| Clin Mol Hepatol > Volume 27(4); 2021 > Article |
|
ABSTRACT
ACKNOWLEDGMENTS
FOOTNOTES
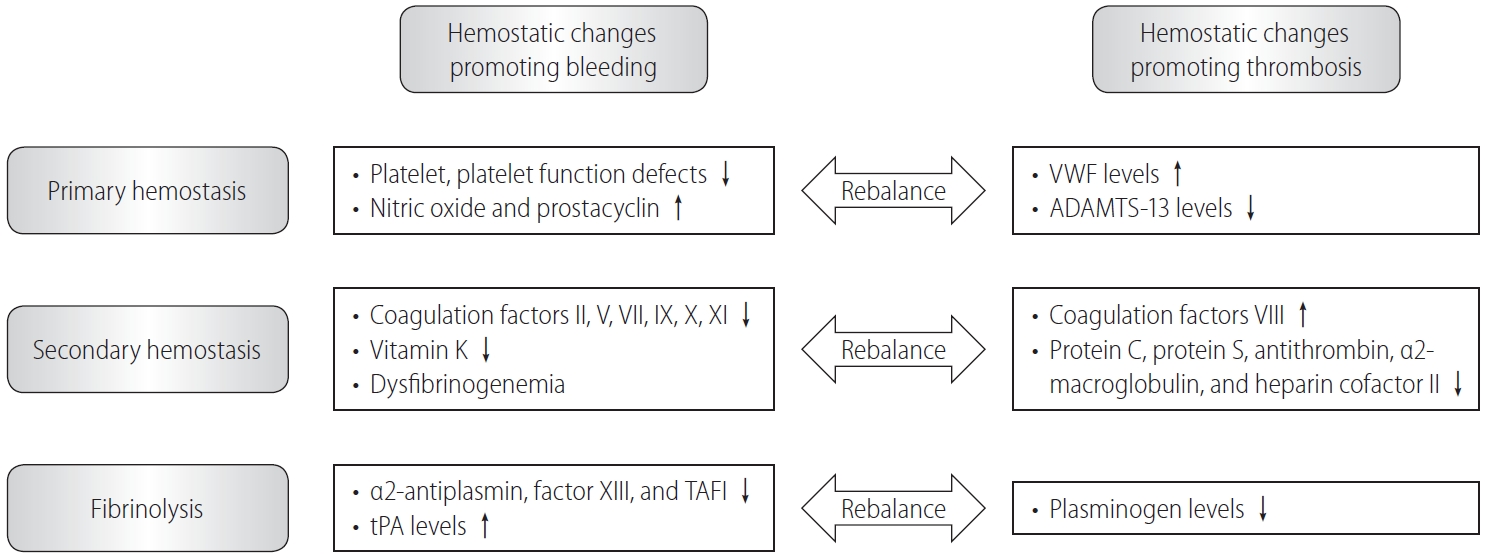
Figure┬Ā1.
Table┬Ā1.
| Guideline | EASL (2016) | AASLD (2020) |
|---|---|---|
| Classification | 1. Acute PVT in patients without malignancy and cirrhosis | 1. Recent PVT (for <6 months) |
| 2. Cirrhotic PVT | 2. Chronic PVT (for >6 months) | |
| Treatment | Acute PVT in patients without malignancy and cirrhosis | Recent PVT |
| Start LMWH + antibiotics if septic thrombophlebitis | Medical anticoagulants ┬▒ local or systemic thrombolytic therapy (very selected cases) | |
| Treat cause when accurate | Recent thrombosis of small intrahepatic sub-branches, minimal occlusive (<50% of lumen) of main PV: observation by serial imaging every 3 months.* | |
| Recent occlusive or partially occlusive (>50% obstruction of the lumen) thrombosis of the main PV or mesenteric veins: antithrombotic therapy should be considered to avoid thrombosis progression.* | ||
| Cirrhotic PVT | Chronic PVT | |
| Medical anticoagulants (applicability of TIPS to treat PVT unknown) | Medical anticoagulants ┬▒ TIPS (with advanced PVT and recurrent bleeding and/or refractory ascites) | |
| Anticoagulation must be started always after implementing an adequate prophylaxis for gastrointestinal bleeding.* | Chronic complete occlusion of the main PV or cavernous transformation of the PV with established collaterals: no established benefit of anticoagulant or interventional therapy, and treatment should focus on the management of portal hypertension complications.* | |
| Type of anticoagulants | LMWH or VKAs (no information currently available for DOACs) | LMWH, VKA, or DOACs (recommendations rely on smaller cohort studies for DOACs) |
| Treatment duration | At least 6 months | Depends on clinical condition |
| Side effect (most common) | Bleeding | Non-portal hypertensive bleeding |
| LT candidates | Consider prolonging anticoagulation for some months and until transplantation, once PVT has been repermeated. | Anticoagulation with the goal to recanalize the portal vascular system before LT. |
| Consider TIPS for those with progressive PVT who are not responding to anticoagulation. | PV recanalization followed by TIPS should be considered. |
PVT, portal vein thrombosis; EASL, European Association for the Study of the Liver; AASLD, American Association for the Study of Liver Diseases; LMWH, low molecular weight heparin; PV, portal vein; TIPS, transjugular intrahepatic portosystemic shunt; VKAs, vitamin K antagonists; DOACs, direct-acting oral anticoagulants; LT, liver transplantation.
Table┬Ā2.
Table┬Ā3.
DOACs, direct-acting oral anticoagulants; AF, atrial fibrillation; TIA, transient ischemic attack; CABG, coronary artery bypass graft; PCI, percutaneous coronary intervention; ECG, echocardiogram; CNS, central nervous system; CHADS2, congestive heart failure, hypertension, age Ōēź75 years, diabetes mellitus, stroke [double weight]; ALT, alanine amino-transferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; ULN, upper limit of normal; HCV, hepatitis C virus.
Table┬Ā4.
| Study | Study design | Aim of study | Indication for anticoagulation therapy | Number of patients | Drug and doses | Treatment duration | Efficacy | Safety |
|---|---|---|---|---|---|---|---|---|
| Intagliata et al. [68] (2016) | 1. Retrospective | To compare the rates of bleeding in cirrhotic patients (DOAC vs. traditional anticoagulants) | Splanchnic thrombosis, non- splanchnic venous thromboembolism, and atrial fibrillation | DOAC (n=20; PVT, n=12) vs. warfarin (n=19) | Rivaroxaban 20 mg daily or apixaban 5 mg bid daily (n=15, 75%) | 10.6 months (mean) | Not studied | Major bleeding (n=1) |
| 2. Cirrhotic, CP A, B | Rivaroxaban 10 mg daily or apixaban 2.5 mg bid daily (n=5, 25%) | |||||||
| 3. DOAC (apixaban and rivaroxaban) vs. traditional anticoagulants (warfarin and LMWH) | ||||||||
| Hum et al. [69] (2017) | 1. Retrospective | To identify of the efficacy and safety of DOAC vs. traditional anticoagulants in cirrhosis | Atrial fibrillation or venous thromboembolism, including PVT and deep vein thrombosis | DOAC (n=27; PVT, n=4) vs. warfarin/LMWH (n=18; PVT, n=3) | Rivaroxaban 15 mg bid ┬▒20 mg daily load (63%), apixaban 5 mg bid ┬▒10 mg bid load (47%), no bridging | 10.6 months (mean) | Recurrence (n=1, 4%) | 1. 10 bleeds in traditional group, 8 bleeds in DOAC group (P=0.12) |
| 2. Cirrhotic, CP A, B & C | 2. More major bleeds in traditional group (n=5) than in DOAC group (n=1) (P=0.03) | |||||||
| 3. DOAC (apixaban and rivaroxaban) vs. traditional anticoagulants (enoxaparin and warfarin) | ||||||||
| De Gottardi et al. [70] (2017) | 1. Retrospective | To identify indications and reasons for starting or switching to DOAC and report adverse effects, complications, and short-term outcomes | Splanchnic vein thrombosis, deep vein thrombosis, atrial fibrillation, and others | Total (n= 94) | Cirrhotic: rivaroxaban 15 mg (range, 5ŌĆō20; 83%), apixaban 5 mg (range, 2.5ŌĆō10; 11%), and dabigatran 165 mg (range, 110ŌĆō220; 5%) | Cirrhotic: 9.6 months (mean) | Cirrhotic: recurrent PVT (n=1, 4.5%) | Cirrhotic: minor bleeding (n=4), major bleeding (n=1) |
| 2. Both cirrhotic and non- cirrhotic, CP A, B | Cirrhosis (n=36) | |||||||
| Cirrhosis with PVT (n=22) | ||||||||
| Nagaoki et al. [71] (2018) | 1. Retrospective | To compare the efficacy and safety of edoxaban and warfarin for treatment of PVT following danaparoid sodium in cirrhotic patients | Portal vein thrombosis | Total (n=50) | Edoxaban 60 mg (CrCl >50) (n=4), edoxaban 30 mg (CrCl 30ŌĆō50) (n=16) | 6 months (maximum) | Recanalization: 90% (complete, n=14; partial, n=1; unchanged, n=1; progression, n=1) | Major gastrointestinal bleeding: edoxaban (n=3) and warfarin (n=2) (P=0.335) |
| 2. Cirrhotic, CP A, B | Edoxaban (n=20) | Edoxaban group had more complete resolution and less PVT progression than warfarin group | ||||||
| 3. Edoxaban vs. warfarin following 2 weeks of danaparoid treatment in cirrhotic PVT | Warfarin (n=30) | |||||||
| Scheiner et al. [72] (2018) | 1. Retrospective | To assess the course of nonmalignant PVT in patients receiving anticoagulants | Portal vein thrombosis | Total (n=10) | Edoxaban 30 or 60 mg once daily, apixaban 5 mg bid daily, rivaroxaban 10 mg once daily, dabigatran 110 mg bid daily | 9.2 months (median) | 20% with regression/resolution of PVT, 80% with stable PVT including unchanged cavernous transformation | Portal hypertensive gastropathy bleeding (n=1) |
| 2. Both cirrhotic and noncirrhotic | Cirrhosis (n=3; edoxaban [n=4], apixaban [n=3], rivaroxaban [n=2], dabigatran [n=1]) | |||||||
| Hanafy et al. [73] (2019) | 1. Prospective | To evaluate the efficacy and safety of rivaroxaban compared to warfarin | Portal vein thrombosis | Rivaroxaban (n=40) vs. warfarin (n=40) | Rivaroxaban 10 mg bid daily | 6 months (maximum) | Recanalization: 100% with rivaroxavan (complete, n=34; partial, n=6) vs. 45% with warfarin | Eight deaths in the warfarin group; none in the rivaroxaban group |
| 2. Cirrhotic, CP A, B | ||||||||
| 3. Rivaroxavan vs. warfarin for acute PVT | ||||||||
| Ai et al. [75] (2020) | 1. Prospective | To investigate the efficacy and safety of DOACs compared to no anticoagulants | Portal vein thrombosis | DOACs (n=40; rivaroxaban [n=26], dabigatran [n=14]) vs. no anticoagulants (n=40) | Rivaroxaban 20 mg once daily | 6 months (maximum) | Recanalization: DOACs vs. no anticoagulation: 12.8% (n=5/39) vs. 0% (n=0/40) at 3 months; 28.2% (n=11/39) vs. 2.6% (n=1/38) at 6 months | DOACs: melena 1, hematuria 1, hemoptysis 1 case vs. no anticoagulation: melena 1 (P>0.05) |
| 2. Cirrhotic, CP A | Dabigatran 150 mg bid daily | |||||||
| 3. Rivaroxaban or dabigatran vs. no anticoagulants | ||||||||
| Lv et al. [74] (2021) | 1. Prospective | To evaluate the management using a waitand-see strategy, anticoagulation, and transjugular intrahepatic portosystemic shunt to treat PVT in cirrhosis | Portal vein thrombosis | Total (n=396) | Rivaroxaban 10 mg daily | 21.0 months (median) | Recanalization: 0% with rivaroxavan only (all with PVT and SMV thrombosis), 100% with TIPS+rivaroxavan | Major bleeding events: AC only (n=14, 22.2%) |
| 2. Cirrhotic, CP A, B & C | All anticoagulants (n=260) | |||||||
| 3. Comparison of anticoagulants and/or TIPS, and no treatment | AC only (n=63; warfarin [n=51], enoxaparin [n=8], rivaroxaban [n=4]) | TIPS+AC (n=30, 15.2%) | ||||||
| TIPS only (n=88) | ||||||||
| TIPS+AC (n=197; rivaroxaban+TIPS [n=18]) |
Table┬Ā5.
Abbreviations
REFERENCES
- TOOLS
-
METRICS

- ORCID iDs
-
Minjong Lee

https://orcid.org/0000-0002-3159-5444Tae Hun Kim

https://orcid.org/0000-0003-3277-3668 - Related articles
-
The use of vaptan in hyponatremic patients with liver cirrhosis2011 December;17(4)




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print



