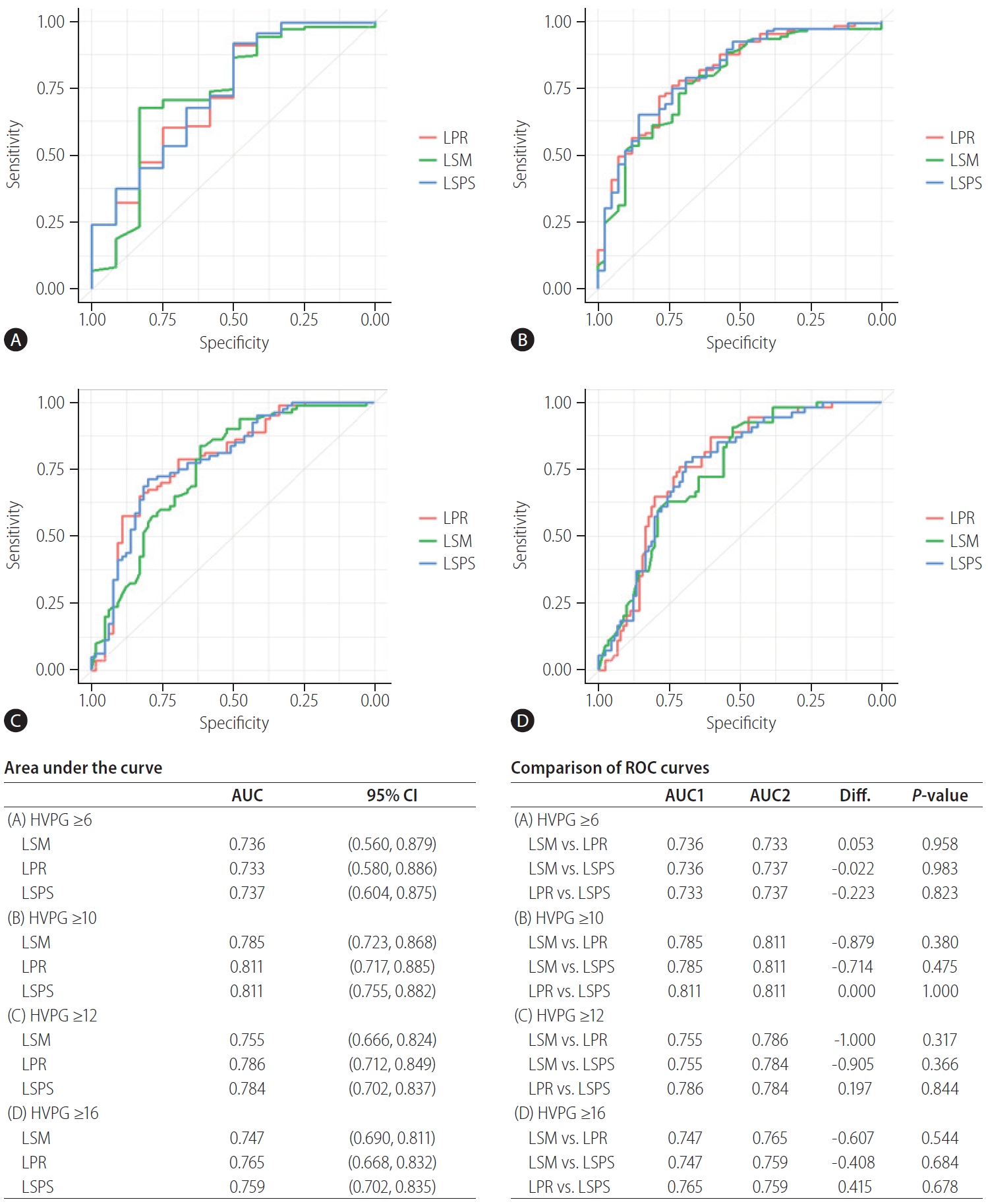
ROC curves of LSM, LPR, and LSPS for HVPG ≥6 (A), ≥10 (B), ≥12 (C), and ≥16 mmHg (D) in all cirrhosis patients. HVPG, hepatic venous pressure gradient; LPR, liver stiffness to platelet ratio; LSM, liver stiffness measurements; LSPS, liver stiffness-spleen diameter-to-platelet ratio score; AUC, areas under the curve; CI, confidence interval; ROC, receiver operating characteristic; Diff., difference.
LS cutoffs according to the HVPG in patients with alcoholic and viral cirrhosis
We calculated LS cutoff values in 334 patients with alcoholic cirrhosis (excluding those with viral cirrhosis). The LS optimal cutoffs according to different HVPG levels were calculated using the Youden index. The optimal LS cutoffs for predicting HVPG ≥6, ≥10, and ≥12 mmHg were 10.0, 32.2, and 36.6 kPa, with a sensitivity, specificity, positive predictive value (PPV), and negative predicting value (NPV) of 98.2%, 77.8%, 99.4%, and 53.9% for HVPG ≥6; 68.1%, 80.7%, 94.5%, and 34.3% for HVPG ≥10; and 66.5%, 82.4%, 91.0%, and 48.1% for HVPG ≥12 mmHg, respectively (Table 3). Although the PPV tended to decrease with increasing HVPG, the PPVs of the LS cutoffs for predicting an HVPG ≥6 and ≥10 were relatively high (99.4% and 91.0%, respectively). We also calculated LS cutoff values in 145 patients with viral cirrhosis. The optimal LS cutoffs for predicting HVPG ≥10, and ≥12 mmHg were 18.0 kPa, respectively with a sensitivity, specificity, PPV, and NPV of 76.7%, 69.1%, 85.9%, and 54.7% for HVPG ≥10; and 83.8%, 61.5%, 72.8%, and 75.5% for HVPG ≥12 mmHg, respectively (Table 3). In comparison of cut-off values using LPR and LSPS in patients with alcoholic and all etiology cirrhosis, the LPR cutoffs of all etiology were higher than those of alcohol in each level of HVPG (Supplementary Table 2).
DISCUSSION
HVPG ≥10 mmHg defined as CSPH, has been associated with formation of esophageal varices and poor prognosis, and HVPG ≥12 mmHg defined as SPH is related to a higher risk of bleeding from varices. However, HVPG measurement is invasive procedure. Therefore, non-invasive measurement such as TE is required to replace the HVPG measurement.
Good correlations between LSM and HVPG values have been reported in many studies [6,18-21]. Bureau et al. [18] measured LS by Fibroscan® in 150 consecutive patients who underwent a liver biopsy with HVPG. Patients were composed of alcohol, viral and other origin. HVPG was correlated with LS (q=0.858, P<0.001) and the LS cut-off value of 21 kPa accurately predicted CSPH in 92% of the 144 patients for whom LS was successful. Hong et al. [21] showed a strong positive correlation between LSM and HVPG in the overall population (r2=0.496, P<0.0001), and reported a CSPH cutoff of 21.95 kPa in their study, which included a higher rate (61.0%) of patients with alcoholic cirrhosis compared to other studies [21]. Vizzutti et al. [20] evaluated LSM to predict HVPG in 61 consecutive patients with HCV-related chronic liver disease. A strong relationship was found in the overall population (r=0.81, P<0.0001), and the LSM cutoff values of CSPH and SPH were 13.6 and 17.6 kPa, respectively.
The cutoff values for CSPH determined in previous studies have ranged widely from 13.6 to 24.6 kPa [20-26]. However, most of those studies involved liver diseases of mixed etiologies, and many included more patients with viral cirrhosis than those with alcoholic cirrhosis.
Lemoine et al. [19] reported that the LSM of patients with alcoholic cirrhosis was higher than that of patients with chronic viral hepatitis. They reported LS cutoffs of 20.5 kPa in 44 HCV cirrhosis patients and 34.9 kPa in 48 alcoholic cirrhosis patients for predicting an HVPG ≥10 mmHg [19]. In our study, the LSM also tended to be higher in patients with alcoholic cirrhosis compared to viral cirrhosis. The LS cutoff value for predicting an HVPG ≥10 mmHg in alcoholic cirrhosis, was 32.2 kPa and that of viral cirrhosis was 18.0 kPa.
In alcoholic cirrhosis, the LS value was higher than that of viral cirrhosis. This may be explained by the different spatial distribution of alcoholic fibrosis, which develops in centrilobular and perisinusoidal as well as in periportal regions [27]. Perisinusoidal fibrosis tends to be more frequent in alcoholics and may increase LSM [28]. In addition, hepatic alcoholic lesions are also characterized by liver cell necrosis, reactive inflammation, steatosis and pericellular fibrosis or steatohepatitis [29]. It is possible that steatosis or inflammation may increase LS values as chronic alcoholics progress to cirrhosis [28].
Some studies have reported that LS combined with the platelet count and spleen size may improve the predictive power of esophageal varices compared to LS alone [14,30-32].
The difference in LS observed between patients with alcoholic cirrhosis and those with viral cirrhosis was no longer apparent when using the LPR and LSPS indices in this study. However, there were no differences in HVPG diagnostic accuracy, according to the AUC value, among the three indices (LSM, LPR, and LSPS). Comparing with the previous studies, there are two progressed points in this study. First, we analyzed the data of large number of patients based on each classification (HVPG classification as ≥6, ≥10, ≥12, and ≥16 mmHg). The number of patients in Lemoine et al.’ study [19] was 92 (44 HCV and 48 alcohol), and that of our study was 551 (145 viral, 334 alcohol, and 72 others). We analyzed each level of HVPG and demonstrated that the cut off value of alcohol group is larger than that of viral group in each level over 10 mmHg of HVPG. We also applied LPR and LSPS whether they can compensate the limitation of LS. Although they did not show the significance, it was a new trial to overcome the limitation of LS. Finally, our study identified the cut off value in each level of HVPG based on large population, and newly applied LS and LSPS to compensate for LS.
According to the Baveno VI recommendations, a LS cutoff of 21 kPa can be used to confirm the presence of CSPH [18,30], but this recommendation was based mostly on studies involving patients with viral cirrhosis [30]. Our LS cutoff value for predicting CSPH in patients with alcoholic cirrhosis was higher than that of the Baveno VI recommendations (32.2 vs. 21 kPa). We calculated LS cutoff values exclusively in patients with alcoholic cirrhosis according to the HVPG: 10.0 kPa for HVPG ≥6 mmHg, 32.2 kPa for HVPG ≥10 mmHg, 36.6 kPa for HVPG ≥12 mmHg, and 45.4 kPa for HVPG ≥16 mmHg. Although the sensitivity and PPV tended to decrease as the HVPG increased, the highest PPVs were associated with HVPG ≥6 mmHg.
Although LS is a measure of fibrosis, it may not adequately reflect the HVPG because it is a hemodynamic parameter [20,33]. In a recent meta-analysis, the measurement of spleen stiffness by ultrasound overcame the limitations associated with LSM, but most of the studies in that analysis evaluated heterogeneous populations, and thus the superiority of spleen stiffness measurement to LSM is not clear [34]. We expected the LPR and LSPS to overcome the limitations of using LSM, but unfortunately they did not. Further studies are needed on noninvasive methods that do not decrease in diagnostic accuracy with increasing HVPG levels. In this study, we measured HVPG mainly in patients with high risk or prior history of variceal bleeding or in patients requiring TIPS treatment. If the LS cut-off value in our study is externally validated, it can be helpful in determining the clinical treatment direction without invasive HVPG treatment. In particular, we expect TE to replace HVPG in situations such as prognostication of variceal bleeding, further optimization of beta-blocker pharmacotherapy, and determination of hemodynamic effects before and after TIPS procedure.
Our study had some limitations. First, it was a retrospective study. Although HVPG and Fibroscan® measurements were performed on the same day in many patients, there was up to a 1-month interval between measurements in some patients. Second, the length of the spleen was measured using ultrasonography, which cannot accurately detect spleen volume. Third, this study included fewer patients with viral cirrhosis than with alcoholic cirrhosis and did not analyze the viral origins of the cirrhosis. We believe that the LSPS and LPR should be used together to overcome the shortcomings of LSM in patients with viral cirrhosis.
In conclusion, the LS cutoff should be determined separately in patients with alcoholic and viral cirrhosis. In patients with alcoholic cirrhosis, the LS cutoff was 32.2 kPa, with a 94.5% PPV, for diagnosing HVPG ≥10 mmHg and 36.6 kPa, with a 91.0% PPV, for diagnosing HVPG ≥12 mmHg. The LPR and LSPS showed strong correlations with HVPG values ≥6 mmHg. However, the LPR and LSPS did not show better diagnostic accuracies than that of LS.
Notes
Authors’ contribution
Study design: Soung Won Jeong, Moon Young Kim
Acquisition and interpretation of data: Se Ri Ryu, Jeong-Ju Yoo, Seong Hee Kang, Su Yeon Park, Young Kyu Cho, Young Chang
Drafting of the manuscript: Se Ri Ryu, Jeong-Ju Yoo, Soung Won Jeong, Moon Young Kim
Critical revision of the manuscript for important intellectual content: Baigal Baymbajav, Sang Gyune Kim, Jae Young Jang, Young Seok Kim, Soon Koo Baik, Yong Jae Kim
Statistical analysis: Jeong-Ju Yoo, Se Ri Ryu, Su Yeon Park
Conflicts of Interest: The authors have no conflicts to disclose.
Acknowledgements
This work was supported by the Soonchunhyang University Research Fund and the National Research Foundation of Korea (NRF) grant funded by the Korea government (2020R1F1A1072449, and 2020R1F1A1076282).
SUPPLEMENTAL MATERIAL
Supplementary material is available at Clinical and Molecular Hepatology website (http://www.e-cmh.org).
The P-values about difference of AUCs between LSM, LPR, and LSPS in total patients
Comparison of cut-off values using LPR and LSPS in patients with alcoholic and all etiology cirrhosis
ROC curves of LSM, LPR, and LSPS for HVPG ≥6 (A), ≥10 (B), ≥12 (C), and ≥16 mmHg (D) for cirrhosis caused by alcohol. HVPG, hepatic venous pressure gradient; LPR, liver stiffness to platelet ratio; LSM, liver stiffness measurements; LSPS, liver stiffness-spleen diameter-to-platelet ratio score; AUC, areas under the curve; CI, confidence interval; ROC, receiver operating characteristic; Diff., difference.
ROC curves of LSM, LPR, and LSPS for HVPG ≥6 (A), ≥10 (B), ≥12 (C), and ≥16 mmHg (D) for cirrhosis caused by viral. HVPG, hepatic venous pressure gradient; LPR, liver stiffness to platelet ratio; LSM, liver stiffness measurements; LSPS, liver stiffness-spleen diameter-to-platelet ratio score; AUC, areas under the curve; CI, confidence interval; ROC, receiver operating characteristic; Diff., difference.
Abbreviations
AUCs
areas under the curves
CI
confidence interval
CSPH
“clinically significant” portal hypertension
FHVP
free hepatic venous pressure
HBV
hepatitis B virus
HCV
hepatitis C virus
HVPG
hepatic venous pressure gradient
LPR
liver stiffness to platelet ratio
LS
liver stiffness
LSM
liver stiffness measurements
LSPS
liver stiffness-spleen diameter-to-platelet ratio score
NPV
negative predicting value
PPV
positive predictive value
PTH
portal hypertension
ROC
receiver operating characteristic
SPH
“severe” portal hypertension
TE
transient elastography
TIPS
transjugular intrahepatic portosystemic shunt
WHVP
wedge hepatic venous pressure
References
Article information Continued
Notes
Study Highlights
The LS value is higher in patients with alcoholic cirrhosis than in those with viral cirrhosis based on HVPG, and the LS cutoff values of alcoholic cirrhosis were 32.2 and 36.6 kPa for predicting an HVPG ≥10 and ≥12 mmHg, respectively.