INTRODUCTION
An adverse liver outcome is one of the leading causes of death in non-alcoholic fatty liver disease (NAFLD) [
1]. Non-alcoholic steatohepatitis (NASH) has become the second leading indication for liver transplantation in the United States among patients with or without hepatocellular carcinoma (HCC) [
2]. The significant morbidity and mortality related to NAFLD can be attributed to the increase in its prevalence, which parallels the rising prevalence of obesity and metabolic syndrome worldwide. Genetic factors are increasingly recognised in the pathophysiology of this multifactorial disease [
3].
Several studies hypothesised that patatin-like phospholipase domain-containing 3 (
PNPLA3) rs738409, the first risk genetic variant discovered from the genome-wide association study (GWAS) of NAFLD, could be utilised as a potential prognostic factor in clinical settings [
4,
5]. Recently, 17╬▓-hydroxysteroid dehydrogenase 13 (
HSD17B13), a liver-specific lipid-droplet associated protein, was found to act in an opposite manner. A recent landmark study involving 46,544 European and Hispanic descendants reported that
HSD17B13 rs72613567, a protein-truncating insertion and deletion (indel) variant, is associated with a lower risk of cirrhosis and HCC [
6]. Similar results were replicated in NAFLD patients of European ancestry and in Argentinian and Danish populations [
7-
9]. Another single amino acid mutation at rs6834314 is also associated with the loss of
HSD17B13 enzymatic activity. The
HSD17B13 rs6834314 variant, which is highly linked with the rs72613567 variant, attenuates the effect of
PNPLA3 on advanced liver fibrosis among the Japanese [
10]. Growing evidence has shown the critical role of
HSD17B13 in the regulation of hepatic lipid homeostasis, which could potentially represent a new target for the treatment of NAFLD.
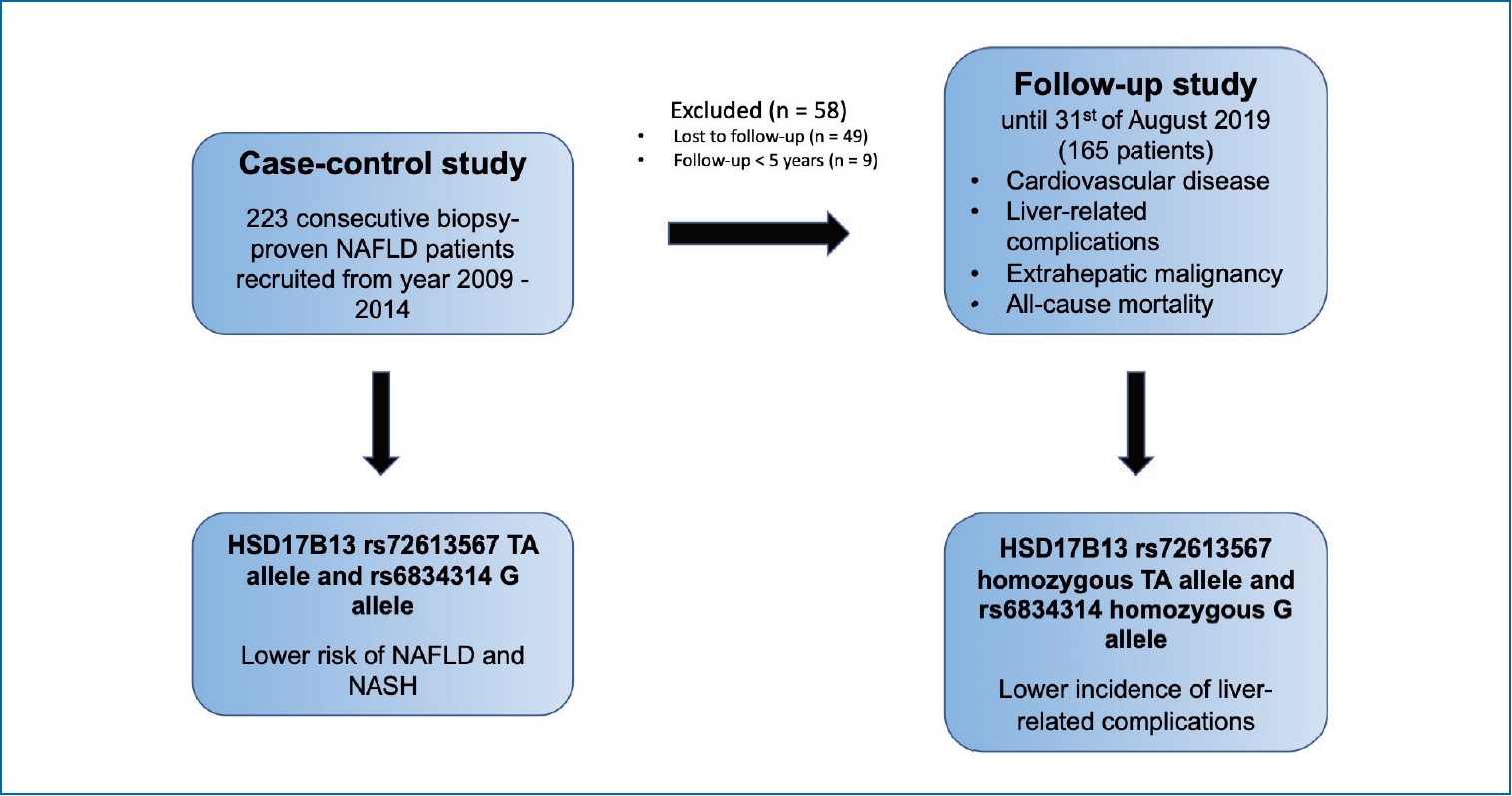
This study aimed to evaluate the clinical impact of HSD17B13 genetic variants (rs72613567 and rs6834314) by applying a case-control and a longitudinal approach. To the best of our knowledge, this is the first study to evaluate the association of HSD17B13 with NAFLD and its clinical outcomes in the Asian population.
DISCUSSION
In this study of a well-characterised cohort of multi-ethnic Asian biopsy-proven NAFLD patients, we found loss-of-function HSD17B13 variants, namely rs72613567 TA allele and rs6834314 G allele, to be inversely associated with NAFLD and NASH in the overall subjects and among ethnic Chinese, but not ethnic Malays and Indians. This study also revealed that the rs72613567 homozygous TA allele and the rs6834314 G/G genotype are associated with reduced risk of liver-related complications and are associated with lower grade of hepatocyte ballooning among the ethnic Chinese.
These novel findings highlight the different impacts of the same genetic variants in persons of different ethnic backgrounds and complements previous interesting observations in genetic studies of NAFLD. Browning and colleagues were the first to report on ethnic differences in the prevalence of significant hepatic steatosis in their landmark paper [
17]. Although Hispanics and Blacks had a similar prevalence of obesity and insulin resistance, the prevalence of significant hepatic steatosis was much higher among Hispanics. The
PNPLA3 gene polymorphism was identified and reported as the underlying cause of the observed ethnic differences in their paper that followed 4 years later [
4]. Since then, many subsequent studies have confirmed the importance of this genetic variant in NAFLD. The
PNPLA3 genetic polymorphism was not only associated with a higher prevalence of NAFLD but also with more severe liver disease [
18]. Studies on multi-ethnic Malaysian populations have consistently shown a higher prevalence of NAFLD among ethnic Malays and Indians compared with that of ethnic Chinese, and this ethnic predilection can be observed as early as young adulthood [
16,
19]. However, interestingly, the frequency of the
PNPLA3 risk allele is relatively high among ethnic Chinese compared with that of the other ethnic groups [
20]. A meta-analysis has similarly reported high frequencies of the
PNPLA3 risk allele among East Asians but a relatively lower prevalence of significant hepatic steatosis [
21].
We set out to determine if the loss-of-function
HSD17B13 variants, recently reported to be protective against liver disease, underlining the lower prevalence of NAFLD despite a relatively high frequency of the
PNPLA3 risk allele among ethnic Chinese in our multi-ethnic population. We found that
HSD17B13 is protective against NAFLD and NASH among ethnic Chinese, but interestingly, not among ethnic Malays and Indians. A recent study among Japanese patients with NAFLD reported an attenuated effect of
PNPLA3 on advanced hepatic fibrosis by
HSD17B13 rs6834314 G allele [
10]. Abul-Husn et al. [
6] also reported that the
HSD17B13 rs72613567-TA allele is associated with decreased
PNPLA3 mRNA expression in an allele dosage-dependent manner. Further research is needed to identify the association between both
PNPLA3 and
HSD17B13 genotypes and their effect on patients with NAFLD.
rs72613567 is an insertion of an adenine adjacent to the donor splice site of exon 6 of
HSD17B13 in high linkage with the rs6834314, which is located downstream of the
HSD17B13 gene. For instance, rs72613567 T/TA and TA/TA are indel variants which produce truncated protein with reduced enzymatic activity.
HSD17B13 was first described as a liver specific lipid-droplet associated protein by Horiguchi et al. [
22] and upregulation of the gene expression in mice and patients with NAFLD was later shown [
23].
HSD17B13 encodes hydroxysteroid 17-╬▓ dehydrogenase, a protein that plays a pivotal role in hepatic lipid metabolism and overexpression of this enzyme contributes to the development of NAFLD [
24]. Hence, disruption of the protein expression that occurs in loss-of-function variants is a mechanism postulated to mediate its protective effect against liver damage. Support for the finding that
HSD17B13 rs72613567 is a protective NAFLD gene variant comes from a recent landmark study involving exome-wide sequencing of 46,544 European descents which reported a substantial decrease in serum alanine aminotransferase and aspartate aminotransferase levels associated with the variant [
6]. The study also demonstrated that the variant is associated with a lower risk of NAFLD, as well as non-alcoholic cirrhosis in an allele dose-dependent manner [
6]. The study has pioneered similar independent studies that confirmed the protective effect of the variant against NAFLD [
7,
8]. In this study, we showed that the
HSD17B13 rs72613567-TA allele is associated with lower odds of NASH in comparison with the controls, which is in line with the previous findings [
6-
8]. Pirola et al. [
7] described how the rs72613567-TA allele is significantly associated with decreased levels of hepatic
HSD17B13 protein in NAFLD patients bearing the heterozygous T/TA and homozygous TA/TA in an allele dose-dependent manner. Additionally, we replicated similar results in rs6834314, showing that patients bearing the minor G allele have a reduced risk of NASH. The rs6834314 variant also demonstrates lower protein levels essential for lipid droplet targeting, which is crucial for protein stability and protection from degradation [
8]. However, as mentioned before, the protective effect of the TA and G alleles was seen only among ethnic Chinese, but not among ethnic Malays and Indians. The cause for this novel finding is uncertain, it may be due to the effect of other genetic variants, and requires further investigation. It is interesting to note that the control frequencies for both rs72613567-TA and rs6834314-G alleles of the East Asian population from the 1000 Genomes Consortium were 0.34, which are similar to our population at 0.34 and 0.39, respectively.
Another highlight of our study is that the loss-of-function
HSD17B13 variants were found to be associated with reduced risk of liver-related complications, suggesting that the loss-of-function variant may contribute to its protective effect in NAFLD patients. Although there was no significant association between both variants with fibrosis, advanced fibrosis is an independent predictor of liver-related complications. The protective role of the
HSD17B13 variants may be lost once advanced fibrosis develops. A recent meta-analysis by Wang et al. [
25] showed that
HSD17B13 rs72613567 is associated with significant liver disease protection among NAFLD patients and milder disease severity among studies that included liver histology.
There was a lack of observed association between the TA allele of rs72613567 and steatosis, which is consistent with the findings from previous studies [
6,
7]. We also observed no interesting finding on the association between the variant and serum lipid levels. In addition, the expression of the protein on the lipid droplet membrane without affecting the intracellular fat content could potentially explain the lack of association between the TA allele and steatosis.
In vitro data reported by Ma and colleagues [
8] lend support to this postulation, whereby overexpressed
HSD17B13 in hepatocytes does not affect their fat-storage capacity. Thus, it seems reasonable to presume that
HSD17B13 does not modulate lipid content in a direct manner and the variant plays a much more important role in the pathogenesis of liver injury. However, the splice variant was shown to protect against liver ballooning degeneration, lobular inflammation and fibrosis [
7]. Similarly, in this study, we have demonstrated that the loss-of-function
HSD17B13 variants are associated with lower grade of hepatocyte ballooning among the ethnic Chinese. Although
HSD17B13 has been discovered as a liver specific lipid-droplet associated enzyme which contributes to the pathogenesis of NAFLD, no firm conclusions can be drawn regarding the underlying mechanism on how the change in
HSD17B13 activity modulates the liver lipid homeostasis due to its unknown substrates and metabolites.
A recent genetic association study in Japan reported that the carriage of the rs6834314 G allele attenuates the effect of
PNPLA3 on advanced fibrosis [
10]. Nonetheless, there exists a longitudinal prospective study for rs72613567 prior to the present study that reports a conflicting result, yet the finding can be argued. Despite having a relatively large number of patients (n=487) in their cohort, the patients were diagnosed with chronic viral hepatitis, alcoholic liver disease, or NAFLD-induced portal hypertension who already had advanced chronic liver disease [
26]. In addition, only 56 patients with NAFLD-induced portal hypertension were included in their study. However, the negative findings of Kaplan-Meier survival analysis of patients with different
HSD17B13 genotyping status in our cohort may be attributed to the small number of events in our study. The mechanism by which loss of
HSD17B13 protects against advanced liver disease remains to be elucidated. Nonetheless, growing evidence provides more certainty to the impact of this genetic variant in disease progression in NAFLD patients.
In this study, we also found that serum GGT level is significantly associated with various clinical outcomes. Serum GGT level is a reliable marker of oxidative stress. Haring et al. [
27] reported that elevated GGT levels are associated with an increased risk of mortality in men with hepatic steatosis. In addition, Ruhl and colleagues [
28] reported that in the USA population, elevated GGT is associated with mortality from all causes, liver disease, cancer, and diabetes. A recent study has also found that individuals with repeated elevated GGT levels have an increased risk of developing fatty liver changes [
29]. Nonetheless, serum GGT also contributes to several algorithms for the diagnosis of NAFLD and liver fibrosis such as the fatty liver index (FLI), SteatoTest, and Fibrotest. These findings explain the clinical importance of serum GGT in NAFLD and its role as a predictor of NAFLD outcome.
An interesting finding from our study showed that the rs72613567 heterozygous mutant genotype is associated with a higher incidence of cardiovascular outcome, but not in the homozygous mutant. We speculate that phenotypic consequences of a dominant mutation can be observed in a heterozygous individual carrying one mutant and one wild-type allele. In 1987, Herskowitz [
30] described dominant negative mutations as mutations in one allele, when overexpressed, may lead to a structural change in the protein that disrupts the activity of the wild-type gene. For instance, in the context of a dimer T/T, if the truncated protein TA renders T/TA and TA/TA dimers inactive, then a heterozygote will produce 25% T/T, 50% T/TA and 25% TA/TA, leading to only 25% of activity with respect to the wild type or homozygous mutant and 50% activity with respect to the heterozygous. The notion of heterozygous dominant negative mutations causes more severe effects than that does simple null alleles or homozygous alleles of the same gene [
30,
31] leads us to believe that the possible mechanism of heterozygous mutations may be more deleterious than that of a truncated protein (TA/TA), which explains the significantly higher incidence of cardiovascular outcome recorded in the rs72613567 T/TA genotype in the present study. However, this novel finding warrants further studies to confirm the impact of rs72613567 on cardiovascular outcomes in patients with NAFLD.
To our knowledge, this study is the first longitudinal cohort study among the Asian population to report a potential protective effect of the protein-truncating HSD17B13 variants on adverse liver outcomes in patients with NAFLD. This novel finding may be useful for future pharmacological development of therapeutic gene inhibition by selective enzyme inhibition techniques or could potentially be utilised as a biomarker to predict the development of advanced liver disease. Moreover, our study was able to compare genetic variants among three Asian ethnic groups. This study has several limitations. First, our study had relatively short follow-up duration in some of our study subjects and hence, a relatively small number of observed events. However, power analysis was conducted prior to the commencement of study to calculate sample sizes and the data available were adequate for statistical significance. Second, we were not able to study the effect of the genetic variants according to ethnic groups due to the even smaller number of observed events when stratified according to ethnicity. Interestingly, there were no liver-related complications among the ethnic Chinese despite the equal proportion of more severe liver disease at baseline compared with that of the other ethnic groups. Third, data on follow-up events were retrieved retrospectively from the medical records; hence, there is a small possibility of missing events while patients seek medical treatment in elsewhere. Due to ethical considerations, liver biopsy was not performed in the controls. Therefore, a list of inclusion and exclusion criteria was used to recruit control subjects, and liver ultrasound was performed to exclude the presence of NAFLD. Lastly, we were unable to follow-up the control subjects and demonstrate a comparison between the NAFLD and non-NAFLD groups in terms of clinical outcomes.
In conclusion, in this two-part study on a cohort of biopsy-proven multi-ethnic Asian NAFLD patients, the loss-of-function variants HSD17B13 rs72613567 and rs6834314 were found to be inversely associated with NASH, specifically among ethnic Chinese, but not among ethnic Malays and Indians. The variants were also found to be independently associated with a lower incidence of liver-related complications. Future studies should examine thoroughly the impact of genetic variants on liver-related outcomes in different ethnic groups, explore the use of genetic variants to predict more severe liver disease, and thereby identify patients for early interventions. We must investigate the underlying mechanism of the pathogenic link between HSD17B13 and adverse liverrelated outcomes in NAFLD, and explore the therapeutic potential of these genetic variants.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Supplement1
Supplement1 Print
Print



