KASL clinical practice guidelines: Management of nonalcoholic fatty liver disease
Article information
INTRODUCTION
Preamble
The prevalence of nonalcoholic fatty liver disease (NAFLD) is increasing rapidly worldwide as the obese and diabetic populations increase, and it has been estimated to be 20–30% in Korea. Considering the increased popularity of a westernized diet and lifestyle, lack of exercise, and the resulting increase in obesity and diabetes, NAFLD is predicted to become more prevalent in the future and to become a major cause of chronic liver disease. In some patients, NAFLD progresses to end-stage liver diseases such as cirrhosis and hepatocellular carcinoma (HCC), and it is an independent cardiovascular risk factor.
In 2013, the Korean Association for the Study of the Liver (KASL) enacted a clinical practice guideline for the diagnosis and treatment of NAFLD to improve understanding of the disease and provide useful clinical information and direction for healthcare providers. The research results that have accumulated since then necessitate a revision. Accordingly, the clinical practice guidelines committee began revising the guidelines to reflect the results of Korean and international research and develop new recommendations based on a systematic approach that reflects evidencebased medicine and expert opinions. However, evidence remains insufficient, and many studies are currently being conducted. As medical evidence and new findings accumulate in the future, these guidelines will require ongoing supplementation and revision.
Target population
Patients diagnosed with NAFLD based on clinical, biochemical, radiological, or pathological findings in the absence of significant alcohol consumption and liver diseases such as viral hepatitis were the primary research population involved in the development of these guidelines. These guidelines are also based on data from pediatric and adolescent NAFLD patients, whose unique findings distinguish them from adult NAFLD patients.
Intended users
The aim of these guidelines is to provide useful clinical information and direction to healthcare providers involved in the diagnosis and treatment of NAFLD patients. Moreover, these guidelines are intended to provide definite and practical information to resident physicians, practitioners, and trainers.
Developer and funding information
The Clinical Practice Guideline Committee for the Management of NAFLD (Committee) was organized in accordance with proposals by the approval of the KASL Board of Executives and consists of 16 gastroenterologists, one pathologist, one radiologist, and two pediatricians specializing in hepatology. All expenses were paid by KASL. Each committee member collected and analyzed the source data in his or her own field, and the members then wrote the manuscript together.
Evidence collection
The committee systematically collected and reviewed the international and domestic literature published in PubMed, MEDLINE, KoreaMed, and other databases. The literature was limited to research papers published in the English and Korean languages. The keywords used were ‘nonalcoholic fatty liver disease,’ ‘nonalcoholic fatty liver,’ ‘nonalcoholic steatohepatitis,’ ‘fatty liver,’ ‘hepatic steatosis,’ and ‘steatohepatitis.’ In addition, keywords related to specific clinical questions were included.
Levels of evidence and grades of recommendations
The literature gathered for data collection was analyzed in a systematic review, and the quality of evidence was classified based on the modified Grading of Recommendations, Assessment, Development and Evaluation (GRADE) system (Table 1). According to the types of studies, randomized, controlled studies were approached from a high level of evidence, while observational studies were approached from a low level of evidence. Subsequently, the level of evidence basis sets in corresponding studies was elevated or lowered by accounting for the factors influencing the quality of the studies. Through follow-up studies, the level of evidence was defined as follows: A, indicating the highest level of evidence with the smallest possibility of any changes in the conclusion; B, indicating a moderate level of potential changes; and C, indicating the lowest level of evidence with the greatest possibility of any changes.
The strength of each recommendation was suggested according to the GRADE system. In addition to the level of evidence, the results of studies were considered based on aspects of clinical multipliers and socio-economic factors, such as cost. Grading of the recommendations was performed as follows: 1, strong recommendation or 2, weak recommendation. A strong recommendation indicated, for example, that the interventions could be applied in most patients with strong certainty, there was a greater possibility of desirable effects, and there was high-quality evidence, as well as presumed patient-important outcomes, cost-effectiveness, preference, and compliance. A weak recommendation indicated a suggestion made with less certainty but that could be considered favorable for many patients. Alternative interventions could be chosen for “weak recommendations”, according to cost and the preferences of the patients or medical practitioners.
These Clinical Practice Guidelines for the Management of NAFLD have been developed through the reviews of medical experts to be used practically for treatment, research, and education. These recommendations are not absolute standards for treatment, and adoption of these guidelines in clinical practice can differ for individual patients.
List of key questions
The committee considered the following clinical questions as key components to be covered in the guidelines.
1. What are the definition and categories?
2. What are the incidence rate and prevalence rate?
3. How does NAFLD progress?
4. What causes NAFLD-related deaths?
5. What are the risk factors of NAFLD?
6. What are NAFLD’s comorbidities?
7. Who should be targeted for NAFLD screening, and how is screening conducted?
8. Which non-invasive surrogates are available to diagnose hepatic steatosis?
9. Which non-invasive surrogates are available to diagnose NASH?
10. Which non-invasive surrogates are available to assess liver fibrosis?
11. Which differential tests are available for advanced fibrosis?
12. What are the indications for liver biopsy?
13. What are the histopathological features of NAFLD?
14. Is surveillance for HCC necessary?
15. How can HCC be prevented?
16. Who should be treated, and what is the aim of the treatment?
17. What do lifestyle modifications include?
18. What is the effect of moderate or less alcohol consumption?
19. What are the types, indications, effects, and side effects of medications for NAFLD?
20. What are the indications for and post-operative management of bariatric surgery?
21. What are the indications for liver transplantation (LT) and post-LT management?
Regarding NAFLD in children and adolescents:
1. What is the prevalence rate?
2. How does NAFLD progress?
3. What genetic diseases are associated with risk factors?
4. Is related NAFLD related to family history and genetic predisposition?
5. Who should be targeted for NAFLD screening, and how is screening conducted?
6. What are the diagnostic methods?
7. Who should be treated, and how?
Review of the manuscript and approval process
The manuscripts written by the committee members were reviewed and approved through meetings of the committee. The quality of the manuscripts was evaluated based on the integrity of the contents and the standards of Appraisal of Guidelines for Research and Evaluation II (AGREE II). The guidelines were also reviewed at a meeting of an external review board consisting of 11 specialists in the field of hepatology and at a symposium open to all KASL members, and they were then further modified prior to publication. The final manuscript was endorsed by the Board of Executives of KASL.
Release of the guidelines and plan for updates
The Korean version of the KASL Clinical Practice Guideline for the Management of NAFLD was released at Liver Week 2021 (May 15, 2021), and published in the Clinical and Molecular Hepatology (July 2021). This guideline in Korean is available on the KASL website (http://www.kasl.org). Updates are planned when new reliable evidence has accumulated. Detailed plans for updates will be posted on the KASL website.
DEFINITION
What are the definition and categories?
NAFLD is a condition characterized by finding fat infiltration of the liver on radiological exams or biopsy without significant alcohol intake, viral hepatitis, medication intake that would cause a fatty liver, or other obvious cause. NAFLD is defined as a disease with findings suitable for clinical, biochemical, imaging, and pathological examinations. NAFLD is a generic term that encompasses the spectrum of nonalcoholic fatty liver (NAFL), nonalcoholic steatohepatitis (NASH), and NASH cirrhosis (Table 2). The significant safe limits of daily alcohol intake that distinguish NAFLD from alcoholic fatty liver disease range from 10–40 g (pure alcohol), though that range varies between studies. Therefore, definite criteria are difficult to recommend. The Clinical Practice Guideline of the European Association for the Study of the Liver agreed to define the amount of significant alcohol consumption as weekly alcohol consumption ≥210 g in men and ≥140 g in women [1]. No ethnic differences have been reported regarding safe alcohol limits that do not produce liver damage. The KASL Clinical Practice Guideline for NAFLD uses the amount of significant alcohol consumption stated above in clinical treatment and in studies for international comparison with the results of future studies.
The term NAFLD was introduced by Schaffner in 1986 [2]. However, as information about the causes and mechanisms of the disease accumulated, an opinion emerged that the term NAFLD does not reflect the condition’s heterogeneous pathogenesis and various courses. Furthermore, the overestimation of the exclusion of alcohol has sparked heated debate about the threshold of “significant” alcohol consumption required for the diagnosis of NAFLD, and the potential (contradictory) role of alcohol consumption in these “non-alcohol” diseases has been repeatedly raised. In 2019, a consensus by 32 experts suggested alternative terminology, metabolic (dysfunction)-associated fatty liver disease (MAFLD), to more accurately reflect the pathogenesis of this disease [3]. The diagnosis of MAFLD is based on evidence of fat accumulation in the liver in the presence of one of the following three criteria: overweight/obesity, type 2 diabetes mellitus (T2DM), and evidence of metabolic dysregulation. Further research results are needed to verify the validity of this term.
EPIDEMIOLOGY
What are the incidence rate and prevalence rate?
Incidence
In 2007, researchers published results from tracking 5,237 men for more than 4 years and reported for the first time that the annual incidence of NAFLD was 74.1 cases per 1,000 persons [4]. The annual incidence rate of NAFLD diagnosed by abdominal ultrasonography in health screening examinees was about 48.2 cases per 1,000 persons (range, 13.4–77.7) [5-11]. As diagnosed by the hepatic steatosis index (HSI), the annual incidence rate was 21.1 cases per 1,000 persons [12]. According to a meta-analysis in Asia, the annual incidence rate in Korea was 45.1 cases per 1,000 persons [13,14].
Prevalence
The prevalence of NAFLD varies depending on the study population, definition of NAFLD, and diagnostic modality. In 2002, the prevalence among 1,074 people receiving health checkups and diagnosed by abdominal ultrasound was 48.6% [15]. The prevalence among 141,610 people receiving health checkups in the Seoul and Gyeonggi area and diagnosed by abdominal ultrasound was 25.2% (male, 34.4%; female, 12.2%) [16], and the prevalence in other studies varied from 21% to 44% [17-23]. In a meta-analysis, the prevalence in Korea, as diagnosed by ultrasonography, was 32.9% [13].
The prevalence diagnosed using the fatty liver index (FLI) was 12.6–16.1% [22,24], and the prevalence diagnosed with transient elastography was 42.9% [25]. The prevalence diagnosed by liver biopsy among 589 living liver donors in Korea was 51% [26].
Lean/non-obese NAFLD
People with normal body weight (body mass index [BMI; kg/m2] of less than 23 kg/m2 for Asians, less than 25 kg/m2 for Westerners) or non-obese weight (BMI of less than 25 kg/m2 for Asians, less than 30 kg/m2 for Westerners) can also be diagnosed with NAFLD, but data on the incidence rate among those people in Korea are limited. In 2004, the prevalence of NAFLD was 16.1% among 460 people with a BMI between 18.5 kg/m2 and 25 kg/m2 who received domestic health checkups [27]. In several domestic studies, the average prevalence of non-obese NAFLD was 18.8% (12.4–27.1%) [28-34].
Summary
1. The annual incidence of NAFLD in Korea is about 45 cases per 1,000 persons.
2. The prevalence of NAFLD in Korea is approximately 30%.
3. The prevalence of nonalcoholic fatty liver disease in the domestic non-obese population is about 19%.
Natural course and cause of death
How does NAFLD progress?
Because the natural course of NAFLD can only be confirmed through repeated liver biopsy, it has been reported only in small studies. The incidence of NASH was reported to vary from 8.5% to 64.0% over a 3.0–6.6 years follow-up period, a large variation that appears to result from differences in the number of patients, various follow-up periods, and diagnostic criteria [35-37].
In a meta-analysis comparing NAFL and NASH, the percentage of patients who progressed to more than one stage of liver fibrosis were similar at 39.1% and 34.5%, respectively, but the time it took those patients to progress more than one stage was 14.3 years and 7.1 years, respectively. NASH thus showed faster progression [38].
In a cohort study of NASH patients, the incidence of cirrhosis varied by race and region, with an average of 21–26% of patients progressing to cirrhosis in eight years [39,40]. According to a study of an LT waitlist, the number of patients with end-stage liver disease because of NAFLD had tripled from 10 years previously, making NAFLD the second most common reason for needing LT [41].
Recently, HCC caused by NAFLD has rapidly increased, becoming the third most common cause of HCC development in United States, and that number is expected to increase by 9% per year [42,43]. This trend is believed to be due to the rapid increase in the prevalence of obesity, which is a risk factor for NAFLD. NAFLD is associated with a higher incidence of HCC than other etiologies of liver disease in the absence of advanced fibrosis or cirrhosis [44-46].
What causes NAFLD-related deaths?
According to data from the U.S. National Vital Statistics System, the mortality rate of NAFLD has increased in the past decade [47]. In a meta-analysis, patients with NAFLD had a 1.6 times higher mortality rate than the general population. The major causes of death were cardiovascular disease (CVD), malignancy, and liver disease, and in the presence of steatohepatitis, liver disease-related deaths increased [48-50]. In several cohort studies, the prognosis of patients with NAFLD was most closely related to the degree of liver fibrosis [51,52]. In a large-scale cohort study based on liver biopsy, the mortality rate of NAFL patients was 1.7 times that of the normal control group; the mortality rate of patients who had steatohepatitis without hepatic fibrosis was 2.1 times that of the normal control group; patients with liver fibrosis had a mortality rate 2.4 times that of the normal control group; patients with cirrhosis had a mortality rate that was 3.8 times that of the normal control group. Thus, the mortality rate increased with the degree of liver fibrosis [53].
Summary
1. Liver fibrosis can progress faster in NASH than in NAFL.
2. The incidence of liver cirrhosis and HCC associated with NAFLD is increasing, and HCC can develop even in the absence of liver cirrhosis.
3. The main causes of death among patients with NAFLD are CVD, malignant tumors, and liver diseases, and liver diseaserelated mortality increases when steatohepatitis and liver fibrosis are present.
What are the risk factors of NAFLD?
NAFLD is closely related to obesity, diabetes, dyslipidemia, and metabolic syndrome (Fig. 1). Obesity is a well-known risk factor for NAFLD, and the prevalence of NAFLD increases as BMI increases [54]. Among obese patients who underwent bariatric surgery, the prevalence of NAFL, NASH, and significant liver fibrosis were 61–91%, 30–37%, and 29.3%, respectively [55,56]. Metabolic syndrome, which consists of abdominal obesity, impaired fasting blood sugar, hypertriglyceridemia, low high-density cholesterolemia, and hypertension, is a major risk factor for NAFLD, just like obesity. In the presence of metabolic syndrome, the prevalence of NAFLD was 50% [57,58]. The prevalence of NAFLD was also high in diabetic patients, 60–75% [14]. In patients with dyslipidemia, another risk factor, the prevalence of NAFLD was 50% [59,60].
In the presence of hypothyroidism, the prevalence of NAFLD increases by 1.6 times [61], and in the presence of polycystic ovary syndrome, the incidence increases by about 2.2 times [62]. In addition, obstructive sleep apnea [63], hypopituitarism [64], hypogonadism [65], pancreatoduodenal resection [66], and psoriasis [67] increase the prevalence of NAFLD.
Decreased physical activity and sarcopenia increase the risk of NAFLD [68,69]. The prevalence and incidence of NAFL increased in the group with reduced physical activity [70-72]. In the presence of sarcopenia, the risk of NAFLD increased by about four times regardless of obesity or metabolic syndrome [68,69]. When sarcopenia was accompanied by NAFLD, the risk of advanced liver fibrosis increased by 1.8 times [69].
Genetic factors play a major role in the occurrence of NAFLD. Typically, the patatin-like phospholipase domain-containing 3 (PNPLA3) and transmembrane 6 superfamily, member 2 (TM6SF2) single nucleotide polymorphisms affect the development and progression of the disease. In Korea, PNPLA3 and sorting and assembly machinery component 50 (SAMM50) were associated with the prevalence and severity of NAFLD [73].
Summary
1. Major risk factors for NAFLD include obesity, diabetes, dyslipidemia, and metabolic syndrome.
What are NAFLD’s comorbidities?
NAFLD is closely related to systemic metabolic diseases [74], and it is an independent risk factor for the occurrence of various non-hepatic diseases, including CVD, T2DM, metabolic syndrome, chronic kidney disease (CKD), and malignant tumors [75-77].
CVD
In a retrospective cohort study of 1,804 patients with NAFLD in Denmark in the 2000s, the mortality rate from CVD was higher than that in the normal control group [50]. Several cohort studies that followed also found NAFLD to be an independent risk factor for CVD [78,79]. In a study that followed patients with histologically diagnosed NAFLD for an average of 26.4 years, the mortality rate increased by 1.3 times, and the risk of CVD increased by 1.6 times compared with the normal control group, with both increases associated with the degree of liver fibrosis [78]. A meta-analysis of patients with NAFLD diagnosed histologically or radiologically concluded that the risk of CVD was increased by about 1.6 times compared with the normal control group. The risk of CVD also increased when liver fibrosis was assessed using the NAFLD fibrosis score (NFS) or liver biopsy [80]. According to two Korean cohort studies, NAFLD was independently associated with the incidence of coronary artery calcification and atherosclerosis, which are direct causes of CVD [18,81].
T2DM
T2DM and NAFLD influence each other [82]. In a large-scale cohort study of healthy Koreans, the prevalence and incidence of T2DM in patients with NAFLD (assessed using the NFS) increased with the degree of hepatic fibrosis [17]. In a meta-analysis of Korean studies, the prevalence of diabetes in patients with NAFLD was 14.2%, higher than the 5.2% of the control group [83]. The results of a meta-analysis of other studies also showed an increase of about 2.2 times in the incidence of T2DM [84].
Metabolic syndrome
Metabolic syndrome is a major risk factor for the occurrence of NAFLD and is a common comorbid disease. A prospective Korean cohort study of 46,874 men reported that patients with mild and moderate NAFLD (diagnosed by abdominal ultrasound) had a risk of metabolic syndrome 1.5 and 2 times higher, respectively, than the control group [85]. In a meta-analysis of Korean studies, the incidence of metabolic syndrome was 40.7% in patients with NAFLD and 11.2% in the control group [83].
CKD
CKD and NAFLD share risk factors such as T2DM and hypertension [86], and CKD frequency increases in patients with NAFLD [87-89]. In a meta-analysis of about 63,000 people, the prevalence of CKD increased by 2.1 times, and the incidence rate increased by 1.7 times compared with the control group [90]. The prevalence and incidence of CKD were 2.5 times and 2.1 times higher in NASH than NAFLD. In a Korean cohort study, patients with NAFLD had a 1.2 times higher risk of developing CKD than the control group, and that risk increased with the stage of liver fibrosis [91].
Other diseases
Arrhythmia, osteoporosis, colon adenoma, colon cancer, and breast cancer all have increased incidence in patients with NAFLD [92-94]. However, more follow-up studies are needed to confirm clear relationships between NAFLD and those diseases.
Summary
1. NAFLD is often associated with comorbidities such as cardiovascular disease, T2DM, metabolic syndrome, CKD, and malignant tumors.
DIAGNOSIS
Who should be targeted for NAFLD screening, and how is screening conducted?
Screening for NAFLD should be considered in cases of persistent liver enzyme elevation. Because the cost effectiveness of screening in diabetic patients has been confirmed [95], patients with diabetes should receive screening tests for NAFLD regardless of their liver enzyme levels. In addition, subjects with metabolic syndrome (which is closely associated with insulin resistance), obesity, and other risk factors for NAFLD can be considered for screening tests [17,96].
The primary screening test for NAFLD is ultrasonography. When NAFLD is suspected in the ultrasonography results, other tests (computed tomography [CT], magnetic resonance imaging [MRI], serologic tests, transient elastography, etc.) can be performed.
[Recommendations]
1. Subjects who have persistent liver enzyme elevation or diabetes should be screened for NAFLD. (A1)
2. Subjects with metabolic syndrome (which is closely associated with insulin resistance, obesity, and other risk factors for NAFLD) can be considered for screening. (B1)
3. Abdominal ultrasonography is the primary screening modality. (B1)
Non-invasive examinations
Because the prognosis of NAFLD differs significantly depending on the histological findings, the diagnosis of hepatic steatosis and fibrosis and assessment of their severity are clinically crucial. To date, liver biopsy is the gold standard in assessing hepatic necroinflammation, steatosis, and fibrosis. However, liver biopsy is expensive, invasive, carries a risk of complications, is subject to intra-/inter-observer interpretational variability, and can suffer from sampling errors when an insufficient amount of liver tissue is collected [97,98]. Therefore, noninvasive radiological surrogates such as ultrasonography and panels of serological tests are widely used.
Which non-invasive surrogates are available to diagnose hepatic steatosis?
Radiological surrogates
Abdominal ultrasonography is the first-line imaging modality for screening asymptomatic patients with abnormal liver enzyme levels. However, it suffers from subjective interpretation, examination difficulties in obese patients, and low sensitivity when the amount of hepatic steatosis is less than 30%. Moreover, ultrasonography cannot distinguish NASH from NAFL [99-101]. When hepatic steatosis assessed using MR spectroscopy (MRS) was used for reference, the diagnostic accuracy of ultrasonography was lower than that of non-contrast CT and MRI [102]. However, ultrasonography is appropriate as a screening test, because it offers 1) robust diagnostic performance for moderate or severe steatosis, 2) the ability to evaluate the entire hepatobiliary system in addition to detecting the presence of hepatic steatosis, and 3) wide availability.
Controlled attenuation parameter (CAP) is a feature of transient elastography that can quantify the degree of fat deposition in the liver parenchyma by measuring ultrasound attenuation [103,104]. According to a recent Korean study enrolling people who received regular health checkups, the normal CAP range is 156–287 dB/m [105]. The area under the receiver operating characteristic curve (AUC) of CAP used to diagnose a moderate to severe degree of steatosis was 0.88 (sensitivity, 83.3%; specificity, 81.6%), and the cutoff value was 276 dB/m in a Korean study of living LT donors [106]. In another Korean study, the AUCs of CAP for mild, moderate, and severe degrees of steatosis in patients with chronic liver diseases, including NAFLD, were 0.885, 0.894, and 0.800, respectively, and the cutoff values were 250 dB/m, 299 dB/m, and 327 dB/m, respectively [107]. In a recent meta-analysis, the AUCs of CAP for mild, moderate, and severe degrees of steatosis were 0.96, 0.82, and 0.70, respectively [108]. CAP can be used as a monitoring tool for hepatic steatosis, and it can be examined simultaneously with transient elastography [109-111]. Other ultrasound-based methods, including image-based quantitative analysis of liver parenchymal echo texture and measuring the attenuation of ultrasound energy, are under investigation [112,113].
Hepatic steatosis is associated with low attenuation on CT scans, which can be used for the quantitative assessment of hepatic steatosis. Because the attenuation of enhanced CT is affected mainly by the amount of blood flow, unenhanced CT is favored for the measurement of hepatic steatosis, and the attenuation of liver and spleen parenchyma are frequently compared. For moderate to severe steatosis, the specificity of CT was reported to be high, but its sensitivity and positive predictive value were low [101,114,115]. Furthermore, its diagnostic performance for mild steatosis was suboptimal. The specificity and sensitivity of unenhanced CT for diagnosing moderate to severe steatosis were 100% and 53.8%, respectively, when CT attenuation of hepatic parenchyma was less than 48 HU [115]. However, CT raises concerns about radiation hazards, and CT evaluations of hepatic steatosis are limited in patients with infiltrative liver diseases that deposit iron, copper, glycogen or amiodarone in the liver parenchyma because CT attenuation is affected by those materials [116,117]. Dual energy CT, which adopts two different tube potentials for image acquisition, can perform material decomposition and has been used to quantify the degree of hepatic steatosis [102].
MRI is superior to ultrasonography for measuring a small amount of fat in the liver, and it is the most precise imaging tool for evaluating NAFLD. Quantitative MRI measurements of hepatic fat deposition using the Dixon technique can be classified into MRS and MRI proton density fat fraction (MRI-PDFF) [118]. MRS can directly measure the signal from acryl groups of triglycerides, and MRS findings correlate closely with histological results and show high sensitivity to hepatic steatosis [119,120]. MRI-PDFF uses differences in the precession frequency of water and fat protons and can map the entire liver for the degree of steatosis. Therefore, it can measure the degree of fat deposition in any part of the liver parenchyma. MRI-PDFF in different MRI units is in high agreement with histological findings, and its diagnostic performance in detecting severe steatosis (≥67%) was high (AUC, 0.95) [121,122]. In a recent meta-analysis, the AUCs for differentiating grade 1–3 steatosis, grade 2–3 steatosis, and grade 3 steatosis were 0.98, 0.91, and 0.90, respectively [123]. MRI-PDFF showed superior diagnostic performance to CAP in assessing hepatic steatosis in a prospective study [124]. MRS and MRI-PDFF can measure the degree of steatosis precisely, irrespective of iron deposition or fibrosis [125]. Despite the superior diagnostic performance of MRS and MRI-PDFF, their limited availability and high cost remain problems.
Panels
In addition to radiological examinations, various panels of serological tests have been proposed to diagnose hepatic steatosis and assess its severity (Table 3). These panels can be calculated using clinical information such as age, sex, and the results from serological tests. These panels do not directly diagnose hepatic steatosis, unlike ultrasonography, but they can help physicians who suspect the presence of hepatic steatosis to decide whether further assessments are justified. Large-scale studies frequently use these noninvasive panels to test for hepatic steatosis instead of ultrasonography, which has a high cost.
The FLI was proposed by Bedogni et al. [126] in an Italian study that examined 216 subjects with liver disease and 280 subjects with healthy livers. In that study, 228 patients had ultrasonography-defined NAFLD. The FLI is calculated based on triglycerides, gamma-glutamyl transpeptidase, BMI, and waist circumference. If the FLI is less than 30, NAFLD can be excluded (negative likelihood ratio, 0.2), and if the FLI is more than 60, NAFLD can be diagnosed (positive likelihood ratio, 4.3). An FLI score of more than 60 has a positive predictive value of 99% and a negative predictive value of 15%. The AUC of the FLI was 0.84. The FLI showed acceptable accuracy among a Korean population [127,128].
The NAFLD liver fat score (NLFS) was proposed by Kotronen et al. [129] in a Finnish study comprising 470 subjects. That study used MRS to diagnose NAFLD. The NLFS is calculated based on metabolic syndrome, T2DM, fasting insulin, aspartate aminotransferase (AST), and the AST/alanine aminotransferase (ALT) ratio. Its cutoff is -0.640 (sensitivity, 86%; specificity, 71%). If the NLFS is less than -0.640, NAFLD can be excluded, and if it is more than -0.640, NAFLD can be diagnosed. The AUC of the NLFS was 0.86–0.87. NLFS showed acceptable accuracy in a Korean population [130].
The HSI was proposed by Lee et al. [131] in a Korean cohort study of 10,724 subjects (5,462 with ultrasonography-defined NAFLD). The HSI is calculated based on sex, BMI, AST, ALT, and T2DM. If the HSI is less than 30, NAFLD can be excluded (negative likelihood ratio of 0.2, sensitivity of 93.1%), and if it is more than 36, NAFLD can be diagnosed with high predictive accuracy (positive likelihood ratio of 6.1, specificity of 92.4%). The AUC of the HSI was 0.81. The HSI showed acceptable accuracy among a Korean population [132].
The noninvasive surrogates just described show acceptable accuracy in most cross-section studies and have been used to trace changes in hepatic steatosis in certain studies. However, the usefulness of those noninvasive surrogates in monitoring disease progression and evaluating treatment response should be further investigated.
[Recommendations]
1. Abdominal ultrasonography, CAP, unenhanced CT, MRS, and MRI-PDFF are acceptable modalities for diagnosing hepatic steatosis. (A1)
2. If radiological examinations are infeasible, panels for hepatic steatosis can be used to assess hepatic steatosis. (B1)
Which non-invasive surrogates are available to diagnose NASH?
NASH is significantly associated with liver fibrosis progression and HCC. Non-invasive modalities to diagnose NASH are limited, but a prediction model based on liver stiffness, CAP (assessed using transient elastography), and ALT levels was recently proposed by a Korean study [133]. Some studies insist that NASH can be distinguished from NAFL using cytokeratin-18 fragments (sensitivity, 66%; specificity, 82%) [134,135]. A few studies claim that CT or MRI can be used to differentiate NASH from NAFLD, but no clear diagnostic criterion has been set [101,136]. Based on the association between the viscosity shown in shear wave dispersion imaging and necroinflammation of the liver parenchyma, a recent study showed that shear wave dispersion imaging could be helpful in diagnosing NASH [137]. The AUC of magnetic resonance elastography (MRE) alone and that for the combined use of MRE and MRIPDFF in differentiating NASH from NAFL was 0.82–0.93 [138,139].
Recently, multiparametric MR indices, which score the results from various MRI techniques, have been under investigation. A recent Korean study found that a multiparametric MRI index using MRE, MRS, and T1 mapping to differentiate NASH from NAFL showed sensitivity of 80%, specificity of 85.2%, and an AUC of 0.883 [140]. In a meta-analysis that systematically reviewed all studies using MRI to differentiate NASH from NAFL, the pooled sensitivity and specificity were 87.4% and 74.3%, respectively [110].
[Recommendations]
1. Non-invasive diagnosis of NASH remains limited, so it should be diagnosed by liver biopsy. (A1)
Which non-invasive surrogates are available to assess liver fibrosis?
The assessment of liver fibrosis is crucial in patients with NAFLD because the degree of liver fibrosis is significantly associated with long-term outcomes such as the development of HCC and liver-related death [78]. Furthermore, it is important to noninvasively assess the regression or progression of liver fibrosis during the course of anti-fibrotic therapy.
Radiological surrogates
Ultrasound-based measurement techniques for liver fibrosis take advantage of shear wave elastography (SWE). They can be divided into two categories: 1) measuring the elasticity of the liver parenchyma using SWE without acquiring imaging data (transient elastography) and 2) image-based sonoelastography that acquires both elasticity and 2D image data (point SWE and 2D SWE). Transient elastography is widely used in clinical practice, and many researchers have reported its high performance in quantifying liver fibrosis in NAFLD patients. In a recent meta-analysis, transient elastography showed high sensitivity and specificity for evaluating the degree of liver fibrosis in NAFLD patients [48,135,141]. However, the accuracy of transient elastography is limited in obese patients, who commonly have NAFLD, making it unavailable in 5–20% of patients [48,142]. A recent study reported that using an XL probe rather than an M probe can significantly lower the failure rate of transient elastography [119,143]. Image-based techniques have the advantage of acquiring both elasticity data and 2D images simultaneously. The failure rate is lower than that of transient elastography because operators can choose the area of the liver parenchyma to acquire elasticity data. The AUC of point SWE for quantifying liver fibrosis in NAFLD patients was higher than 0.8 [144,145]. The performance of point SWE for advanced liver fibrosis was excellent (100% sensitivity and 91% specificity) [146]. In a recent meta-analysis, the diagnostic performance of point SWE for liver fibrosis was similar to that of transient elastography [147]. 2D SWE can obtain elasticity data from a wider area than point SWE, and the reported failure rate of 2D SWE was lower than that of point SWE [148,149]. In a prospective study, 2D SWE, MRE, and transient elastography had similar AUCs for advanced hepatic fibrosis (0.920, 0.929, and 0.915, respectively) [150].
MRE shows high diagnostic accuracy for liver fibrosis [138,151,152]. In contrast to transient elastography, which can examine the elasticity of only a small portion of liver tissue, MRE can evaluate the entire liver parenchyma [152]. MRE also has other advantages: operator non-dependency and no limitations for obese patients. MRE is the most accurate non-invasive test for liver fibrosis, with a diagnostic performance superior to that of transient elastography [124,141,153]. In a meta-analysis, MRE correlated well with each stage of liver fibrosis, with AUCs for each stage of 0.84–0.93 [154,155]. The failure rate of MRE was less than 5%, which is significantly better than that of transient elastography. MRE was not significantly affected by the MRI manufacturer or the strength of the magnet [156]. In addition, MRE showed robust reproducibility in repetitive examinations [157]. However, the high cost of MRE limits its availability in clinical practice. In patients with iron deposition in the liver parenchyma, it is difficult to perform MRE, and the presence of other infiltrative diseases, such as profound hepatic steatosis, hepatic congestion, or acute inflammation, can attenuate the diagnostic accuracy of MRE [158,159].
Panels
In addition to radiological examinations, various panels of serological tests have been proposed to diagnose liver fibrosis (Table 4). Well-validated panels are summarized here.
Of the noninvasive panels for liver fibrosis, the NFS has been studied the most. The NFS was proposed in a US study by Angulo et al. [160] that comprised 733 subjects with biopsy-proven NAFLD. Two cutoffs were proposed: <-1.455 (low probability, negative predictive value of 88–93%) and >0.676 (high probability, positive predictive value of 82–90%) [160]. A meta-analysis based on 13 studies with 3,064 subjects showed that the AUC of the NFS for advanced liver fibrosis was 0.85. If the NFS is less than -1.455, advanced liver fibrosis can be excluded with a sensitivity of 90% and a specificity of 60%, and if NFS is more than 0.676, advanced liver fibrosis can be diagnosed with a sensitivity of 67% and a specificity of 97% [48,160-172]. Among 412 Korean subjects with biopsy-proven NAFLD, an NFS of less than -1.455 allowed advanced liver fibrosis to be excluded with a high negative predictive value (86.6%), and an NFS of more than 0.676 allowed advanced liver fibrosis to be diagnosed with a positive predictive value of 50% [173]. In another Korean study, which recruited 315 subjects with biopsy-proven NAFLD, the cutoff values (<-1.455 and >0.676) showed an AUC of 0.84 in diagnosing advanced liver fibrosis (negative predictive value of 89.3–95.7%) [174]. However, cases that fall between the cutoff values (indeterminate probability) still require a liver biopsy [48].
The fibrosis-4 index (FIB-4) was proposed by Sterling et al. in a study comprising 832 subjects with human immunodeficiency virus/hepatitis C virus co-infection. FIB-4 is calculated using the platelet count, age, AST, and ALT. The AUC of FIB-4 for advanced liver fibrosis was 0.765. When the FIB-4 is less than 1.30, advanced liver fibrosis can be excluded (accuracy, 90%), and when FIB-4 is more than 2.67, advanced liver fibrosis can be diagnosed (accuracy, 80%) [175]. A recent study of subjects with biopsy-proven NAFLD showed that the diagnostic accuracy of the NFS and FIB-4 was significantly higher than that of other noninvasive panels for liver fibrosis and similar to that of MRE in diagnosing advanced liver fibrosis [141]. However, because the diagnostic accuracy of FIB-4 was inferior to that of the NFS in a Korean study, further validation studies are required [173].
The enhanced liver fibrosis (ELF) panel has recently been used to assess liver fibrosis in Europe. ELF was proposed by Guha et al. [163] in a UK study comprising 192 subjects with biopsy-proven NAFLD. ELF is calculated based on three proteins associated with liver fibrosis: hyaluronic acid, tissue inhibitor of metalloproteinase 1, and amino terminal peptide of procollagen III. The cutoff value and AUC of ELF for advanced liver fibrosis were 0.3576 and 0.90, respectively (sensitivity, 80%; specificity, 90%; positive predictive value, 71%; negative predictive value, 94%) [163].
Other serological surrogates for liver fibrosis, such as M2BPGi and AsAGP, have been proposed [176-179]. However, because few data for subjects with NAFLD are available, further validation studies are required. The noninvasive surrogates described above show acceptable accuracy and prognostic values in most cross-section studies. However, the usefulness of noninvasive surrogates in monitoring disease progression and evaluating treatment response should be further investigated.
[Recommendations]
1. Radiological examinations such as transient elastography, point SWE, 2D SWE, and MRE are helpful in assessing hepatic fibrosis. (A1)
2. If radiological examinations are infeasible, panels such as NFS or FIB-4 can be used to diagnose liver fibrosis. (B1)
Which differential tests are available for advanced fibrosis?
In NAFLD, liver fibrosis testing uses serologic tests, image tests, or liver biopsy. Because liver biopsy cannot be performed in all patients, an algorithm can be used to differentiate advanced fibrosis (Fig. 2). Advanced fibrosis can be differentiated primarily using transient elastography, FIB-4, and the NFS [180].
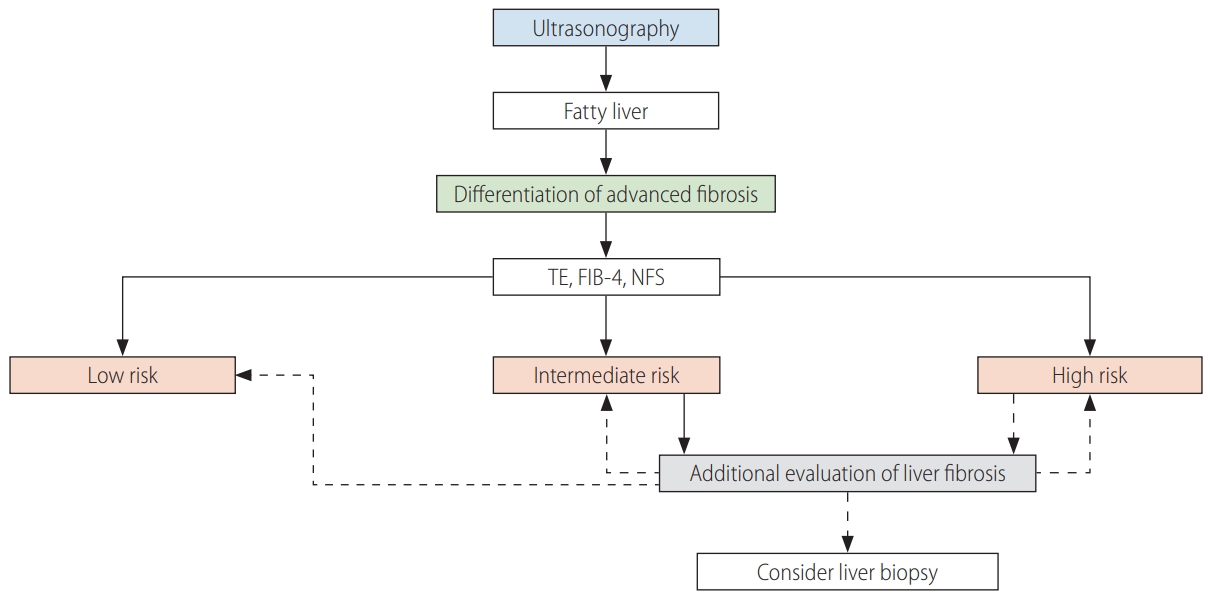
Algorithm to differentiate advanced fibrosis. TE, transient elastography; FIB-4, fibrosis-4 index; NFS, nonalcoholic fatty liver disease fibrosis score.
When subjects are classified as intermediate risk by transient elastography, FIB-4, or the NFS, additional tests such as M2BPGi, AsAGP, ELF, SWE, or MRE can be performed. If the algorithms suggest advanced fibrosis, additional tests to re-evaluate the liver fibrosis or a liver biopsy should be considered.
[Recommendations]
1. Non-invasive methods such as transient elastography, FIB-4, and NFS are prioritized to discriminate advanced liver fibrosis. (A1)
2. Serological tests, imaging tests, and liver biopsies may be performed as additional liver fibrosis evaluations. (B1)
Liver biopsy
What are the indications for liver biopsy?
Non-invasive tests to replace liver biopsy have been developed and shown high accuracy [122,181,182]. However, liver biopsy remains the gold standard for diagnosing NAFLD [183]. In practice, it is difficult to perform liver biopsy in all patients suspected of having NAFLD [184,185]. However, liver biopsy can help with diagnosis, treatment planning, and distinguishing between NAFLD and other liver diseases (autoimmune hepatitis, drug-induced hepatitis, Wilson’s disease, etc.). Liver biopsy is necessary when NASH or advanced liver fibrosis is suspected, as well as when other liver diseases cannot be excluded [186-188].
Liver biopsy has several limitations. First, sampling error is a concern because only a small portion of the liver tissue is sampled during liver biopsy. Second, intra- and inter-observer variability occurs [189-191]. Third, it carries the risk of complications such as bleeding and infection, as well as increased medical costs. Therefore, it is difficult to perform or repeat liver biopsy in all patients [192]. To minimize discrepancies, it is recommended that a sufficient amount of tissue be collected, a thick needle (16–18 gauge) be used, and two or more samples of a sufficient length be collected [97].
[Recommendations]
1. Liver biopsy should be considered in patients suspected of having NASH or advanced liver fibrosis. (B1)
2. Liver biopsy should be considered when the presence or severity of coexisting chronic liver disease cannot be excluded. (B1)
What are the histopathological features of NAFLD?
The role of liver biopsy in NAFLD diagnosis lies in differentiating simple steatosis (NAFL) from NASH, evaluating the extent of fibrosis (stage), and excluding the possibility of other liver diseases. NAFL is defined as the presence of ≥5% steatotic hepatocytes without evidence of hepatocellular injury in the form of hepatocyte ballooning [193]. The degree of steatosis is graded as 1+ (mild, 5–33%), 2+ (moderate, 34–66%), and 3+ (severe, ≥67%). NASH is diagnosed when there is evidence of hepatocellular injury (hepatocellular ballooning) and lobular inflammation in addition to steatosis [193-195]. Fibrosis, when present, is staged as stage 1 (perivenular, perisinusoidal, or periportal fibrosis), stage 2 (both zone 3 and periportal fibrosis), stage 3 (bridging fibrosis), and stage 4 (cirrhosis) [195].
HCC surveillance and prevention
Is surveillance for HCC necessary?
Because the incidence of liver cirrhosis and HCC associated with NAFLD is increasing rapidly, the risk of HCC should be assessed and surveillance should be established in all NAFLD patients. Because the incidence of HCC in patients with NAFLD-related cirrhosis is more than 1.5% per year [196,197], HCC surveillance is recommended if liver cirrhosis is clinically suspected [198-201].
Although the incidence of HCC in patients with NAFLD was 10 times higher than that in the normal control group [202], the incidence of HCC is very low in patients with early liver fibrosis (F0–2). However, when patients with early liver fibrosis have HCC risk factors (obesity, metabolic syndrome, diabetes, etc.), they become more likely to develop HCC. Thus, surveillance should be individualized [203,204].
Abdominal ultrasound is the primary surveillance test for HCC. However, in overweight or obese patients, it can be difficult to perform accurately [205,206]. In those cases, CT or MRI can be used instead.
How can HCC be prevented?
Smoking is associated with liver fibrosis and is known to be a risk factor for the development of HCC. In meta-analyses and cohort studies, smoking increased the risk of developing HCC by 1.5 and 1.8 times, respectively [207,208]. Therefore, smoking cessation is recommended for NAFLD patients. The effect of alcohol consumption on the development of HCC varies between studies, but in a meta-analysis, drinking increased the incidence of HCC by 1.2–2.1 times. Patients with liver cirrhosis associated with NAFLD should abstain from alcohol because drinking it increases the risk of HCC and liver-related mortality [209,210].
In a 32-year cohort study, diabetes increased the incidence of HCC by 4.6 times [211]. A meta-analysis also showed that diabetes increased the incidence of HCC [211-215]. Metformin decreased the incidence of HCC, but the use of sulfonylurea and insulin increased the incidence of HCC by 1.6 and 2.6 times, respectively [216]. Peroxisome proliferator activated receptor gamma (PPAR-γ) agonist and glucagon-like peptide-1 (GLP-1) agonist were effective in the recovery of NASH, but they did not show a significant association with the development of HCC [217-220].
Dyslipidemia is associated with NASH and CVD, but the relationship with liver disease-related mortality or HCC is still lacking. In a meta-analysis, statin use reduced the risk of developing HCC by 37% [221]. However, results in patients with NAFLD remain insufficient [222,223].
Obesity is associated with both liver fibrosis and HCC [224]. Although weight loss and exercise improve both steatosis and fibrosis, there are insufficient studies showing that weight loss and exercise therapy reduce the incidence of HCC. Further research is thus needed on the association between weight loss and HCC incidence.
[Recommendations]
1. Patients with liver cirrhosis associated with NAFLD need HCC surveillance. (A1)
2. To reduce the development of HCC in patients with NAFLD, smoking cessation, alcohol abstinence, and weight loss are recommended. (B1)
TREATMENT
Who should be treated, and what is the aim of the treatment?
NAFLD is commonly associated with metabolic diseases such as obesity, diabetes, and insulin resistance. Given that NAFLD is associated with increased mortality from CVD and liver-related complications [225,226], NAFLD patients require management and treatment to improve their prognosis. Treatments for NAFLD aim to reduce the incidence and mortality of CVD and liver-related complications, and they include both pharmacologic and non-pharmacologic options to improve intrahepatic inflammation and fibrosis and treat comorbid metabolic diseases. Lifestyle modifications such as weight reduction, dietary control, and exercise and treatment of comorbidities such as diabetes, obesity, hypertension, and dyslipidemia are the cornerstones of treatment for NAFLD and should be applied to all NAFLD patients, regardless of the degree of inflammation or fibrosis. However, pharmacologic treatments should be applied selectively because NAFLD progresses slowly and encompasses a spectrum of conditions from NAFL to NASH cirrhosis.
The most important histologic marker indicating long-term prognosis is the severity of fibrosis [51,52,227], with stage 2 or more fibrosis (≥F2) being an independent predictor of liver-related complications and mortality [51,52,228]. The most important factor in the progression of fibrosis is the presence of steatohepatitis. Fibrosis progresses more rapidly in patients with steatohepatitis than in those without it [36,38], and changes in steatohepatitis are associated with the progression of fibrosis [229,230]. Therefore, patients with hepatic fibrosis or steatohepatitis can receive pharmacologic treatment to improve their long-term prognosis.
[Recommendations]
1. Patients with NAFLD need lifestyle modifications and treatment for comorbidities. (A1)
2. Patients with NASH or hepatic fibrosis need management or treatment for histologic improvement. (A1)
Lifestyle modifications
What do lifestyle modifications include?
Weight reduction
Among overweight or obese (BMI >25.0 kg/m2) NAFLD patients, weight loss through lifestyle changes significantly reduced their liver fat content as revealed by imaging [231,232] or liver biopsy [233-235]. In patients histologically diagnosed with NAFLD, weight loss of more than 5–7% resulted in decreased intrahepatic fat content and inflammation [233,234], with greater weight loss correlating with greater histologic improvement [234]. Liver fibrosis also improved in 45% of patients whose weight loss was more than 10% [234]. In a meta-analysis of studies about weight loss through lifestyle modification, anti-obesity drugs, and surgical treatment, weight loss was associated with a decrease in intrahepatic fat, NAFLD activity score (NAS), and liver enzymes [236]. Even in nonobese NAFLD patients, intrahepatic fat content improved with a weight loss of 3–5% [237]. Therefore, weight loss is important in NAFLD patients regardless of the presence of obesity.
In NAFLD patients with obesity, the rate of weight loss affects steatohepatitis. A study showed that weight reduction that targeted a gradual decrease (maximum of 1 kg/week of body weight) improved both NASH and NAS [238]. However, a rapid decrease (more than 1.6 kg/week of body weight) worsened portal inflammation and fibrosis in some morbidly obese patients [239], and rapid weight loss through bariatric surgery can lead to acute hepatic failure [240]. Therefore, progressive weight loss of less than 1 kg/week is recommended over rapid weight loss in NAFLD patients with obesity.
Dietary therapy
Reducing the intake of total energy and controlling food intake are crucial aspects of NAFLD treatment. In prospective, randomized, controlled studies, reductions in energy intake caused weight loss, decreased intrahepatic fat content, decreased liver enzyme levels, and decreased insulin resistance [232,241,242]. A daily intake of 1,500–1,800 kcal in men and 1,200–1,500 kcal in women can reduce total energy intake by more than 500 kcal/day [243]. However, daily caloric intake should be optimally adjusted according to age, sex, weight, and physical activity.
Recently, the association between the ratio of macronutrients (carbohydrates, fats, and proteins) and the development of obesity and NAFLD has been studied. Carbohydrate intake was associated with metabolic syndrome and the severity of intrahepatic inflammation [244,245]. In Western studies, low-carbohydrate diets were more effective than low-fat diets in reducing liver fat content [242]. In Korea, increased carbohydrate and fructose intake was associated with an increased prevalence of fatty liver and elevated liver enzymes [246], and low-carbohydrate dietary training was more effective than low-fat dietary training in reducing both liver enzymes and liver fat content [247]. However, both low-fat and low-carbohydrate diets effectively reduced liver fat content [241]. The decrease in liver fat content did not differ depending on the type of diet; liver fat content decreased in patients who lost more than 7% of their body weight regardless of whether they ate a low-carbohydrate or high-carbohydrate diet [232]. In a meta-analysis comparing low-carbohydrate and low-fat diets, the two did not differ in reducing liver fat content [248]. Therefore, total energy intake is a more important factor in NAFLD treatment than the composition ratio of macronutrients.
The Mediterranean diet pattern emphasizes vegetables, fruits, whole grains, and legumes, and the principal source of dietary lipids is olive oil. It also includes the moderate consumption of fish and shellfish, white meat, eggs, and dairy products, with red meat and processed meats eaten rarely and in small quantities [249]. It has a high content of monounsaturated fatty acids. The Mediterranean diet reduces liver fat content and makes insulin resistance significantly better than low-fat diets regardless of body weight [250,251], and adherence to the Mediterranean diet was more important than adherence to a low-fat diet [251,252].
Dietary control along with weight loss can help reduce hepatic fat content. However, studies of specific nutrients and dietary habits have been conducted on only small numbers of patients, and few have shown histologic improvement to hepatic inflammation or fibrosis. It is difficult to maintain adherence to appropriate dietary habits in the long run. Therefore, it is necessary to study an appropriate diet to which patients can maintain adherence for a long time that produces histological improvement. In addition, dietary effects can vary depending on genetic predisposition [253], such as the presence of PNPLA3 or TM6SF2 variants, and dietary control needs to be individualized for each patient.
Exercise
NAFLD is associated with a low level of physical activity. In large-scale Korean cohort studies, prolonged sitting time and decreased physical activity were positively associated with the prevalence of NAFLD regardless of BMI [254], and moderate to vigorous exercise decreased the risk of developing a fatty liver or improved the resolution of an existing fatty liver [255]. In biopsy-proven NAFLD patients, vigorous exercise (≥6 metabolic equivalents of task [METs]) was associated with a lower frequency of NASH and advanced fibrosis [256]. Exercise itself decreases insulin resistance and reduces liver fat content regardless of body weight changes [257-262]. In a meta-analysis, exercise was found to be effective in reducing the liver fat content [263-265]. Aerobic exercise was mainly recommended at moderate or vigorous intensity (greater than 50–70% of maximal heart rate), and exercise for 30–60 minutes more than three times per week for at least 6 weeks was found to be effective [258,262,266,267]. Resistance exercise was recommended at 50–70% of maximal strength (1-repetition maximum) for 30–60 minutes more than three times per week [257,266,267]. According to the World Health Organization and United States Department of Health and Human Services, moderate-intensity physical activities include brisk walking, dancing, gardening, and carrying or moving an object of less than 20 kg, and vigorous-intensity physical activities include running, fast cycling, aerobics, fast swimming, and carrying or moving objects of more than 20 kg (Table 5) [268,269].
It is unclear which exercise is most effective. Some randomized controlled studies comparing aerobic exercise and resistance exercise have shown that aerobic exercise more effectively reduces liver fat content than resistance exercise [270], but some studies show similar effects from both exercise types [266,267]. In recent systematic reviews, both aerobic and resistance exercise similarly reduced the liver fat content [271,272]. However, resistance exercise could be more feasible than aerobic exercise for NAFLD patients with poor cardiorespiratory fitness or those who cannot tolerate aerobic exercise because it requires much lower energy consumption [271]. Because exercise-mediated improvements in liver fat can be reversed to baseline levels after cessation, it is necessary to maintain exercise habits [273]. Therefore, the selection of exercise needs to be individualized so that it can be maintained continuously considering each patient’s preferences and cardiopulmonary fitness.
[Recommendations]
1. In overweight or obese NAFLD patients, weight loss of more than 5–7% results in decreased intrahepatic fat content, and weight loss of more than 7–10% is required to improve hepatic inflammation and fibrosis. (A1)
2. To reduce intrahepatic fat content, a reduction in the total energy intake of more than 500 kcal/day is required. (A1)
3. To reduce intrahepatic fat content, at least moderate-intensity exercise for more than 30 minutes more than 3 times per week is required. (B1)
What is the effect of moderate or less alcohol consumption?
The effects of moderate or less alcohol consumption should be considered because NAFLD, by definition, includes patients whose alcohol consumption is insignificant. However, alcohol consumption is not easy to distribute randomly, so the effects of alcohol consumption on NAFLD can be evaluated only through longitudinal observational studies. Significant alcohol consumption (male ≥210 g/week, female ≥140 g/week) can cause alcohol-related liver disease and should be avoided. However, the effects of light or moderate drinking vary. In some studies, light or moderate drinking (male <210 g/week, female <140 g/week) appears to be protective against fatty liver and hepatic fibrosis [274-279], but in other studies it was associated with the progression of NAFLD [196,280-282]. In a large cohort study in Korea, light drinking (less than 10 g/day) was associated with worsening in noninvasive markers of fibrosis [283,284], but further studies are needed.
[Recommendations]
1. Moderate or less alcohol use in NAFLD patients requires attention. (B1)
What are the types, indications, effects, and side effects of medications for NAFLD?
Insulin sensitizers
Pioglitazone
Pioglitazone, a PPAR-γ agonist, reduces insulin resistance in the liver, muscle, and adipose tissue, and also reduces the amount of fat in the liver and hepatocellular injury by alleviating hepatic mitochondrial oxidative dysfunction [285-288]. In four randomized controlled studies, histologic improvement of steatohepatitis was observed in patients with or without diabetes who were treated with pioglitazone (30 or 45 mg/day) compared to placebo-treated patients [217,289-291]. However, no improvement was observed in liver fibrosis, a major indicator predicting the progression of liver disease [289-293]. In the Pioglitazone versus Vitamin E versus Placebo for the Treatment of Nondiabetic Patients with Nonalcoholic Steatohepatitis (PIVENS) trial, 247 nondiabetic NASH patients were randomized to pioglitazone (30 mg/day), vitamin E (800 IU/day), or placebo and followed for 96 weeks. The primary endpoint of this study was a ≥2 point reduction in the NAS (one or more point improvement in hepatocellular ballooning and one or more point improvement in either the lobular inflammation or steatosis scores). Compared with placebo, pioglitazone showed improvement in NASH (34% vs. 19%, P=0.04). This study concluded that the improvements observed with pioglitazone were not statistically significant because they did not reach the prespecified level of significance (P<0.025) for the primary outcome. However, there were discrepancies in the presence of hepatocellular ballooning between the results of pathological assessments performed locally and those performed centrally from deeper cuts prepared from the biopsy specimens obtained, and those classification errors were much higher in the subjects taking pioglitazone. The resolution of steatohepatitis, the secondary outcome, was higher in the pioglitazone group than the placebo group (47% vs. 21%, P=0.001). In conclusion, pioglitazone can be considered as a treatment option for NASH diagnosed by liver biopsy in patients with or without diabetes mellitus.
However, weight gain [294] is a common side effect of pioglitazone treatment. Other potential side effects of long-term pioglitazone use in NASH patients with diabetes mellitus include lower extremity edema, muscle cramps, as well as an increased risk of fractures [295], bladder cancer [296], and congestive heart failure [297].
Metformin
Metformin, a commonly prescribed drug for T2DM, was expected to be beneficial in treating NASH patients, as it reduces insulin resistance in the liver and muscles. Moreover, metformin inhibits hepatic fat accumulation and glucose excretion by activating adenosine monophosphate-activated protein kinase, and also decreases the expression of tumor necrosis factor-α [298,299]. However, metformin has little or no effect on liver histology [300,301]. Since metformin has a weight loss effect, it can compensate for the weight gain associated with pioglitazone when the two medications are used together. However, several studies, including randomized controlled studies, showed that the co-administration of metformin and pioglitazone did not improve histological findings in the liver, insulin resistance in the liver, or liver enzyme levels compared with controls [300,302-307]. Only in a retrospective study, long-term use (more than 6 years) of metformin in patients with diabetes mellitus and histologically proven NASH or advanced fibrosis lowered the risk of overall mortality, LT, and HCC [308]. Another retrospective study showed that diabetes mellitus increased the risk of death and liver-related complications such as HCC, and that metformin use lengthened the survival and decreased the risk of decompensated cirrhosis and HCC in 299 patients with NAFLD-associated Child-Pugh class A cirrhosis [309].
GLP-1 agonist
Liraglutide, a synthetic long-acting GLP-1 receptor agonist, has been approved to treat diabetes mellitus and obesity. In a small phase 2 clinical trial of 52 patients with biopsy-proven NASH, patients receiving subcutaneous injections of liraglutide (1.8 mg/day for 48 weeks) achieved greater weight loss and resolution of NASH than those receiving placebo [218]. However, its development as a medication for NASH has been interrupted by its frequent gastrointestinal side effects such as diarrhea, constipation, and loss of appetite.
In a phase 2 trial of semaglutide, a GLP-1 analogue, 320 patients with NASH were randomized to receive daily subcutaneous semaglutide (0.1 mg, 0.2 mg, or 0.4 mg) or placebo for 72 weeks. The proportions of patients who experienced NASH resolution without the exacerbation of hepatic fibrosis were 40%, 36%, and 59%, respectively, in the treatment groups and 17% in the placebo group (P<0.001, semaglutide of 0.4 mg vs. placebo) [310]. However, the groups did not differ significantly with regard to fibrosis improvement. The mean percentage of weight loss was 13% for the 0.4 mg semaglutide group and 1% for the placebo group, with the semaglutide group reporting more frequent nausea, constipation, and vomiting than the placebo group. Based on those results, semaglutide is expected to prove its benefit as a treatment for NASH through additional phase 3 clinical trials.
[Recommendations]
1. Pioglitazone is effective in improving steatohepatitis in NASH confirmed by liver biopsy, regardless of diabetes mellitus, but safety concerns about long-term treatment should be considered. (B1)
2. Metformin can be used as a first-line treatment for diabetes mellitus in patients with concomitant NAFLD who also have diabetes mellitus. (B1)
Antioxidants
Vitamin E (alpha-tocopherol)
Vitamin E decreases oxidative stress (which worsens NASH) and improves liver inflammation [311,312]. In the PIVENS study, which was a large-scale phase 3 trial, the administration of 96-week highdose vitamin E (800 IU/day) produced significant improvement in liver histology compared to placebo (43% vs. 19%, P=0.001) [291], but it did not improve liver fibrosis [292]. The proportion of NASH resolution, a secondary endpoint, was 36% in the vitamin E group, which was higher than that in the control group (21%). Therefore, the use of vitamin E can be considered as biopsy-proven NASH treatment in patients without diabetes mellitus.
However, the long-term use of vitamin E also carries safety concerns because of the increased risk of prostate cancer or hemorrhagic stroke [313]. Although the finding is controversial, high doses of vitamin E (>400 IU/day) are associated with an increased mortality rate, requiring safety precautions [314-316]. According to a retrospective study of 236 patients with biopsy‐proven NASH with bridging fibrosis or compensated cirrhosis, the use of 800 IU/day of vitamin E for more than 2 years decreased the risk of death, LT and decompensated cirrhosis in patients both with and without diabetes mellitus. However, the incidence of HCC, vascular disease, and non-hepatic cancers did not differ between the vitamin E users and controls [317].
[Recommendations]
1. High-dose vitamin E (800 IU/day) can improve NASH confirmed by liver biopsy in non-diabetic patients, but safety should be considered for long-term administration. (B1)
Lipid-lowering drugs
CVD is the most common cause of death for NAFLD patients, so it is important to modify its risk factors [78,318-320]. An increase in plasma lipoprotein increases the carotid intima-media thickness and atherosclerotic plaques, which cause CVD, and thus it is necessary to prevent and treat dyslipidemia [321]. Lipid lowering agents such as statins (hydroxy-methyl-glutaryl coenzyme A reductase inhibitors) [322] can be considered in NAFLD patients with dyslipidemia [323,324]. In a post-hoc analysis of the GREek Atorvastatin and Coronary-heart-disease Evaluation (GREACE) study, statin use decreased aminotransferases and poor cardiovascular outcomes in NAFLD patients with aminotransferases up to three times higher than the upper normal limit. Less than 1% of patients (seven of 880 patients) withdrew from the study due to hepatotoxicity associated with the statin treatment; thus statin treatment seems to safely lower liver enzymes and reduce cardiovascular morbidity in patients with NAFLD [325]. In a study using data from the National Health Information database of South Korea, statin treatment decreased not only the risk of NAFLD occurrence but also the development of fibrosis attributed to NALFD, regardless of diabetes mellitus [326]. Strict control of low density lipoprotein cholesterol (LDL-C) is emphasized because many NAFLD patients treated with statins still did not meet their LDL-C treatment targets, which can itself increase the incidence of CVD [327]. A common adverse effect of statins is the asymptomatic elevation of aminotransferases, which usually appears within 1 year of starting statins and recovers spontaneously [328]. This increase in liver enzyme levels depends on the statin dose [329]. However, because statin users and controls did not differ in terms of persistent and significant elevation of liver enzyme levels [328] or the incidence of liver and biliary tract disease [330], the administration of a statin is possible in chronic liver diseases, including NAFLD [331,332]. However, the administration of statins to patients with decompensated cirrhosis or acute liver failure should be avoided [333-337]. Statins can be used in NAFLD and NASH, and they are considered as a first-line treatment to lower LDL-C and prevent atherosclerotic CVD. If the response to the statin is insufficient, ezetimibe can be added [338]. Omega-3 fatty acids are not recommended as a treatment for NASH because only some study results showed an effect on NASH [339], whereas others did not [340,341]. However, they may be considered for use in hypertriglyceridemia with NAFLD [340,342,343].
[Recommendations]
1. Because the incidence and mortality rate of CVD in NAFLD are high, it is necessary to actively control the risk factors of CVD. (A1)
2. In the case of dyslipidemia in NAFLD, a statin can be used to prevent CVD. (B1)
3. Omega-3 fatty acids are not recommended as a treatment for NASH, but they can be used in NAFLD with hypertriglyceridemia. (B1)
New NASH drugs in development
The pathophysiology of NASH is complicated, and its interactions with other metabolic diseases have not been fully elucidated. Therefore, NASH treatments are currently under development for a wide range of targets. The main targets are changes in intestinal microflora and intestinal permeability, oxidative stress, insulin resistance, apoptosis, lipotoxicity, inflammation, bile acid metabolism, and liver fibrosis. At the time of this writing, six new drugs are in phase 3 clinical trials or have published intermediate results (Table 6). Among them, the STELLAR-3, 4 trial of an apoptosis signal-regulating kinase 1 inhibitor (selonsertib) in NASH subjects with advanced liver fibrosis [344] and the RESOLVE-IT trial of a PPAR-α/δ agonist (elafibranor) in NASH patients with stages 1–3 of liver fibrosis have failed to demonstrate therapeutic efficacy in the interim results. Consequently, the development of both drugs was discontinued. In the REGENERATE trial in NASH subjects with hepatic fibrosis stage 1–3, the farnesoid X receptor agonist (obeticholic acid; Ocaliva®) showed dose-dependent primary treatment efficacy, an improvement in liver fibrosis of at least 1 stage, in the 10 mg and 25 mg treatment groups (18% and 23%) compared with the control group (12%) after 18 months of treatment [345]. However, the US Food and Drug Administration rejected conditional approval of the drug as a treatment for NASH, judging that severe itching and the increased risk of CVD caused by increased LDL-C outweighed the benefits of treatment. To date, no drugs under development have met their efficacy targets in more than 50% of patients. Considering the complicated pathophysiology of NASH and the various treatment responses observed in clinical trials for individual drugs, it is highly likely that combination treatments or personalized treatments will become the standard.
Bariatric surgery and LT
What are the indications for and post-operative management of bariatric surgery?
Bariatric surgery has been performed in NASH patients with obesity who did not respond to medical treatment for weight loss. In Western countries, bariatric surgery is considered to be indicated for patients with a BMI greater than 35 kg/m2 that is accompanied by hypertension or diabetes mellitus or a BMI greater than 40 kg/m2 [284,318,346]. Several studies reported significant weight loss in addition to improvement in NAFLD [347-351]. In Korea, the Health Insurance Review and Assessment Service allows bariatric surgery to be covered by the national health insurance for patients who have had no response to medical treatment and lifestyle modifications and whose BMI is greater than 35 kg/m2 or 30 kg/m2 and associated with hypertension, diabetes mellitus, or NAFLD.
In a 5-year follow-up study of biopsy-confirmed NASH patients who underwent bariatric surgery, BMI, the amount of fat in the liver, and NAS were all found to be reduced, and histological improvement in the fibrosis stage was noted after surgery [352]. Recent meta-analyses identified that bariatric surgery effectively reduced the amount of fat in the liver, inflammation, and fibrosis in NASH patients [353,354]. However, histological worsening after surgery was observed in some patients. Therefore, well-designed, randomized controlled studies should be performed to confirm the benefits of bariatric surgery in NASH patients [348]. The limitation of bariatric surgery is the risk of lethal liver failure that can be generated by rapid weight loss. Also, the safety of bariatric surgery for cirrhotic patients is still controversial, so a careful approach is needed [355-358]. Before deciding to undertake bariatric surgery, intraoperative complications, long-term malnutrition, and other factors need to be considered comprehensively [359-361].
[Recommendations]
1. Bariatric surgery can be considered for NASH patients with obesity who do not respond to medical treatment and lifestyle modification. (B1)
2. The effectiveness and safety of bariatric surgery have not been established in patients with cirrhosis. (B1)
What are the indications for LT and post-LT management?
LT can be considered in patients with end-stage liver disease caused by NAFLD-associated cirrhosis, liver failure, or HCC, according to the clinical practice guideline for LT. NASH patients experience a high risk of mortality from cardiovascular complications, so a meticulous pretransplant cardiovascular evaluation is needed [80,362,363]. Posttransplant outcomes for NAFLD patients, including 3- and 5-year survival, were comparable to those of nonNAFLD patients, whereas the risk of graft failure was lower [364]. Overall survival was associated with BMI and the presence of diabetes mellitus. LT patients with a BMI greater than 35 kg/m2 experienced a higher transplant failure rate and lower 1-year survival rate than LT patients with a BMI lower than 35 kg/m2 [365].
Posttransplant management is similar to that for other NASH patients. Maintaining a healthy weight and diet is important, especially given that weight gain is common following LT [363,366]. Hepatic steatosis [367] or metabolic syndrome [368] is very common after LT, especially in patients with a history of NASH. Thus, careful attention should be paid to posttransplant management.
[Recommendations]
1. Liver transplantation could be considered in NASH patients with end-stage liver disease or HCC, according to the clinical practice guideline for LT. (A1)
NAFLD IN CHILDREN AND ADOLESCENTS
Epidemiology
What is the prevalence rate?
The National Health and Nutrition Survey conducted between 2015 and 2017 defined cases with ALT of 26 IU/L (boys) and 22 IU/L (girls) as NAFLD, and the estimated prevalence of this disease in children was 11.2% (14.7% for boys and 7.4% for girls) [369]. In 2001–2005, the prevalence was 7.8% (10.6% for boys and 4.6% for girls), so the prevalence is increasing, and 40–45% of obese adolescents have NAFLD [369,370]. The obesity rate of children and adolescents aged 7–18 years increased from 8.4% in 2008 to 14.3% in 2016, so the prevalence of NAFLD is expected to increase rapidly in the future [371,372].
Summary
1. The prevalence of NAFLD among children and adolescents in Korea is increasing as their obesity rate increases.
How does NAFLD progress?
The natural course and prognosis of NAFLD in children and adolescents are not well known. Unlike NAFL, NASH can progress to cirrhosis in children and adolescents [373]. In a foreign study that followed 66 children and adolescents with NAFLD for 20 years, the exacerbation of liver disease and risk of early death increased by 14 times compared with the control group [374]. There was also a case in which HCC occurred in a 7-year-old child diagnosed with NAFLD [375]. In recent years, LT has increased in children, adolescents, and young adults due to end-stage liver disease related to NAFLD [376,377].
Summary
1. NASH in children and adolescents can progress to end-stage liver disease, including cirrhosis, in young adults.
What genetic diseases are associated with risk factors?
The risk factors for NAFLD in children and adolescents are obesity, adolescent age, and being male [378,379]. This disease is common in adolescence because the increased sex hormones that occur at puberty cause changes in insulin resistance and body composition, with the hormones of boys perhaps causing more NAFLD than those of girls. In patients with abdominal obesity and obstructive sleep apnea, NAFLD is likely to be associated with acanthosis nigricans [376,377].
Unlike in adults, in children and adolescents, steatosis sometimes appears as a phenotype of genetic disease. Therefore, through medical history, examination, and testing, Wilson’s disease, Bardet-Biedl syndrome, polycystic ovary syndrome, Prader-Willi syndrome, Turner syndrome, Cohen syndrome, alpha1-antitrypsin deficiency, glycogen storage disease, genetic tyrosinemia type 1, homocystinuria, Refsum disease, citrullinemia, and lysosomal acid lipase deficiency should be differentiated [373].
Summary
1. Obesity, adolescent age, and being male are risk factors for nonalcoholic fatty liver disease in children and adolescents.
2. If steatosis is found in children and adolescents, potential accompanying genetic diseases should be considered.
Genetic factors
Is related NAFLD related to family history and genetic predisposition?
Children and adolescents with a family history of NAFLD are at high risk of developing it. In children and adolescents diagnosed with NAFLD, 59% of their siblings and 78% of their parents also had NAFLD. On the other hand, in obese children and adolescent patients without NAFLD, NAFLD was found in only 17% of their siblings and 37% of their parents [380]. NAFLD occurs from a variety of causes, but family outbreaks, twin studies, and differences in prevalence among races confirm that more than 50% of patients with this disease are likely to have a genetic predisposition to it [381].
In a genome-wide association study, an increase in liver fat mass was associated with mutations in the PNPLA3, TM6SF2, LYPLAL1, and GCKR genes [382]. Genetic polymorphisms reported in children and adolescents include MBOAT7, PNPLA3, TM6SF2, and GCKR [383-390]. Studies also showed an association with monoallelic ABHD5 mutations [391].
Summary
1. Children and adolescents are at increased risk of NAFLD if there is a family history of it.
2. NAFLD in children and adolescents can be associated with genetic variations and genetic polymorphism.
Screening and diagnosis
Who should be targeted for NAFLD screening, and how is screening conducted?
NAFLD is common in children and adolescents who are overweight (above the 85th percentile and below the 95th percentile of BMI) or obese (above the 95th percentile of BMI). Therefore, screening is necessary for those groups. Overweight and obesity are checked during school health checkups in the 4th and 6th grades of elementary school, the 1st grade of middle school, and the 1st grade of high school in Korea. In overweight young people, screening tests are performed using liver enzyme levels [392].
ALT is used as a screening method for NAFLD in overweight and obese children and adolescents. In 2007, the US Expert Committee also recommended AST and ALT as screening tests [393-395]. However, because the normal ranges of ALT by age and sex are unclear, European authorities recommended abdominal ultrasound along with ALT as a screening test [396]. In the 2019 guidelines for obesity in children and adolescents, those who were overweight or obese and had an ALT of 26 IU/L (boys) or 22 IU/L (girls) or more were diagnosed with NAFLD and recommended for abdominal ultrasound when necessary [397]. In 2017, the North American Society of Pediatric and Gastrointestinal Nutrition recommended ALT as a screening test for obese and overweight children aged 9–11 years with insulin resistance, pre-diabetes, diabetes, or dyslipidemia [398]. Based on the limited research results available for the cost-effectiveness of screening tests in overweight and obese children and adolescents, the American Liver Association did not recommend screening for NAFLD using ALT in obese children and adolescents [399]. However, the 2019 guidelines for pediatric and adolescent obesity did recommend screening tests for NAFLD and other concomitant diseases in overweight and obese children and adolescents.
[Recommendations]
1. NAFLD screening tests can be performed in overweight and obese children and adolescents. (B1)
2. The preferred screening test for NAFLD in children and adolescents is ALT, and abdominal ultrasound can also be performed. (B1)
What are the diagnostic methods?
The standard test for diagnosing NAFLD is liver biopsy. However, because that method is invasive, its application to children and adolescents is limited. The pathologic findings of NAFLD in children and adolescents can differ from those found in adults. Representative differences are that steatosis is more widely observed, balloon degeneration of hepatocytes and hepatic lobular inflammation are milder, and inflammation and fibrosis of the portal region are often present. Sinusoid fibrosis in zone 3, which is commonly observed in adults, is relatively rare in NAFLD in children and adolescents.
As a non-invasive method for diagnosing NAFLD, a method using cytokeratin-18, which is produced during hepatocyte death, can be used [400]. In addition, various non-invasive panels are being studied, but they have not yet been recommended for clinical practice. Other additional methods include abdominal ultrasound, liver fibrosis scans, and MRI-PDFF.
[Recommendations]
1. In addition to liver biopsy, non-invasive tests such as abdominal ultrasound, liver fibrosis scans, and MRI-PDFF can be performed. (B1)
Treatment
Who should be treated, and how?
Overweight and obese children and adolescents with NAFLD are subject to treatment, and these patients are recommended to correct their lifestyle habits first. There are insufficient studies on the long-term prognosis of NAFLD in children and adolescents, and few prospective randomized controlled studies have been done. However, when the disease is diagnosed at an early age, the possibility of long-term complications is high.
Most of children and adolescents with NAFLD are associated with obesity, so it is important to correct lifestyle habits to improve obesity. Various studies have been conducted on the correction of lifestyle habits. Based on randomized controlled studies, the North American Society of Pediatric Gastrointestinal Nutrition recommended in 2017 that simple sugar-added beverages be restricted, moderate physical activity be increased, and screen time (time exposed to the screens of electronic devices such as TVs, computers, and smartphones) be reduced to less than 2 hours per day [401-403].
According to the TONIC trial, a large multicenter randomized controlled study comparing vitamin E, metformin, and placebo in NAFLD patients aged 8–17 years, the three groups did not differ in terms of a sustained decrease in ALT. In the vitamin E group, histological improvement was observed. However, long-term use of high-dose vitamin E is not recommended because of concerns about side effects [404-407]. In addition, a small randomized controlled study tested ursodeoxycholic acid, docosahexaenoic acid, and fish oil, and no significant effect was found [408,409]. In conclusion, no drug treatment is currently recommended for NAFLD in children and adolescents.
Although there is no guideline for surgical treatment of NAFLD, it can be performed in cases of severe obesity (BMI 97th percentile or higher) based on the results of research in adults [410]. In the domestic guidelines for obesity in children and adolescents, obesity surgery is recommended when BMI is 40 or more, or 35 or more and accompanied by major complications related to obesity, and lifestyle improvements and drug treatment have produced no effects [397]. Because children and adolescents are growing, growth should be considered when deciding on the timing of surgery. In general, surgery should be considered only when skeletal growth is almost complete (i.e., 13–14 years old for girls, 15–16 years old for boys), and it is recommended that surgery be performed when the Tanner stage (division stage considering the degree of development of male and female genitals according to the stage of puberty) is 4 or higher [411,412].
[Recommendations]
1. In NAFLD in children and adolescents who are overweight or obese, lifestyle correction such as improvement of dietary habits, increase in physical activity, and restriction of screen time is needed first. (A1)
Acknowledgements
This study was supported by the Korean Association for the Study of the Liver (KASL).
This manuscript was reviewed by native speakers for English proof readings (Eworld Editing; KASL2104-01).
Notes
Authors’ contributions
Study conception and design: Seong Hee Kang, Hye Won Lee, Jeong-Ju Yoo, Yuri Cho Seung Up Kim and Yong Kyun Cho; Report writing: Seong Hee Kang, Hye Won Lee, Jeong-Ju Yoo, Yuri Cho Seung Up Kim, Tae Hee Lee, Byoung Kuk Jang, Sang Gyune Kim, Sang Bong Ahn, Haeryoung Kim, Dae Won Jun, Joon-Il Choi, Do Seon Song, Won Kim, Soung Won Jeong, Moon Young Kim, Hong Koh, Sujin Jeong, Jin-Woo Lee and Yong Kyun Cho; Review: Seong Hee Kang, Hye Won Lee, Jeong-Ju Yoo, Yuri Cho Seung Up Kim and Yong Kyun Cho; Final approval of the submission of the manuscript: Seong Hee Kang, Hye Won Lee, Jeong-Ju Yoo, Yuri Cho Seung Up Kim, Tae Hee Lee, Byoung Kuk Jang, Sang Gyune Kim, Sang Bong Ahn, Haeryoung Kim, Dae Won Jun, Joon-Il Choi, Do Seon Song, Won Kim, Soung Won Jeong, Moon Young Kim, Hong Koh, Sujin Jeong, Jin-Woo Lee and Yong Kyun Cho.
Conflicts of Interest
Yong Kyun Cho is a speaker for Samil Pharm, Bukwang and DaeWoong. He has received a research grant from DaeWoong, Dong-A, Celltrion and Ildong.
Seung Up Kim has served as an advisory committee member of Gilead Sciences, Bayer, Eisai, Novo Nordisk, and GreenCross. He is a speaker for Gilead Sciences, GSK, Bayer, Eisai, Abbvie, EchoSens, MSD, Eisai, Otsuka, Bristol-Myers Squibb, Yuhan, Samil Pharm, Ildong, Celltrion, PharmaKING, DaeWoong, Samjin, DongA, Hanmi, BuKwang, Echme medical, Hanwha, Sysmax, and ChongKunDang. He has also received a research grant from Abbvie, Bristol-Myers Squibb, ChongKunDang, DaeWoong, Hanmi, Samil Pharm, and Echme Medical.
Jeong-Ju Yoo is a speaker for DaeWoong, Ildong, Pharmaking, Dong-A, BMS, Samil Pharm and Bukwang.
Tae Hee Lee is a speaker for Abbvie, Gilead, Samil Pharm and Yuhan. He has received a research grant from Eisai, Celltrion, PharmaKING and GC Wellbeing.
Byoung Kuk Jang is a speaker for Gilead. He has received a research grant from PharmaKING, Novo Nordisk Pharma, Vaccitech and GC Wellbeing.
Sang Gyune Kim has received grants from GE healthcare, Samsung Medison, BMS, Samil, Ildong; received honoraria from BMS, MSD, Gilead, Daewoog, Hanwha; consulted Samsung Medison.
Sang Bong Ahn has received grants from Hanwha, Samjin, Ildong, and Hanmi.
Dae Won Jun has served as an advisory committee member of Sysmax, J2H, and Future medicine. He is a speaker for Gilead Sciences, Bristol-Myers Squibb, Yuhan, Chong Kun Dang, Ildong, Donga, Samil, Phamaking, Celltrion, and Daewoong. He has also received a research grant from Yuhan.
Joon-Il Choi previously received grants from Bayer Healthcare, Guerbet Korea, Bracco Korea and Samsung Medison. He previously received honorariums from Bayer Healthcare, Guerbet Korea and Bracco Korea.
Won Kim has served as an advisory committee member of Boehringer-Ingelheim, HK inno.N, GreenCross, Standigm, Pharmaking, KOBIOLABS, Eisai, Zydus and Novonordisk. He is a speaker for Gilead, Boehringer-Ingelheim, Samil, Il-dong, LG chemistry and Bukwang. He has also received a research grant from Gilead, Il-dong, GreenCross, Bukwang, Roche, Galmed, Novartis, Pfizer, Springbank, Altimmune, MSD, BMS, Novonordisk, Hitachi Aloka, JW medical, Dicerna and Enyo.
Soung Won Jeong is a speaker for Daewoong, Samil and Pharmaking. He has received a research grant from Dong-A ST, Ildong and Chongkundang.
Moon Young Kim is a speaker for Samjin and Gilead. He has received a research grant from Yuhan.
Jin-Woo Lee has served as an advisory committee member of Abbvie and Novo Nordisk. He has received a research grant from Galectin Therapeutics Inc, BMS, MSD and Ipsen.
The other authors declare that they have no competing interests.
Abbreviations
AGREE II
Appraisal of Guidelines for Research and Evaluation II
ALT
alanine aminotransferase
AST
aspartate aminotransferase
AUC
area under the receiver operating characteristic curve
BMI
body mass index
CAP
controlled attenuation parameter
CKD
chronic kidney disease
CT
computed tomography
CVD
cardiovascular disease
ELF
enhanced liver fibrosis
FIB-4
fibrosis-4 index
FLI
fatty liver index
GLP-1
glucagon-like peptide-1
GRADE
Grading of Recommendations
GREACE
GREek Atorvastatin and Coronary-heart-disease Evaluation
HCC
hepatocellular carcinoma
HIS
hepatic steatosis index
KASL
the Korean Association for the Study of the Liver
LDL-C
low density lipoprotein cholesterol
LT
liver transplantation
MAFLD
metabolic (dysfunction)-associated fatty liver disease
METs
metabolic equivalents of task
MRE
magnetic resonance elastography
MRI-PDFF
MRI proton density fat fraction
MRI
magnetic resonance imaging
MRS
MR spectroscopy
NAFL
nonalcoholic fatty liver
NAFLD
nonalcoholic fatty liver disease
NAS
NAFLD activity score
NASH
nonalcoholic steatohepatitis
NFS
NAFLD fibrosis score
NLFS
NAFLD liver fat score
PIVENS
Pioglitazone versus Vitamin E versus Placebo for the Treatment of Nondiabetic Patients with Nonalcoholic Steatohepatitis
PNPLA3
the patatin-like phospholipase domain–containing 3
PPAR-γ
peroxisome proliferator activated receptor gamma
SAMM50
sorting and assembly machinery component 50
SWE
shear wave elastography
T2DM
type 2 diabetes mellitus
TM6SF2
transmembrane 6 superfamily







