| Clin Mol Hepatol > Volume 27(2); 2021 > Article |
|
ABSTRACT
Background/Aims
Methods
Results
FOOTNOTES
SUPPLEMENTAL MATERIAL
Supplementary┬ĀTable┬Ā1.
Supplementary┬ĀTable┬Ā2.
Supplementary┬ĀTable┬Ā3.
Supplementary┬ĀFigure┬Ā1.
Supplementary┬ĀFigure┬Ā2.
Supplementary┬ĀFigure┬Ā3.
Supplementary┬ĀFigure┬Ā4.
Supplementary┬ĀFigure┬Ā5.
Figure┬Ā1.
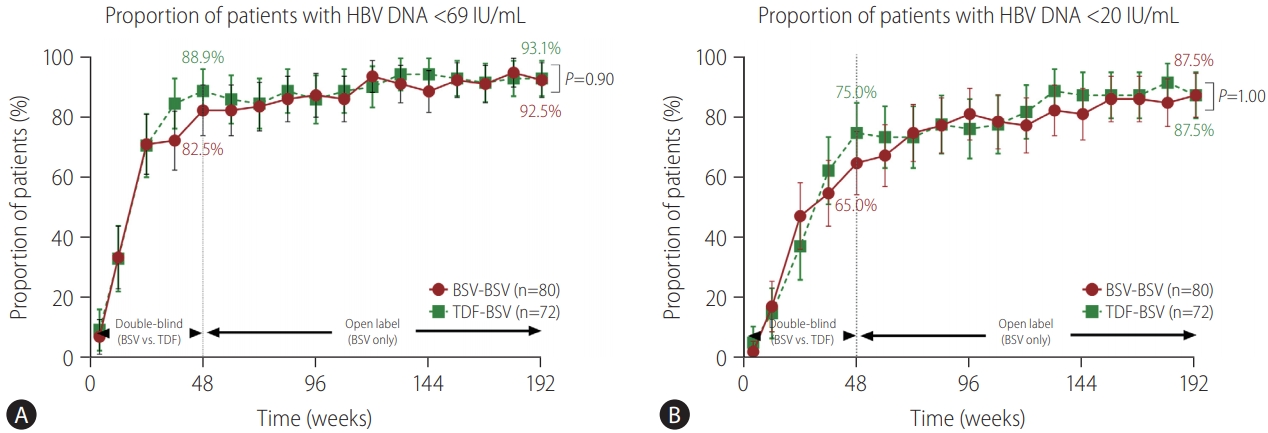
Figure┬Ā2.
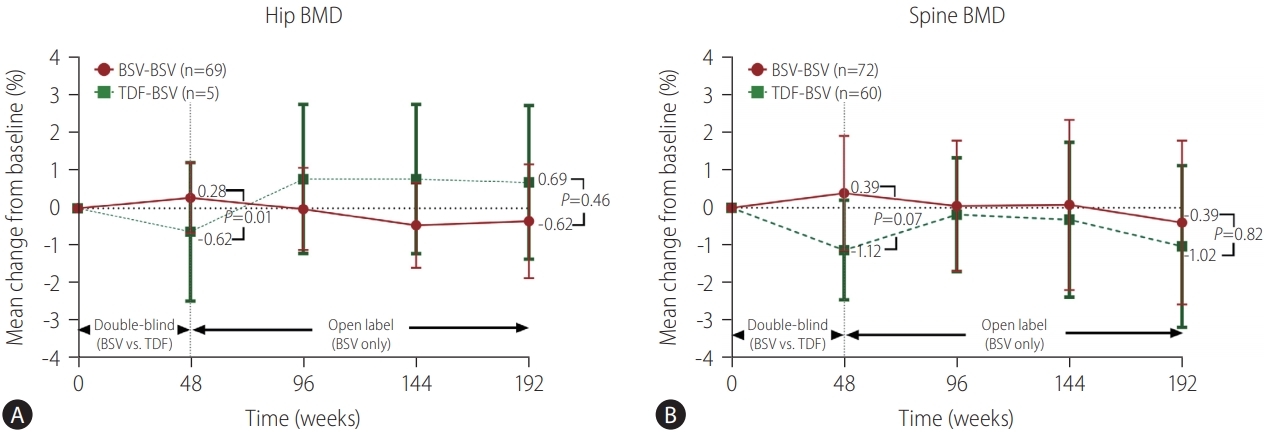
Figure┬Ā3.
Figure┬Ā4.

Table┬Ā1.
| BSV-BSV (n=80) | TDF-BSV (n=72) | P-value | |
|---|---|---|---|
| Male | 53 (66.3) | 46 (63.9) | 0.76 |
| Age (years) | 46.16 (10.8) | 44.04 (9.9) | 0.21 |
| HBeAg positive | 50 (62.5) | 40 (55.6) | 0.38 |
| HBV genotype | 0.86 | ||
| ŌĆāA | 1 (1.3) | 0 | |
| ŌĆāC | 78 (97.5) | 71 (98.6) | |
| ŌĆāD | 0 (0.0) | 1 (1.4) | |
| Not determined | 1 (1.3) | 0 (0.0) | |
| HBV DNA (log10 IU/mL) | 6.31 (1.7) | 6.55 (1.5) | 0.35 |
| ALT (U/L) | 105.6 (102.1) | 127.4 (143.7) | 0.38 |
| ALT normal* by central laboratory criteria | 4 (5.0) | 6 (8.3) | 0.52 |
| BMI (kg/m2) | 0.05 | ||
| ŌĆāNormal, <25 kg/m2 | 53 (66.3) | 49 (68.1) | |
| ŌĆāOverweight, Ōēź25 to Ōēź30 kg/m2 | 25 (31.3) | 15 (20.8) | |
| ŌĆāObese, >30 kg/m2 | 2 (2.5) | 8 (11.1) | |
| eGFR by MDRD (mL/min) | 89.5 (14.6) | 92.4 (13.9) | 0.22 |
| Creatinine (mg/dL) | 0.86 (0.2) | 0.83 (0.1) | 0.31 |
| Phosphate (mg/dL) | 3.53 (0.5) | 3.45 (0.6) | 0.56 |
| Fib-4 score | 2.14 (2.08) | 1.91 (1.14) | 0.98 |
| Total hip BMD clinical statusŌĆĀ | 0.88 | ||
| ŌĆāNormal, T-score Ōēź-1.0 | 57 (82.6) | 46 (83.6) | |
| ŌĆāOsteopenia, -2.5Ōēż T-score <-1.0 | 12 (17.4) | 9 (16.4) | |
| ŌĆāOsteoporosis, T-score <-2.5 | 0 (0.0) | 0 (0.0) | |
| ŌĆāData not collected | 11 | 17 | |
| Spine BMD clinical status | 0.71 | ||
| ŌĆāNormal, T-score Ōēź-1.0 | 49 (68.1) | 39 (65.0) | |
| ŌĆāOsteopenia, -2.5Ōēż T-score <-1.0 | 19 (26.4) | 19 (31.7) | |
| ŌĆāOsteoporosis, T-score <-2.5 | 4 (5.6) | 2 (3.3) | |
| ŌĆāData not collected | 8 | 12 | |
| Concurrent medical history | |||
| ŌĆāMild renal impairmentŌĆĪ | 44 (55.0) | 33 (45.8) | 0.26 |
| ŌĆāCirrhosis | 19 (23.8) | 13 (18.1) | 0.39 |
| ŌĆāDiabetes mellitus | 10 (12.5) | 3 (4.2) | 0.07 |
| ŌĆāHypertension | 10 (12.5) | 9 (12.5) | 1.00 |
| Prior antiviral therapy | 0 | 1 (1.4) | 0.47 |
Values are presented as number (%), n/N (%), or mean (standard deviation) unless otherwise stated.
P-value: two-sample t test or Wilcoxon rank-sum test for continuous variables, and chi-square test or FisherŌĆÖs exact test for categorical variables.
BSV, besifovir dipivoxil maleate; TDF, tenofovir disoproxil fumarate; HBeAg, hepatitis B e antigen; HBV, hepatitis B virus; ALT, alanine aminotransferase; BMI, body mass index; eGFR, estimated glomerular filtration rate; MDRD, modification of diet in renal disease; Fib-4, fibrosis-4; BMD, bone mineral density.
Table┬Ā2.
|
FAS |
PPS |
|||||
|---|---|---|---|---|---|---|
| BSV-BSV (n=80) | TDF-BSV (n=72) | P-value | BSV-BSV (n=77) | TDF-BSV (n=67) | P-value | |
| HBV DNA <69 IU/mL | 74 (92.5) | 67 (93.1) | 0.90 | 73 (94.8) | 63 (94.0) | 1.00 |
| HBV DNA <20 IU/mL | 70 (87.5) | 63 (87.5) | 1.00 | 69 (89.6) | 59 (88.1) | 0.77 |
| HBeAg loss*,ŌĆĀ | 16/49 (32.7) | 14/40 (35.0) | 0.82 | 14/47 (29.8) | 14/37 (37.8) | 0.44 |
| HBeAg seroconversion*,ŌĆĀ | 5/49 (10.2) | 5/40 (12.5) | 0.75 | 3/47 (6.4) | 5/37 (13.5) | 0.29 |
| HBsAg lossŌĆĀ,ŌĆĪ | 0/79 (0.0) | 1/72 (1.4) | 0.48 | 0/77 (0.0) | 1/67 (1.5) | 0.47 |
| HBsAg seroconversionŌĆĀ,ŌĆĪ | 0/79 (0.0) | 1/72 (1.4) | 0.48 | 0/77 (0.0) | 1/67 (1.5) | 0.47 |
| ALT normalization┬¦ | 71 (88.8) | 67 (93.1) | 0.36 | 69 (89.6) | 62 (92.5) | 0.54 |
Values are presented as n/N (%) or number (%).
P-value: PearsonŌĆÖs chi-squared test or FisherŌĆÖs exact test.
FAS, full analysis set; PPS, per protocol set; BSV, besifovir dipivoxil maleate; TDF, tenofovir disoproxil fumarate; HBV, hepatitis B virus; HBeAg, hepatitis B envelope antigen; HBsAg, hepatitis B surface antigen; ALT, alanine aminotransferase.
Table┬Ā3.
|
HBeAg-positive patients (n=90) |
HBeAg-negative patients (n=62) |
|||||
|---|---|---|---|---|---|---|
| BSV-BSV (n=50) | TDF-BSV (n=40) | P-value | BSV-BSV (n=30) | TDF-BSV (n=32) | P-value | |
| HBV DNA <69 IU/mL | 44 (88.0) | 35 (87.5) | 1.00 | 30 (100.0) | 32 (100.0) | ŌĆō |
| HBV DNA <20 IU/mL | 40 (80.0) | 32 (80.0) | 1.00 | 30 (100.0) | 31 (96.9) | 1.00 |
| HBeAg loss*,ŌĆĀ | 16/49 (32.7) | 14/40 (35.0) | 0.82 | ŌĆō | ŌĆō | ŌĆō |
| HBeAg seroconversion*,ŌĆĀ | 5/49 (10.2) | 5/40 (12.5) | 0.75 | ŌĆō | ŌĆō | ŌĆō |
| HBsAg lossŌĆĀ,ŌĆĪ | 0/49 (0) | 1/40 (2.5) | 0.45 | 0/30 (0) | 0/32 (0) | ŌĆō |
| HBsAg seroconversionŌĆĀ,ŌĆĪ | 0/49 (0) | 1/40 (2.5) | 0.45 | 0/30 (0) | 0/32 (0) | ŌĆō |
| ALT normalization┬¦ | 42 (84.0) | 37 (92.5) | 0.33 | 29 (96.7) | 30 (93.8) | 1.00 |
Values are presented as n/N (%) or number (%).
P-value: PearsonŌĆÖs chi-squared test or FisherŌĆÖs exact test.
HBeAg, hepatitis B envelope antigen; FAS, full analysis set; BSV, besifovir dipivoxil maleate; TDF, tenofovir disoproxil fumarate; HBV, hepatitis B virus; HBsAg, hepatitis B surface antigen; ALT, alanine aminotransferase.
Table┬Ā4.
| Variable |
0ŌĆō192 weeks |
||
|---|---|---|---|
| BSV-BSV (n=80) | TDF-BSV (n=72) | P-value | |
| Adverse events | 69 (86.3) | 57 (79.2) | 0.25 |
| Adverse drug reactions | 38 (47.5) | 35 (48.6) | 0.89 |
| Serious adverse events | 12 (15.0) | 9 (12.5) | 0.66 |
| Serious adverse drug reactions | 2 (2.5) | 2 (2.8) | 1.00 |
| Serious adverse events leading to drug discontinuation | 1 (1.3) | 0 (0.0) | 1.00 |
| Death | 0 (0.0) | 0 (0.0) | - |
| Adverse drug reaction recorded in Ōēź3% of all patients | |||
| ŌĆāNasopharyngitis | 2 (2.5) | 3 (4.2) | 0.67 |
| ŌĆāDyspepsia | 3 (3.8) | 6 (8.3) | 0.31 |
| ŌĆāOsteopenia | 1 (1.3) | 5 (6.9) | 0.10 |
| ŌĆāAlanine aminotransferase increased | 4 (5.0) | 3 (4.2) | 1.00 |
| ŌĆāHeadache | 4 (5.0) | 1 (1.4) | 0.37 |
| ŌĆāDizziness | 3 (3.8) | 0 (0.0) | 0.25 |
| ŌĆāSomnolence | 3 (3.8) | 1 (1.4) | 0.62 |
| ŌĆāFatigue | 2 (2.5) | 4 (5.6) | 0.42 |
| ŌĆāPruritus | 1 (1.3) | 4 (5.6) | 0.19 |
| ŌĆāBenign hepatic nodules | 3 (3.8) | 1 (1.4) | 0.62 |
| ŌĆāHypertension | 5 (6.3) | 0 (0.0) | 0.06 |
| Renal related adverse events | |||
| ŌĆāProteinuria | 0 (0.0) | 1 (1.4) | 0.47 |
| ŌĆāPhosphate <2.5 mg/dL | 10 (12.5) | 15 (20.8) | 0.17 |
| ŌĆāeGFR <50 mL/min | 1 (1.3) | 2 (2.8) | 0.60 |
| ŌĆāSerum creatinine increase Ōēź0.5 mg/dL above baseline | 0 (0.0) | 1 (1.4) | 0.47 |
| Bone-related adverse events | |||
| ŌĆāFracture | |||
| ŌĆāSpontaneous* | 1 (1.3) | 0 (0.0) | 1.00 |
| ŌĆāTraumatic | 4 (5.0) | 1 (1.4) | 0.37 |
| Dyslipidemia | |||
| ŌĆāTriglyceride, above 300 mg/dL | 0 (0.0) | 0 (0.0) | - |
| ŌĆāTotal cholesterol, above 300 mg/dL | 0 (0.0) | 0 (0.0) | - |
| Total carnitine level at week 192ŌĆĀ | |||
| ŌĆāLow | 2 (2.5) | 5 (6.9) | 0.26 |
| ŌĆāHigh | 10 (12.5) | 7 (9.7) | 0.59 |
Abbreviations
REFERENCES
- TOOLS
-
METRICS

- ORCID iDs
-
Soon Ho Um

https://orcid.org/0000-0002-6390-2218 - Related articles




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Supplement1
Supplement1 Print
Print



