INTRODUCTION
Hepatocellular carcinoma (HCC) was the sixth most common malignancy and the third most common cause of cancer-related death in 2020 [
1]. The HCC treatment options depend on many factors, such as tumor characteristics (size, number, or location), the presence or absence of liver cirrhosis (LC), liver function, portal vein invasion, comorbidity, performance status, and the presence or absence of an extrahepatic metastasis [
2].
The Barcelona clinic liver cancer system classifies HCC into five categories, including very early, early, intermediate, advanced, and terminal stage [
3]. Surgical resection (SR) is the first-line treatment for very early and early-stage patients who not only have solitary HCC unaccompanied by LC, but also have cirrhosis with sufficient hepatic functional reserve [
4]. Liver transplantation (LT) is the primary treatment choice for patients with single tumor Ōēż5 cm or those with small multinodular tumors (Ōēż3 nodules Ōēż3 cm) and advanced liver dysfunction [
5]. On the other hand, SR or LT cannot be applied to very early or early HCC patients in some cases due to associated diseases or shortage of donor livers [
5]. In these cases, radiofrequency ablation (RFA) has been performed as an effective nonsurgical curative treatment [
5]. Based on many guidelines from America, Europe, and Asia, RFA can be used for Child-Turcotte-Pugh (CTP) class A or B patients with very early or early-stage HCC who are ineligible for SR [
4-
6].
However, until now, comparative results of the overall survival (OS) and recurrence-free survival (RFS) between SR and RFA in CTP class A patients with single small HCC according to the presence of LC remain unclear, particularly for those who are suitable candidates for SR. The results from studies that examined the outcomes of RFA and SR for small HCC have been inconsistent and had several limitations [
7-
10]. Huang et al. [
7] reported that SR provides superior survival and lower recurrence rates than RFA in HCC patients within the Milan criteria (single tumor with diameter Ōēż5 cm or up to 3 tumors, each with diameter Ōēż3 cm). On the other hand, the study was not double-blinded, and the tumor size between the SR and RFA groups was different. In addition, the considerable loss to follow-up was significantly different between the SR group (15.6%) and RFA group (6.1%) [
7]. Feng et al. [
8] reported that SR was more effective in the treatment of small HCCs than RFA, but the median follow-up duration was also only 3 years, and there were considerable post-RFA residual tumors. Fang et al. [
9] reported that RFA had similar efficacy in local tumor control, but the outcome was also reported just as a 3-year survival. A prospective randomized trial conducted by Chen et al. [
10] showed that local ablative therapy was as effective as SR in small solitary HCC. Nevertheless, approximately 20% of patients withdrew from local ablative therapy, and the study only reported relatively short-term outcomes (median follow-up duration of 2 years) [
10]. Therefore, it is still difficult to confirm whether RFA could be a first-line treatment for CTP class A patients with single small (Ōēż3 cm) HCC, regardless of the presence of LC. Moreover, no study has compared the long-term survival outcomes after SR and RFA in those with and without cirrhosis, respectively.
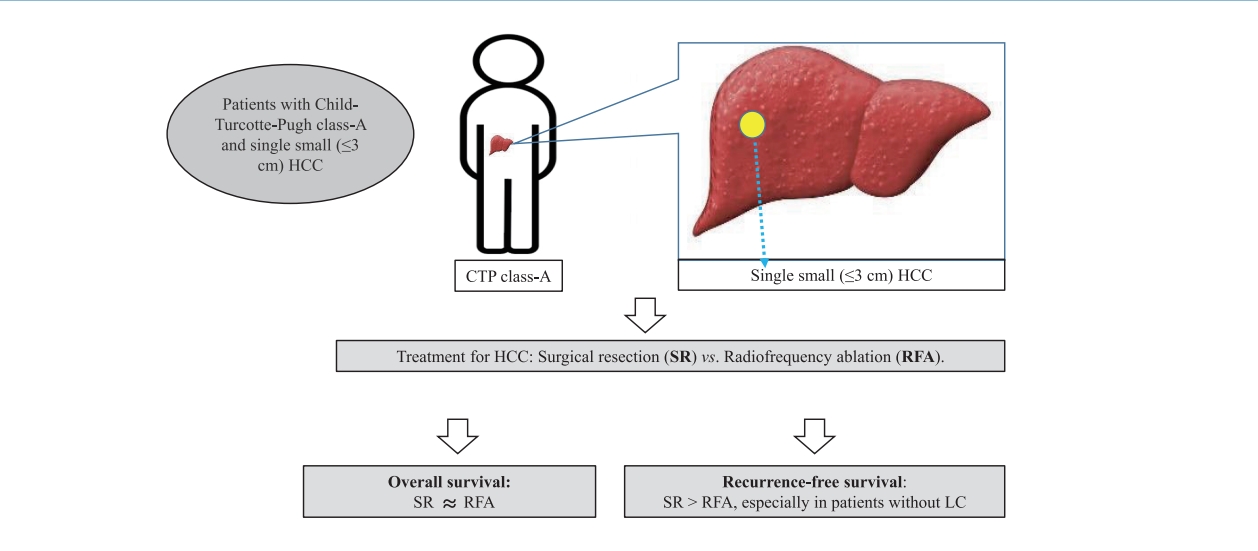
Therefore, this multicenter retrospective cohort study compared the long-term therapeutic effectiveness of SR and RFA as a first-line treatment in patients with CTP class A cirrhosis or non-cirrhosis and single small (Ōēż3 cm) HCC by analyzing the post-treatment long-term OS and RFS.
MATERIALS AND METHODS
Study subjects
Between January 2005 and December 2015, 651 consecutive patients with an initial diagnosis of primary HCC at four university hospitals were analyzed respectively. HCC was histologically proven or diagnosed based on the criteria issued by the Korean Liver Cancer Study Group and National Cancer Center Korea Practice Guideline for the Management of HCC [
11]. None of the patients had been treated previously for HCC. Patients who met the following criteria were enrolled in the present study: single tumor Ōēż3 cm in the largest diameter; well-preserved liver function with CTP class A; good health status (Eastern Cooperative Oncology Group Performance Status 0); no vascular invasion or extrahepatic metastasis; and first-line treatment with SR or RFA. Those with combined other malignancies, CTP class B or C, or aged <18 years were excluded, and those with HCC sized >3 cm or multiple HCCs, as well as extrahepatic, vascular, or lymph node metastasis, were also excluded. Of the 651 patients, those who underwent treatments other rather than SR or RFA were excluded. Finally, the data of 391 patients with CTP class A and single small (Ōēż3 cm) HCC, but without vascular/lymphatic/extrahepatic metastasis treated by SR or RFA were enrolled in this retrospective cohort study.
The tumor size was recorded as the longest diameter of the tumor lesions in at least one dimension on liver dynamic computed tomography (CT) or magnetic resonance imaging (MRI) scans. LC was clinically diagnosed based on clinical evidence of portal hypertension (HTN) (encephalopathy, esophageal varices, ascites, or splenomegaly), a low platelet count (<100,000/mm3), or radiologic findings. Of these 391 patients, 232 and 159 patients underwent SR and RFA, respectively. The Institutional Review Board of each hospital approved this study (approval number: 2021-10-013).
Initial treatment and follow-up after initial treatments
SR was carried out under general anesthesia. The surgical extent was determined anatomically, aiming to remove all macroscopic HCC considering the reserved liver function, the subsequent remnant liver volume, and tumor-free resection margins of at least 1 cm. Percutaneous RFA procedure was performed under local anesthesia or conscious sedation using ultrasonographic or computed tomographic guidance. Single electrode with a 2ŌĆō3 cm exposed tip (COVIDIEN Valleylab, Boulder, CO, USA) or multitined expandable electrode (LeVeen needle Electrode; Boston Scientific, Marlborough, MA, USA) was used for tumor ablation. The radiofrequency current was emitted with a 100ŌĆō200 W generator set for 12 or 15 minutes to deliver the maximum power using the automatic impedance control method. The RFA procedure was performed to ablate the visible HCC covering a larger area than the tumor. The whole course of the surgery and RFA procedures was performed by two expert surgeons and two intervention radiologists, respectively, at each institution, who had at least 5ŌĆō10 years of professional experience in conducting the procedures.
Patients who underwent SR or RFA were followed up regularly by liver dynamic CT or MRI at 1 to 3-month intervals for the first 6 months, and subsequently, at 3 to 6-month intervals for 2 years, and then, every 6 to 12 months, if HCC did not recur. The follow-ups after initial treatments continued until the date of death, recurrence, last follow-up, or December 31, 2019. SR or RFA is a curative-intent treatment, but patients were considered to be cured if the resection margin was free of tumor or if there was no evidence of enhanced lesions on the liver dynamic CT or MRI scans performed immediately after or 1 month after the treatments.
Statistical analyses
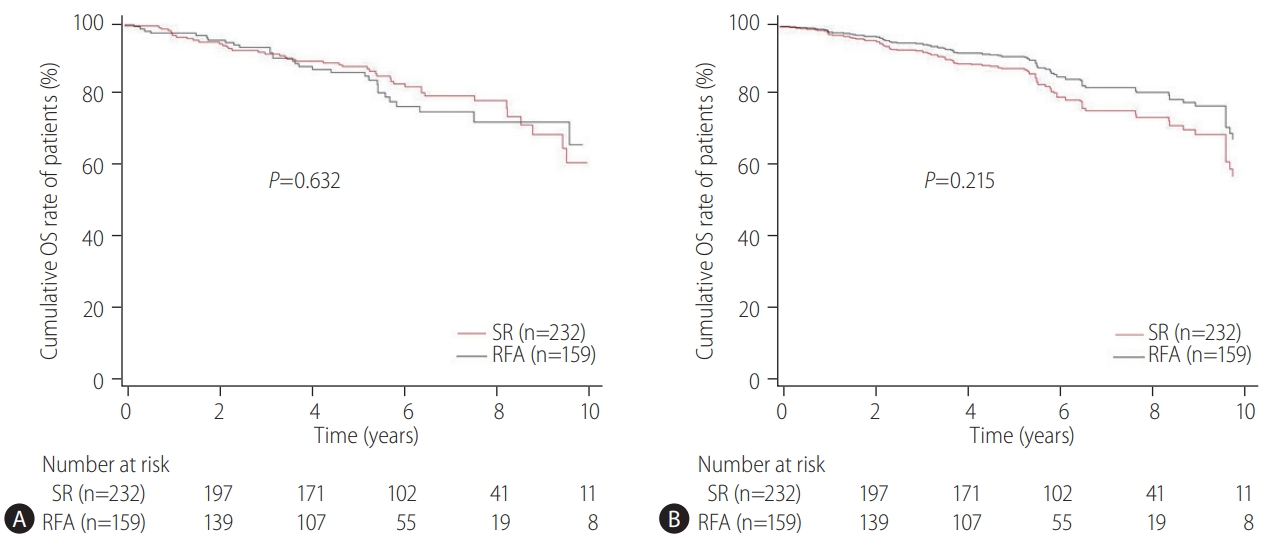
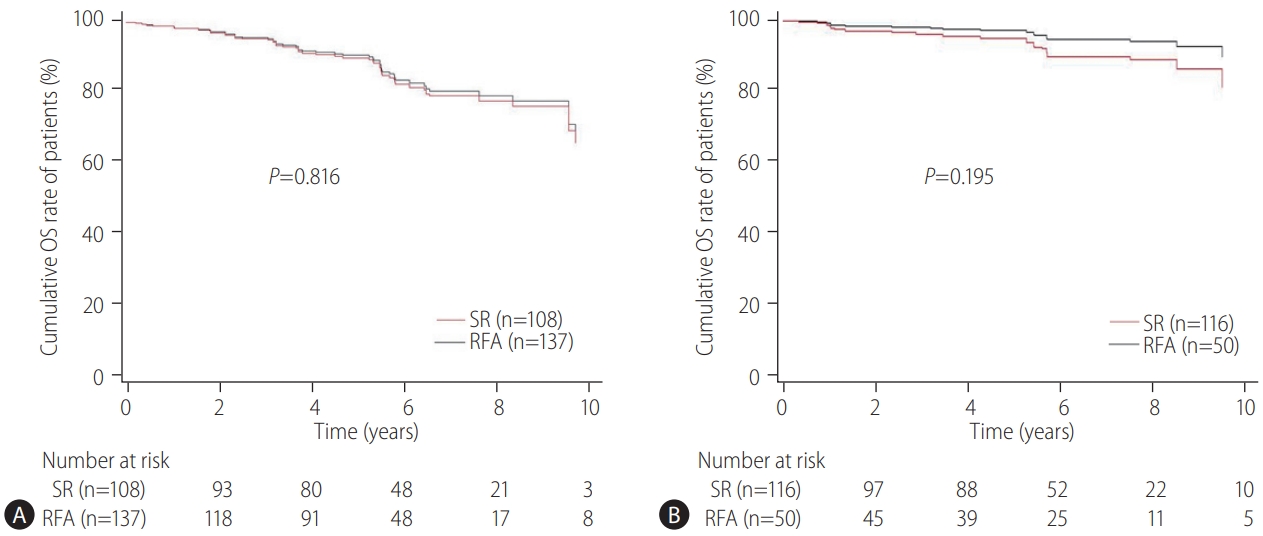
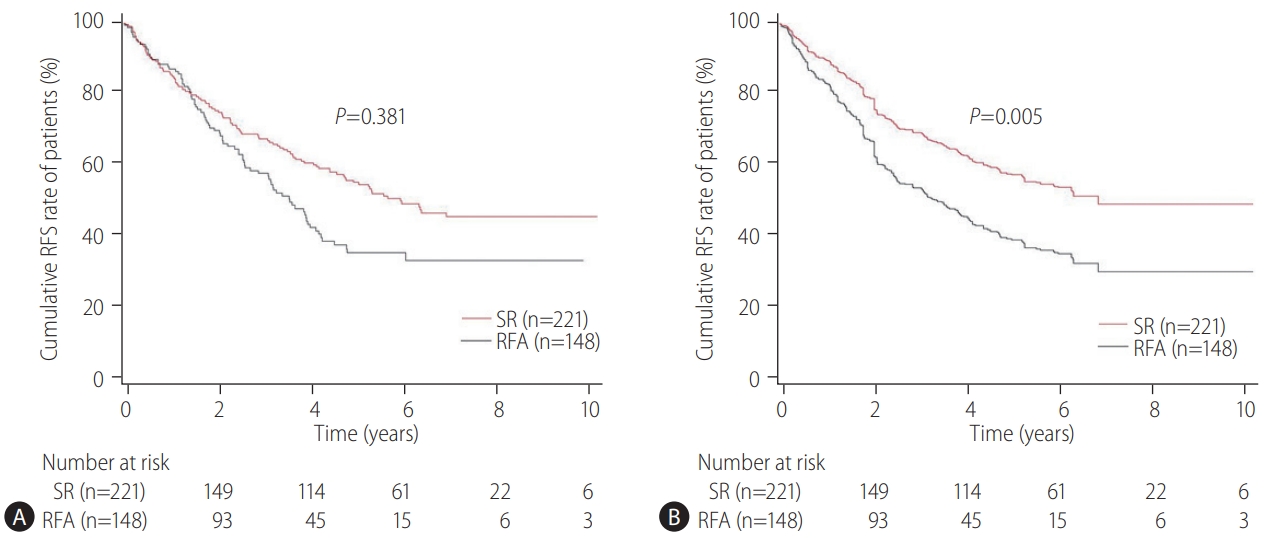
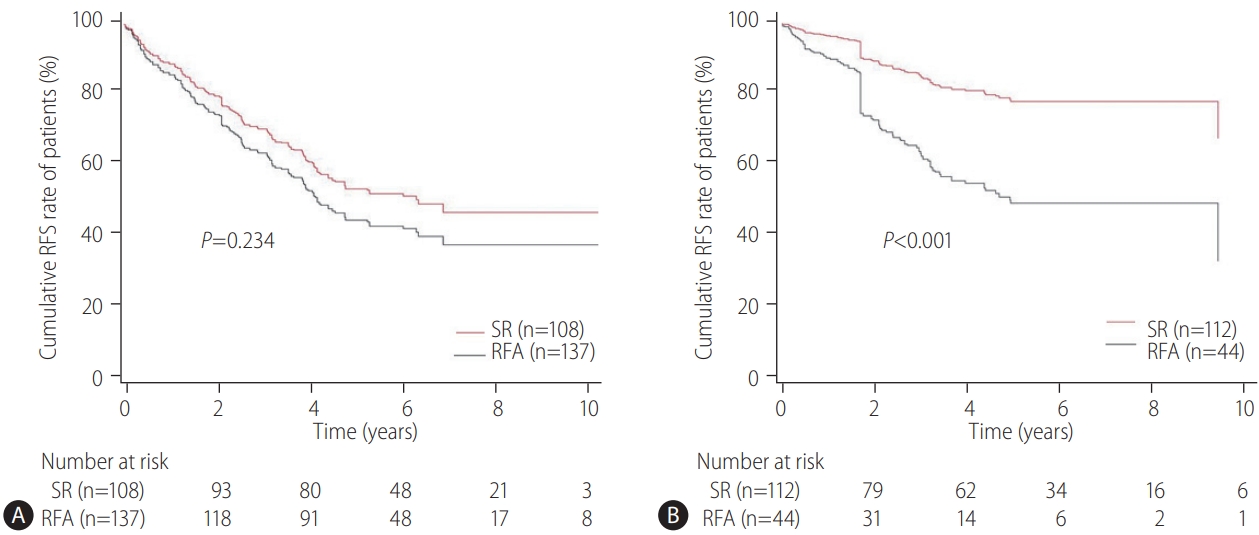
The primary endpoint of this study was the difference in the OS rates in patients with CTP class A and single small (Ōēż3 cm) HCC and without vascular/lymphatic/extrahepatic metastasis according to the treatment types (SR vs. RFA). The secondary endpoint was the RFS differences according to the treatment types (SR vs. RFA). The OS and RFS between the two treatment groups were also assessed according to the presence or absence of LC and the tumor size (Ōēż2 cm or 2ŌĆō3 cm), respectively. The prognostic factors associated with the OS or RFS in these patients were evaluated. Adverse events (AEs) of each treatment were compared.
The following variables were obtained: age, gender, weight and height, comorbidity of HTN or diabetes mellitus (DM), HCC etiology, aspartate aminotransferase (AST), alanine aminotransferase (ALT), albumin, total bilirubin, prothrombin time (PT), alpha-fetoprotein (AFP), CTP class, tumor size, and treatment types. This study examined the potential relationships between these factors and the primary and secondary outcomes. For evaluation of AE, data on abdominal pain, fever, infection, bleeding, blood pressure (BP) drop, care at the intensive care unit (ICU), and post-treatment death were also collected. Severe AE (SAE) was defined as abdominal pain Ōēźgrade 3ŌĆō4, fever Ōēź38┬░C, bleeding requiring transfusion, systolic BP drop (SBP <90 mmHg) requiring vasopressor, ICU care, or post-treatment death within 30 days.
Baseline characteristics of the study subjects and HCCs are expressed as the mean (┬▒standard deviation) for continuous variables, and as number (percentage) for categorical variables. The differences between groups were analyzed using the StudentŌĆÖs ttest for continuous variables or the chi-square test for categorical variables. The average causal effect of treatment was estimated by generating the inverse probability of treatment weighting (IPW) method based on the propensity score (PS) analysis [
12,
13]. An attempt was made to minimize imbalances of the distributions of baseline characteristics between the two treatment groups using the stabilized IPW. After using IPW, the distribution between groups was well-balanced using the absolute standardized differences. The balance was considered to be achieved when the absolute standardized difference between the groups was Ōēż0.1. The weighted Cox proportional hazards model was performed using IPW after adjusting the variables with the absolute standardized difference greater than 0.1. This model was used to estimate the hazard ratios (HRs) and 95% confidence intervals (CIs) for mortality. The two-tailed P-values of <0.05 were considered significant. Statistical analyses were performed using SAS ver. 9.4 (SAS Institute Inc., Cary, NC, USA) and SPSS ver. 19.0 (SPSS Inc, Chicago, IL, USA).
DISCUSSION
Our multicenter study analyzed the post-treatment long-term OS and RFS of patients with CTP class A and single small (Ōēż3 cm) HCC using the IPW method. In the present study, the long-term OS was comparable between the SR and RFA groups, regardless of the presence of LC or tumor size (Ōēż2 cm or 2ŌĆō3 cm). On the other hand, RFS was better in the SR group than in the RFA group, particularly in those without LC. Interestingly, the difference in RFS between the two treatment groups was determined by LC rather than the tumor size in the enrolled patients. Therefore, in the non-cirrhotic patients with CTP-class A and single small (Ōēż3 cm) HCC, SR is better than RFA as a first-line treatment to increase the RFS, even though there was no difference in OS. The present study is distinctive in that it attempted to avoid related confounding variables by selectively analyzing patients who met the same criteria (single small HCC sized 3 cm or less) among the well-known indications between SR and RFA, even in those with and without cirrhosis. Moreover, the long-term treatment OS and RFS between SR and RFA treatments were compared using the multicenter data and with the IPW method based on PS analysis to minimize the shortcomings of retrospective observational studies.
SR has been the primary treatment option in many guidelines for patients with small HCCs, and it can be preferred in patients without severe portal HTN, with peripheral tumors, or with tumors located near other organs, in which thermal ablation may be less effective [
4-
6,
14,
15]. On the other hand, SR for small tumors may require resection of a significant portion of normal liver parenchyma, contributing to an increase in treatment-related complications [
14]. RFA can be preferred in patients with severe portal HTN or centrally located tumors. Moreover, RFA has recently gained popularity owing to its ease of use, safety, effectiveness, and minimal invasiveness for the treatment of small HCC [
8]. Therefore, the choice between SR and RFA as a first-line treatment for single small HCC still remains controversial.
The previous studies that examined the survival benefits of SR and RFA in the treatment of small HCC reported inconsistent results [
8,
14]. Feng et al. [
8] reported that the SR and RFA groups had similar OS or RFS for patients with HCC sized less than 4 cm and no more than two tumor nodules. However, there was no analysis on whether the presence or absence of LC affects the OS or RFS. Moreover, unlike the present study, CTP-class B patients constituted approximately 50% of the enrolled patients, and the median follow-up duration was only 3 years, which is relatively short [
8]. Pompili et al. [
14] reported that RFA could provide similar OS and RFS rates compared to SR in treating patients with single small (Ōēż3 cm) HCC and compensated LC. These were similar to the results of the present study, but the previous study included only cirrhotic patients with CTP-class A. Hence, it could not reveal the data for those without cirrhosis [
14], unlike the current study. The present study enrolled patients with CTP class A and single small (Ōēż3 cm) HCC, which are tumor characteristics that can be applied equally to SR and RFA treatments [
4-
6]. This was different from the registration criteria of the previous study [
8], and may help maintain the homogeneity of the patient selection criteria between the SR and RFA groups.
Some studies reported that the SR group had a better OS and RFS compared to the RFA group [
7,
16]. In a previous study, HCC patients within the Milan criteria were enrolled. As a result, patients with HCC >3 cm in size underwent RFA, which is beyond the usual criteria for applying RFA to HCC patients [
7]. In addition, solitary tumors sized <3 cm were more common in the SR group than in the RFA group (44/115 vs. 27/115,
P=0.021) [
7]. These factors may have affected the superiority of the SR group. In another study, the therapeutic effects between the two treatments were not evaluated in each patient with and without LC, even though the study reported that the presence of LC was a poor prognostic factor for OS or RFS [
16]. Moreover, the previous study enrolled only patients with single HCC of <2 cm, without including those with HCC of 2ŌĆō3 cm [
16], even though these patients could also be candidates for SR or RFA treatment. Another meta-analysis reported that patients in the SR group provided better 5-year OS and were less likely to develop recurrence than those in the RFA group for small HCC, but it showed similar 1-year and 3-year OS between the two treatment groups [
17]. In addition, the enrollment criteria of the meta-analysis were patients within the Milan criteria, including those with CTP class A or B [
17]. This study concluded that the indication for RFA as a first-line treatment for early-stage HCC patients for whom SR is appropriate is unclear [
17]. Therefore, the present study aimed to elucidate the factors affecting the OS or RFS by evaluating patients with the same characteristics in the two treatment groups.
The therapeutic choice between SR and RFA in non-cirrhotic patients with CTP class A and single small HCC has not been well-established, likely due to the rarity of the disease. Unlike the previous studies [
7-
10,
14,
16], the current study revealed a prolonged RFS with SR compared to RFA, but it did not show a benefit in OS in these patients with or without LC. The lack of difference in the OS rates between the treatment groups in the present study may be due to the fact that additional treatments were actively performed for recurred HCC even after the RFA treatment, potentially resulting in an overestimation of the effectiveness of RFA. Regarding RFS, it is believed that tumor recurrence may be affected more by a cirrhotic liver itself if the SR or RFA procedure was performed successfully with curative intent, resulting in a similar outcome between the two treatment groups in cirrhotic patients. On the other hand, in patients with non-cirrhotic liver background, SR or RFA procedure may affect the tumor recurrence. For example, it may be difficult to sufficiently widen the ablation range with RFA as much as the SR range for HCC [
18]. Therefore, it may be reasonable to apply SR rather than RFA as first-line treatment for patients with CTP class A and single small (Ōēż3 cm) HCC to improve the RFS, particularly in non-cirrhotic patients. On the other hand, for cirrhotic patients with CTP class A, RFA may be a comparable first-line treatment option to SR.
According to the current guidelines of HCC management, RFA could be an effective treatment option for single nodular HCC Ōēż3 cm in diameter [
4-
6]. Previous studies that compared SR and RFA based on the tumor size reported varying results [
7,
19-
21]. Some studies reported that SR was better in patients with HCC sized >2 cm [
19,
20], or even in those with HCC Ōēż2 cm [
7,
21], whereas there were no differences between the two groups for HCC sized <2 cm [
20]. On the other hand, these results were obtained by analyzing subjects such as patients with HCC >3 cm in diameter or two or more tumors, or those with CTP class B or C [
7,
19-
21]. Therefore, these data may not be applied accurately to patients with CTP class A and single HCC Ōēż3 cm in diameter. In the present study, subgroup analysis was performed for patients with HCC of Ōēż2 cm or 2ŌĆō3 cm in size, respectively, and the study found that RFS was unaffected by the tumor size, but by the presence or absence of LC. This suggests that when applying SR or RFA in patients with CTP class A and single small (Ōēż3 cm) HCC, the presence of LC rather than the tumor size should be considered more important.
The present study had several limitations. First, due to the retrospective nature of this study, some confounding factors might still be unavoidable even after minimizing the influence of the variables using IPW based on PS analysis. A direct comparison between SR and RFA in a wellŌĆÉdesigned prospective randomized controlled trial (RCT) would be the best way. On the other hand, RCT would be a challenge for patients with CTP class A and single small (Ōēż3 cm) HCC, due to the requirement of a long follow-up period until death and a large sample size. Nevertheless, our results could offer useful data for conducting future prospective RCTs. Second, LC was not diagnosed pathologically in all of the patients. Unlike patients who underwent SR, it was difficult to obtain pathologic tissue from all patients undergoing RFA; and in these patients, LC had to be diagnosed clinically. Third, in the present study, approximately 85% and 66% of patients in the SR and RFA groups, respectively, were infected with HBV. Given that the leading cause of HCC is related to the hepatitis C virus (HCV) in the Western population [
22], unlike South Korea [
23], where HBV is the main cause of HCC, these results may not be generalized to the HCV-endemic areas.
In conclusion, SR showed similar OS rates compared to RFA in patients with CTP class A and single small (Ōēż3 cm) HCC regardless of the presence of cirrhosis, but provided a better RFS than RFA, particularly in those without cirrhosis. RFS was determined by the presence or absence of cirrhosis rather than the tumor size. Therefore, SR rather than RFA should be considered in non-cirrhotic patients with CTP class A and single small (Ōēż3 cm) HCC to prolong the RFS, even if there is no difference in the OS.






 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Supplement1
Supplement1 Print
Print



