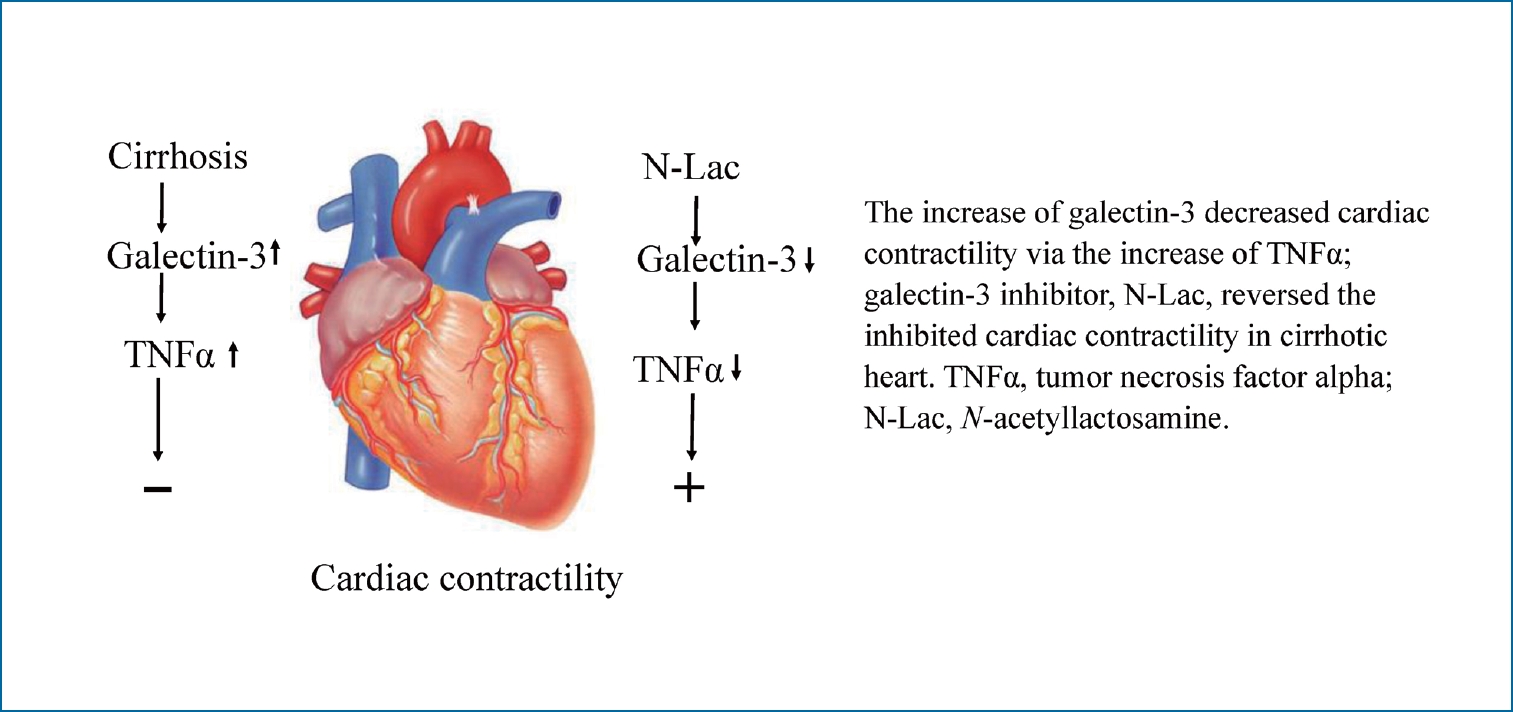
Galectin-3 inhibits cardiac contractility via a tumor necrosis factor alpha-dependent mechanism in cirrhotic rats
Article information
Abstract
Background/Aims
Galectin-3 plays a key pathogenic role in cardiac hypertrophy and heart failure. The present study aimed to investigate the effects of galectin-3 on cardiomyopathy – related factors and cardiac contractility in a rat model of cirrhotic cardiomyopathy.
Methods
Rats were divided into two sets, one for a functional study, the other for cardiac contractile-related protein evaluation. There were four groups in each set: sham operated and sham plus N-acetyllactosamine (N-Lac, a galectin-3 inhibitor; 5 mg/kg); bile duct ligated (BDL) and BDL plus N-Lac. Four weeks after surgery, ventricular level of galectin-3, collagen I and III ratio, tumor necrosis factor alpha (TNFα), and brain natriuretic peptide (BNP) were measured either by Western blots or immunohistochemistry or enzyme-linked immunosorbent assay. Blood pressure was measured by polygraph recorder. Cardiomyocyte contractility was measured by inverted microscopy.
Results
Galectin-3 and collagen I/III ratio were significantly increased in cirrhotic hearts. TNFα and BNP were significantly increased in BDL serum and heart compared with sham controls. Galectin-3 inhibitor significantly decreased galectin-3, TNFα, and BNP in cirrhotic hearts but not in sham controls. N-Lac also significantly improved the blood pressure, and systolic and diastolic cardiomyocyte contractility in cirrhotic rats but had no effect on sham controls.
Conclusion
Increased galectin-3 in the cirrhotic heart significantly inhibited contractility via TNFα. Inhibition of galectin-3 decreased the cardiac content of TNFα and BNP and reversed the decreased blood pressure and depressed contractility in the cirrhotic heart. Galectin-3 appears to play a pathogenic role in cirrhotic cardiomyopathy.
Graphical Abstract
INTRODUCTION
Cirrhosis is associated with several cardiovascular disturbances. These disturbances include cardiac contractile dysfunction when challenged, and decreased peripheral vascular resistance and arterial pressure. Despite the baseline increase in cardiac output, cardiac function in patients with cirrhosis is abnormal in several respects. Patients show attenuated systolic and diastolic contractile responses to stress stimuli, electrophysiological repolarization changes including prolonged QT interval, and enlargement or hypertrophy of cardiac chambers. This constellation of cardiac abnormalities is termed cirrhotic cardiomyopathy [1,2].
The mechanisms underlying impaired cardiac contractile responsiveness to stressful stimuli in cirrhotic cardiomyopathy remain incompletely clarified. Galectin‐3, a beta‐galactoside‐binding lectin, is predominantly expressed by activated macrophages. We previously showed that macrophages/monocytes are increased in the murine cirrhotic heart [3]. Galectin‐3 is significantly increased in patients with cirrhosis [4] and fibrotic/cirrhotic animal models [5]. Numerous studies implicate a potential pathogenic role of galectin-3 in noncirrhotic forms of heart failure and cardiac dysfunction, as well as being a possible biomarker of cardiac diseases [6-10]. However, a possible role of galectin-3 in pathogenesis of cirrhotic cardiomyopathy has not previously been examined. We therefore aimed to investigate the effects of galectin-3 on a rat model of cirrhotic cardiomyopathy.
MATERIALS AND METHODS
Reagents and enzymes
Rabbit polyclonal anti-galectin-3 antibody, goat polyclonal anti-COL I antibody, rabbit polyclonal anti-COL III antibody and rabbit anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibody were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Donkey anti-goat immunoglobulin (horseradish peroxidase conjugated, HRP) and goat anti-rabbit immunoglobulin (HRP) were from Santa Cruz and were used as a secondary antibody. Isoproterenol was purchased from Sigma (St. Louis, MO, USA). Other reagents were purchased from Sigma, Bio-Rad (Hercules, CA, USA), or Fisher Scientific (Waltham, MA, USA) and were the highest available grade.
Animal model
Male Sprague-Dawley rats between 200 and 250 g, bred by Charles River (St Laurent, Canada), were used in the present study. The protocol was approved by the Faculty of Medicine Animal Experimentation Committee in the University of Calgary Cumming School of Medicine in accordance with guidelines established by the Canadian Council on Animal Care. Cirrhosis was induced according to previously described methods [3,11]. Briefly, after anesthetized by isoflurane inhalation, the common bile duct of the rat was exposed after the abdominal midline incision and doubly ligated with 4-0 silk suture and cut between the ligatures. The muscle and skin were separately closed with 3-0 silk. Immediately after surgery, rats were administered 30,000 U penicillin G intra-muscularly to prevent infection and buprenorphine (0.03 mg/kg) for analgesia. Rats were relocated to an individual cage for recovery. Rats for sham-operated controls received the same laparotomy except that the bile duct was not ligated nor sectioned. Our previous study demonstrated that 4 weeks after bile duct ligation, rats display typical biliary cirrhotic changes in histology [11]. We therefore performed all experiments 4 weeks after surgery.
Two sets of rats were used in this study, one to study cirrhotic cardiomyopathy – related protein changes and another for a functional study. There were four groups (n=6 in each group) in each set: sham operation and sham plus galectin-3 inhibitor (N-acetyllactosamine [N-Lac], 5 mg/kg, every other day at week 4); bile duct ligation (BDL) and BDL plus N-Lac. Four weeks after surgery, the rats were sacrificed and the blood and hearts were collected; plasma and tissues were stored at -80°C for further use. Cardiac protein expression of galectin-3, GAPDH (internal control), collagen I and III were semi-quantitated by Western blots. Heart and serum tumor necrosis factor alpha (TNFα) were measured by enzyme-linked immunosorbent assay (ELISA). The functional study (cardiac contractility) was carried out under IonOptix microscopy (cell-shortening video system; IonOptix Corporation, Milton, MA, USA) with isolated cardiomyocytes.
Expression of galectin-3, collagen I and collagen III quantified by Western blotting
Western blots were performed as previously described [12]. Briefly, ventricular tissues were homogenized with a Kinamatica homogenizer (Brinkmann Instruments, Rexdale, Canada) in a buffer containing 20 mM Tris HCl (pH 7.2), 0.2 mM phenylmethylsulfonyl fluoride, and 1 mM dithiothreitol. The supernatants from 2,000 g centrifugation were collected. The protein concentrations were determined with the Bio-Rad protein assay using bovine serum albumin as a standard. The proteins were denatured with sample buffer at 100°C for 5 minutes. Equal amounts (30 μg) of the denatured proteins per lane were loaded and separated on sodium dodecyl sulfate-12% polyacrylamide gels by electrophoresis. Proteins in gel were transferred to nitrocellulose membrane by wet electroblotting at 4°C for 2.5 hours. Nitrocellulose membrane with proteins were blocked for 1.5 hours at room temperature with 10% skim milk in 0.1% Tween Tris-buffered saline buffer (TBS-T) at pH 7.5 containing 20 mM Tris base, 137 mM NaCl, and 0.1% Tween 20. The membranes were washed with TBS-T (3 times, 10 minutes each) and then incubated at 4°C overnight with goat polyclonal anti-COL I antibody, rabbit polyclonal anti-COL III, rabbit polyclonal anti-galectin-3 (1:1,000). Rabbit polyclonal anti-GAPDH was used as internal controls. After three times washing with TBS-T, the membranes were subsequently incubated with anti-goat or anti-rabbit horseradish peroxidase-linked immunoglobin (1:1,000). The blots were detected with the enhanced chemiluminescence method (enhanced chemiluminescence Western blot kit from Thermo Scientific; Rockford, IL, USA). The relative expressions of galectin-3/GAPDH, collagen I/III were quantified by computerized optical densitometric scanning of the images using a Hewlett-Packard Scan Jet IIc scanner, DeskScan II software, and analyzed with the NIH Image program [13]. The morphology of the liver was evaluated by haematoxylin and eosin (HE) and Masson stains.
Immunohistochemistry of galectin-3
Hearts embedded in paraffin were cut to 7 µm sections which were de-paraffined by xylene and graded concentrations of alcohol. Antigens were retrieved by citrate buffer. H2O2 (3%) was used to eliminate the endogenous peroxidase activity. After washing, the sections were blocked with 5–10% normal goat serum. Subsequently, the slices were incubated with rabbit anti-rat antibody at 4°C overnight. Following the wash in phosphate buffered saline, slices were incubated with goat anti-rabbit, biotin-labelled secondary antibody for one hour at room temperature. After an additional wash with PBS, slices were incubated with Vectastain ABC Elite kit (Vector Laboratories, Burlingame, CA, USA). The color was developed with DAB reagent (Vector Laboratories), hematoxylin was used for counterstain. Spleen was used as a positive control.
Serum and ventricular TNFα and brain natriuretic peptide (BNP) measurement
TNFα and BNP were measured by ELISA using a commercially available kit (Biosource, Camarillo, CA, USA for TNFα and Novus Biologicals, Littleton, CO, USA for BNP). The serum was used directly; left ventricles were homogenized in ice-cold phosphate-buffered saline and centrifuged at 2,000 g, 4°C for 10 minutes. The supernatants were placed on ice. Protein concentration was determined with Bio-Rad protein assay using bovine serum albumin as standard. The assay of TNFα and BNP for serum and supernatants from left ventricles was performed as follows. Samples and standard (50 μL) were pipetted into wells precoated with rat TNFα-specific antibody. Biotinylated anti-TNFα or anti-BNP solution (50 μL) was added to each well, plate was covered and incubated at room temperature for 1.5 hours (BNP, 2 hours). The liquid was then discarded, and the wells were washed with washing buffer for four times. Streptavidin-peroxidase (100 μL) was added to each well and incubated at room temperature for 45 minutes (for BNP, SP conjugate, 50 μL), and the washing process was repeated four times. Subsequently, 100 μL stabilized chromogen was added to each well in a dark room and incubated for 20 minutes (50 μL for BNP, incubation time was 25 minutes). Finally, 100 μL stop solution (50 μL for BNP) was added and the wells were read at 450 nm with a spectrometer (Molecular Devices, Menlo Park, CA, USA).
Blood pressure measurement
Under isoflurane anesthesia, a polygraph recorder (Grass Instruments, West Warwick, RI, USA) was used to measure blood pressure via a femoral artery cannula. The blood pressure was measured twice, 3 weeks and 4 weeks after surgery. We adjusted the isoflurane concentration to record the highest mean blood pressure.
Cardiomyocyte isolation
Ventricular myocytes were isolated using methods that have been described previously [14]. Briefly, animals were sacrificed by decapitation, and the heart was removed, and the aorta was cannulated and retrogradely perfused at a rate of 10 L/min using a standard Langendorff apparatus. The heart was initially perfused for 5 minutes with standard Tyrode solution containing 1 mmol/L CaCl2, then subsequent 5‐minute perfusion was performed with Ca2+ free Tyrode solution. Tyrode solution with collagenase (0.02 mg/mL), protease (0.004 mg/mL; type XIV) and CaCl2 (50 mmol/L) was used to perfuse the heart for about 7 minutes. The soft left ventricular free wall was cut and minced in 10 mL of Tyrode solution containing collagenase (0.5 mg/mL), protease (0.1 mg/mL), bovine serum albumin (5 mg/mL) and CaCl2 (50 mmol/L). This tissue was gently agitated in a shaking water bath for 10–20 minutes at 37°C. The tissue-cell mixture was monitored under microscopy until there were free healthy cells. Cardiomyocytes were washed to eliminate the collagenase and protease, and kept in storage solution with the calcium concentration gradually adjusted to 1.1 mM.
Cardiomyocyte systolic and diastolic contractility
The protocol of measuring systolic and diastolic contractility of isolated cardiomyocytes was performed as previosly described [14]. Briefly, unloaded cell shortening was quantified using a video sarcomere detector. The standard Tyrode solution was composed of 140 mmol/L NaCl, 5.4 mmol/L KCl, 1 mmol/L MgCl2, 1 mmol/L Na2HPO4, 5 mmol/L HEPES, and 10 mmol/L glucose. pH was adjusted to 7.4 with 1 mol/L NaOH and continuously gassed with 100% O2. Left ventricular myocytes were loaded into the recording chamber which were continuously field‐stimulated at a rate of 1 Hz. The contractile response to this stimulation was used as the baseline value. Cardiomyocytes were allowed to equilibrate to this stimulus for a minimum of 10 minutes before further stimulating with isoproterenol (10-5 mol/L). Calibrated output of the video sarcomere detector was digitized for off‐line analysis. Maximal systolic contractile velocity and diastolic relaxation velocity, time from basal length to 50% of peak contractility and time from peak contraction to 50% of basal length in the diastolic relaxation phase were recorded and analysed.
Statistical analysis
Results are expressed as means±standard deviation. For analysis of two independent groups, a Student’s t-test was used. For analysis of three or more independent groups, one-way ANOVA with post hoc Newman-Keuls testing was used to compare group means. Statistical analyses were done using GraphPad Prism software, version 5.02 for Windows (GraphPad, San Diego, CA, USA). The significance level was set at P<0.05.
RESULTS
Left ventricular protein expression of galectin-3, collagen I, and collagen III
Ventricular galectin-3 protein expression in each group was confirmed by immunohistochemistry (Fig. 1). Sham-controls (Fig. 1A) showed scant galectin staining. In contrast, BDL cardiomyocytes (Fig. 1B) displayed significant cardiomyocyte cytoplasmic staining, and N-Lac treated BDL myocytes (Fig. 1C) showed attenuated amounts of staining.

Representative immunohistochemistry of galectin-3 protein expression in hearts (×200). (A) Sham. (B) BDL. (C) BDL+N-Lac. (D) Spleen was used as positive control. (A) Scant staining is visible in cardiomyocytes. (B) Significantly increased galectin-3 staining is predominantly in cardiomyocyte cytoplasm, although a few macrophages also stain. (C) N-Lac significantly decreased galectin-3 staining in BDL heart. N-Lac did not affect galectin-3 postive staining in heart from sham operated controls (data not shown). (D) Spleen shows expected heavy galectin-3 staining, as a positive control. Sham, sham operated; BDL, bile duct ligation; N-Lac, N-acetyllactosamine.
Western blot analysis also showed that the expression of galectin-3 in left ventricular tissue was significantly increased in BDL rats compared with sham rats (galectin-3/GAPDH, 0.2±0.05 vs. 0.82±0.18, P<0.01). Galectin-3 inhibitor N-Lac significantly decreased galectin-3 expression in left ventricles from BDL rats (0.82±0.18 vs. 0.59±0.16, P<0.05), whereas N-Lac had no significant impact on galectin-3 expression in sham operated rats (Fig. 2).
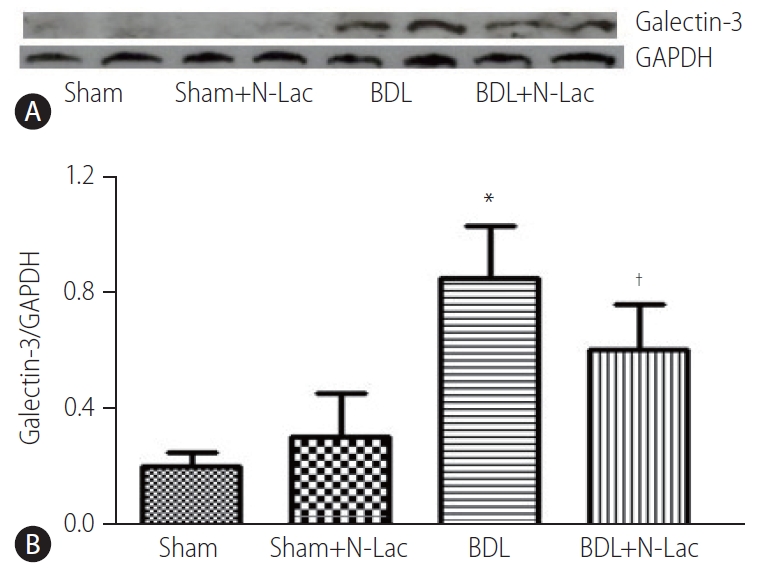
(A) Representative Western blot analysis of galectin-3 protein expression in hearts. From left, 1st and 2nd lanes, sham-operated controls; 3rd and 4th lanes, sham+N-Lac, 5th and 6th lanes, BDL, 7th and 8th lanes, BDL+N-Lac. (B) Computerized optical densitometry showed that galectin-3 protein expression was significant increased in BDL heart, N-Lac significantly decreased galectin-3 protein expression in BDL, N-Lac did not affect galectin-3 protein expression in sham operated rats (n=6 in each group, *P<0.01 compared with sham; †P<0.01 compared with BDL). Sham, sham operated; N-Lac,N-acetyllactosamine; BDL, bile duct ligation.
Collagen I was significantly increased, and collagen III significantly decreased in cirrhotic left ventricles; the ratio of collagen I/III was significantly increased from 0.44±0.04 in sham operated rats to 1.83±0.28 (P<0.01). N-Lac did not change the expression of collagen I/III in either sham-operated or cirrhotic rats (Fig. 3). HE and Masson stains (Supplementary Figs. 1 and 2) showed that N-Lac did not change the fibrosis extent or pattern in the liver of BDL rats.
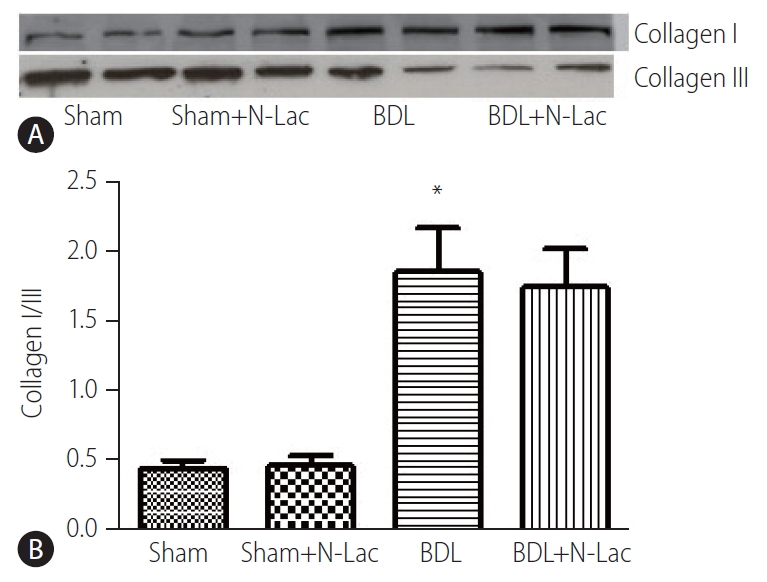
(A) Representative Western blot analysis of collagen I/III protein expressions in hearts. From left, 1st and 2nd lanes, sham-operated controls; 3rd and 4th lanes, sham+N-Lac, 5th and 6th lanes, BDL, 7th and 8th lanes, BDL+N-Lac. (B) Computerized optical densitometry showed that the ratio of collagen I/III was shifted from collagen III dominant to collagen I dominant in BDL heart, N-Lac had the tendency to decrease the ratio of collagen I/III in cirrhotic heart (n=6 in each group, *P<0.01 compared with sham). Sham, sham operated; N-Lac, N-acetyllactosamine; BDL, bile duct ligation.
TNFα levels in left ventricles and serum
TNFα levels in cardiac homogenates showed significant increases in the BDL cirrhotic rats compared with those of sham-operated controls (310.4±33.5 vs. 189.9±20.9 pg/mg, P<0.01). N-Lac significantly decreased TNFα levels in left ventricles of BDL rats (310.4±33.5 vs. 191.3±32.5 pg/mg, P<0.01). N-Lac had no significant effects on sham-operated rats. The changes of serum TNFα tended to be similar to those in left ventricles (Fig. 4).
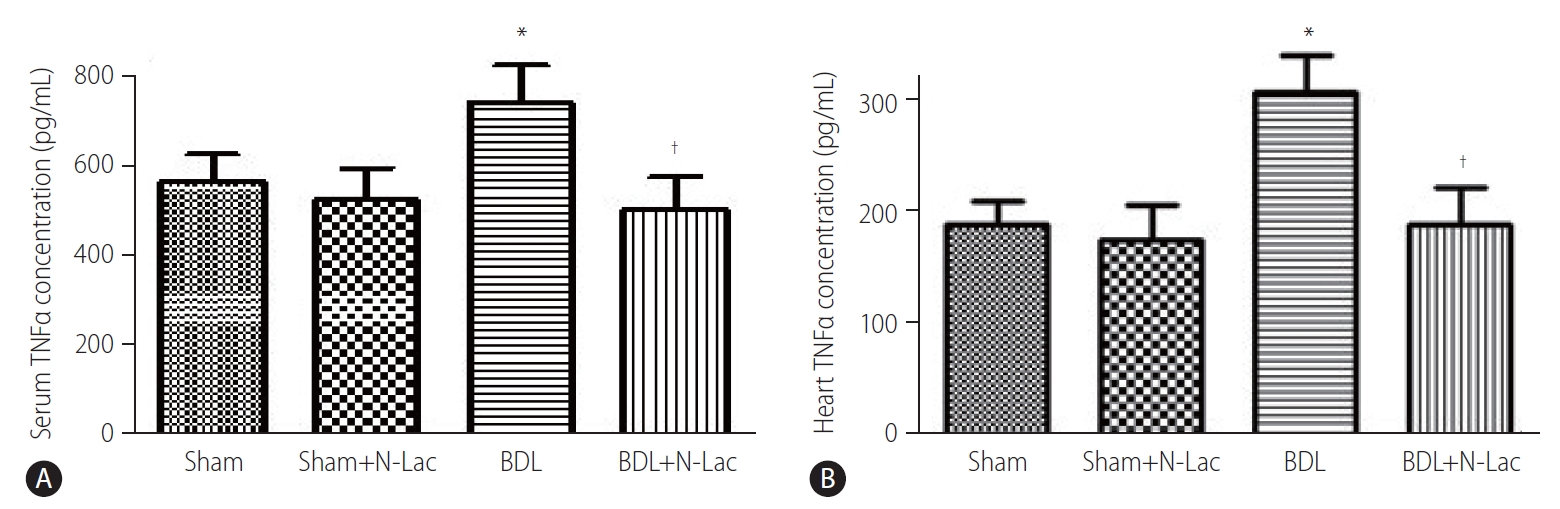
The concentrations of TNFα in serum (A) and heart (B). From left, 1st and 2nd lanes, sham-operated controls; 3rd and 4th lanes, sham+N-Lac, 5th and 6th lanes, BDL, 7th and 8th lanes, BDL+N-Lac. The concentrations of TNFα in serum and heart were significantly increased in BDL, N-Lac significantly decreased TNFα concentrations in both serum and heart from cirrhotic rats (n=6 in each group, *P<0.01 compared with sham; †P<0.01 compared with BDL). TNFα, tumor necrosis factor alpha; Sham, sham operated; N-Lac, N-acetyllactosamine; BDL, bile duct ligation.
BNP levels in left ventricles and serum
BNP levels in cardiac homogenates showed significant increases in the BDL cirrhotic rats compared with those of sham-operated controls (18.6±3.8 ng/g vs. 10.6±5.0 ng/g, P<0.05). N-Lac significantly decreased BNP levels in left ventricles of BDL rats (18.6±3.8 vs. 12.5±3.6 ng/g, P<0.05). N-Lac had no significant effects on sham-operated rats. The changes of serum BNP tended to be similar to those in left ventricles (Fig. 5).

The concentrations of BNP in serum (A) and heart (B). The concentrations of BNP in serum and heart were significantly increased in BDL rats. N-Lac significantly decreased BNP concentrations in both serum and heart from cirrhotic rats (n=6 in each group, *P<0.05 compared with sham; †P<0.05 compared with BDL). Sham, sham operated; BNP, brain natriuretic peptide; N-Lac, N-acetyllactosamine; BDL, bile duct ligation.
Systolic and diastolic contractility
The isolated cardiomyocyte contractility studies confirmed attenuated systolic and diastolic contractility of cardiomyocytes from cirrhotic rats. The maximum velocity of systolic contraction was significantly decreased in cirrhotic cardiomyocytes compared with controls (5.76±1.43 vs. 7.76±1.90 µm/s, P<0.01); N-Lac restored systolic contractility in cirrhotic cardiomyocytes (5.76±1.43 vs. 7.24±1.45 µm/s, P<0.05). N-Lac had no effect on cardiomyocyte contractility in sham operated rats. On the contrary, the time to reach half‐peak contractility (T1/2 peak) was significantly prolonged in the cirrhotic cardiomyocytes. N-Lac restored T1/2 peak in cirrhotic cardiomyocytes but had no effect on T1/2 peak in sham controls (Fig. 6). Diastolic function showed that the maximum diastolic relaxation velocity was significantly reduced compared with sham controls (3.50±1.22 vs. 5.55±1.50 µm/s, P<0.01). N-Lac restored maximum diastolic relaxation velocity in cirrhotic cardiomyocytes (3.50±1.22 vs. 5.08±1.41 µm/s, P<0.05). The half‐maximal relaxation time in cirrhotic cardiomyocytes showed the same pattern as half‐maximal contractile time. N-Lac restored the half‐ maximal relaxation time but did not change this parameter in control rats (Fig. 7).
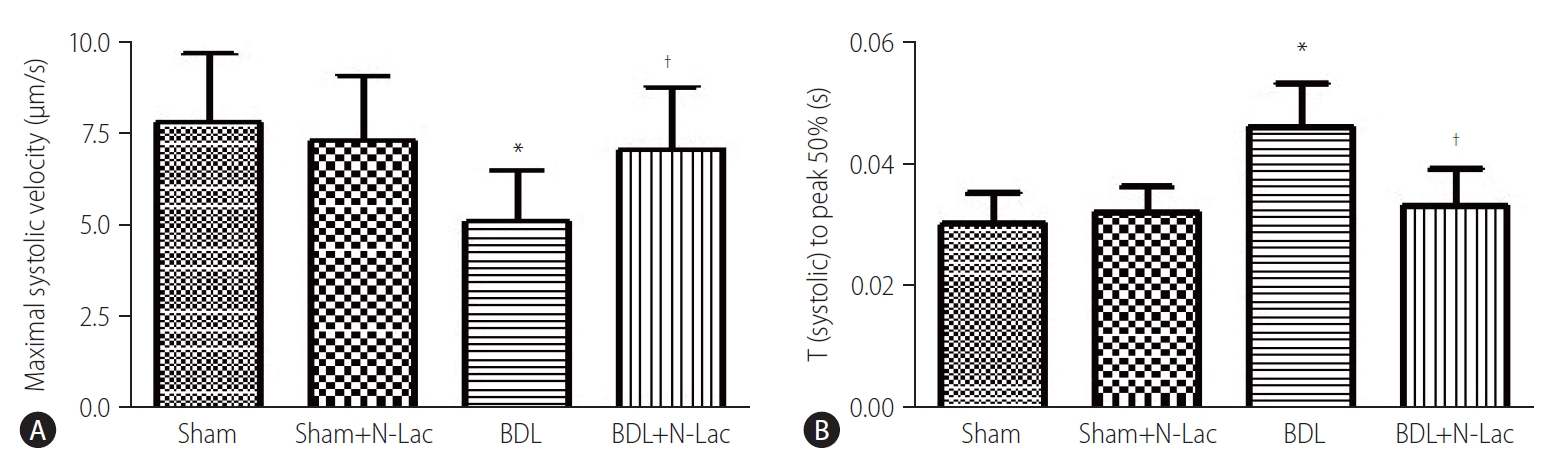
(A) Isoproterenol-stimulated maximal systolic velocity in isolated cardiomyocytes. Systolic velocity was significantly decreased in cirrhotic rats compared to the sham control group (P<0.05). Treatment of the cirrhotic group with N-Lac significantly reversed the reduced systolic contractility in BDL rats (†P<0.05 compared to the BDL group) (n=6 in each group). (B) Time to peak 50% under stimulation of isoproterenol. Time to peak 50% was significantly prolonged in BDL cardiomyocytes compared with sham control (*P<0.01), N-Lac reversed this delayed time to peak 50% (†P<0.05 compared with untreated BDL), N-Lac had no effects on sham control group (n=6 in each group). Sham, sham operated; N-Lac, N-acetyllactosamine; BDL, bile duct ligation.
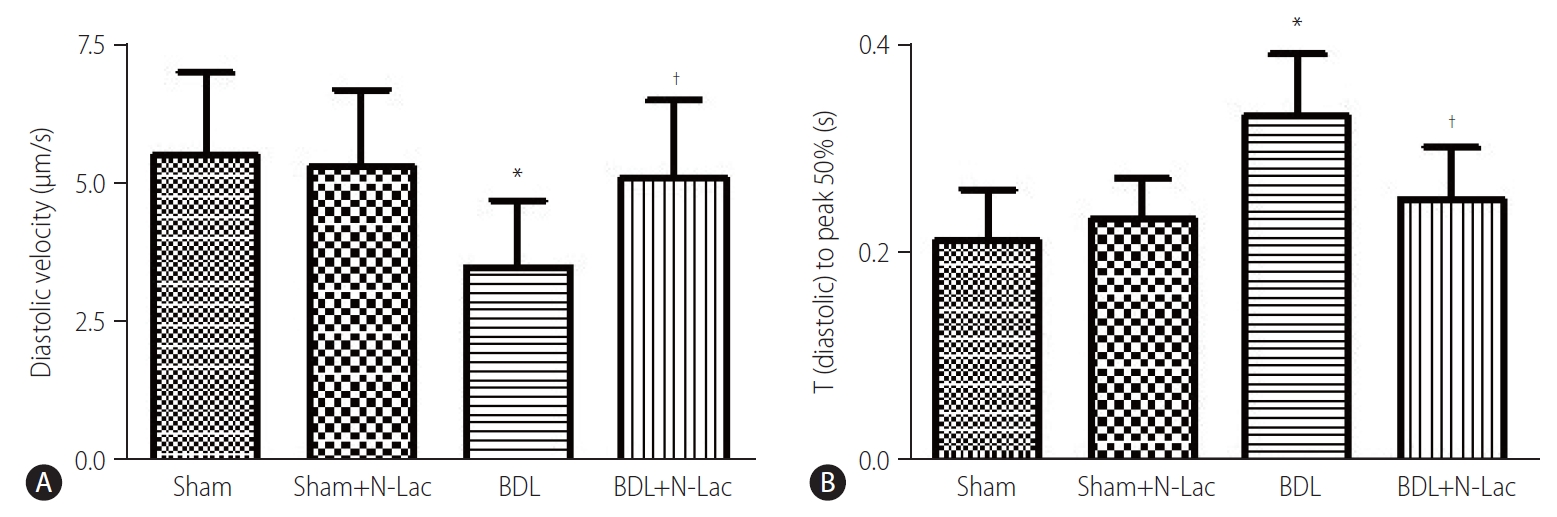
(A) Diatolic relaxation velocity in isolated cardiomyocytes under the environment of isoproterenol stimulation. Diatolic relaxation velocity was significantly decreased in cirrhotic rats compared to the sham control group (P<0.01). Treatment of the cirrhotic group with N-Lac significantly reversed the reduced diatolic relaxation velocity in BDL rats († P<0.05 compared to the BDL group) (n=6 in each group). (B) Time from peak contractility to 50% baseline under stimulation of isoproterenol was significantly prolonged in BDL cardiomyocytes compared with sham control (*P<0.01), N-Lac reversed this delayed time (†P<0.05 compared with untreated BDL), N-Lac had no effects on sham control group (n=6 in each group). Sham, sham operated; N-Lac, N-acetyllactosamine; BDL, bile duct ligation.
Effect of N-Lac on blood pressure
There were no significant changes of blood pressure in sham, and BDL groups measured twice, at 3 weeks after surgery, and 4 weeks after surgery. N-Lac significantly reversed the decreased blood pressure in BDL rats, not in sham controls (Fig. 8).
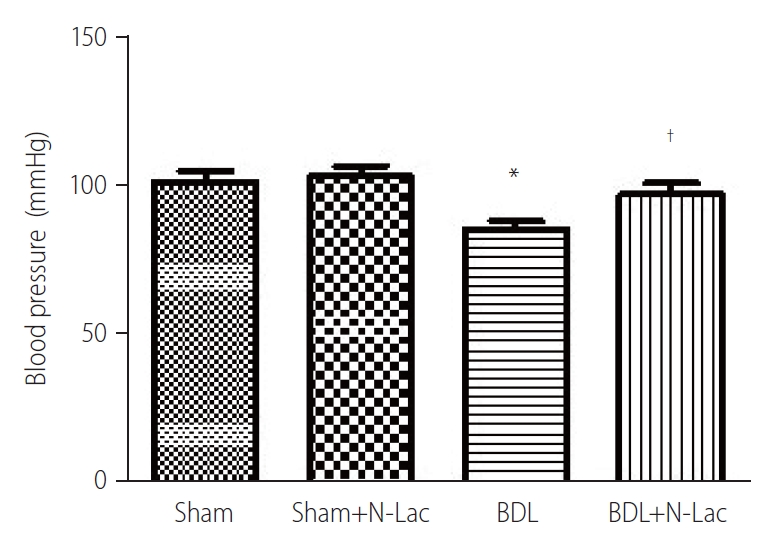
Blood pressure changes. The blood pressure was significantly decreased in cirrhotic rats, N-Lac significantly increased blood pressure in BDL rats. N-Lac had no effect on blood pressure in sham rats. The two measurements had no significant difference in BDL rats (n=6 in each group, *P<0.05 compared with other three groups). Sham, sham operated; N-Lac, N-acetyllactosamine; BDL, bile duct ligation. †Sham: 101±9.5 mmHg, Sham+N-Lac: 103±8.9 mmHg, BDL: 85.5±7.8 mmHg, BDL+N-Lac: 97.0±10.1 mmHg.
DISCUSSION
To our knowledge, the current study is the first to examine a possible regulatory role of galectin-3 in cirrhotic cardiomyopathy. Galectin-3 is a protein encoded by the LGALS3 gene in humans [15]. It is a member of the lectin family and a total of 15 mammalian galectins have been described [16]. Galectin-3 has pleiotropic roles in biological processes and diseases such as immune and inflammatory responses [17], tumor growth, progression and metastasis [18], liver fibrosis [19], and cardiac fibrosis [16,20].
Several studies have clarified a key role of galectin-3 in physiological and pathophysiological processes of the heart. van Kimmenade and colleagues [6] demonstrated a correlation between galectin‐3 and cardiac fibrosis and heart failure. Galectin‐3 predicts 18‐month mortality and heart failure re-hospitalization [8]. Increased galectin‐3 is associated with both diastolic [9] and systolic dysfunction [10]. Infusing recombinant galectin‐3 into the pericardial space in rats depresses left ventricular ejection fraction, fractional shortening, and the amplitude of the negative slope of dP/dtmax. Galectin‐3 also directly increases cardiac collagen I and collagen I/III ratio [21]. Blockade of galectin-3 with N-Lac significantly reduced cardiac fibrosis and inflammation in the heart in mice [22]. The inhibition of galectin-3 by N-Lac attenuates cardiac fibrosis and improves left ventricular function and subsequent heart failure [20]. In view of this significant literature demonstrating a pathogenic role of galectin-3 in heart failure, since 2014 the U.S. Food and Drug Administration has included galectin-3 in a list of validated cardiovascular biomarkers [7].
Cardiac dysfunction is latent in cirrhotic patients. However, when challenged, the cardiac dysfunction manifests, leading to increased morbidity and mortality in cirrhotic patients who undergo procedures or surgeries such as transjugular intrahepatic portosystemic shunt and liver transplantation [23]. The mechanisms underlying cirrhotic cardiomyopathy remain to be fully clarified.
The evidences of involvement of galectin-3 in cirrhotic cardiomyopathy are as follows: 1) galectin is produced by monocytes/macrophages, and we previously showed that monocytes/macrophages are increased in cirrhotic hearts [3]. Furthermore, in normal rat heart, galectin-3 levels are low. However, as heart disease develops and progresses, galectin-3 is significantly upregulated in the myocardium [24]. 2) Galectin-3 levels are significantly increased in patients with chronic heart failure, independent of etiology [6,25]. 3) Galectin-3 stimulates TNFα production in dendritic cells in a dose and time-dependent manner [26], and our previous studies demonstrated that TNFα plays an important mechanistic role in cirrhotic cardiomyopathy [27,28]. And 4) galectin-3 upregulates collagen I and increases collagen I/III ratio [21], and we previously demonstrated that an increased collagen I:III ratio contributes to diastolic dysfunction in a murine model of cirrhotic cardiomyopathy [13]. Baseline serum galectin-3 levels are associated with a higher risk for incident heart failure with preserved ejection fraction (HFpEF), mortality, and cardiovascular hospitalization [29]. The type of heart failure of cirrhotic cardiomyopathy is HFpEF [30].
Effective treatments that improve cardiac function in patients with cirrhosis are not yet available. Conventional remedies for other heart diseases, such as vasodilators, are not applicable because of the significant baseline vasodilatation in cirrhotic patients. Similarly, angiotensin-converting enzyme inhibitors are also not usable due to the marked vasodilating effect which may precipitate profound hypotension. Direct inotropic agents such as cardiac glycosides were proven ineffective in improving any contractile parameters [31]. Therefore, exploration of new treatment modalities for cirrhotic cardiomyopathy beyond the traditional therapies for heart diseases is of utmost importance.
The present study demonstrated that galectin-3 indeed was increased in the cirrhotic heart; this upregulation paralleled the cardiac content of TNFα. Although the inhibition of galectin-3 did not significantly change the ratio of cardiac collagen I/III, or liver fibrosis, this may have been due to the short time frame of galectin-3 application (only three doses). However, N-Lac, a galectin-3 inhibitor, not only downregulated galectin-3 but also decreased the cardiac level of TNFα. Another biomarker related to cardiac function, BNP, was also increased in both heart and serum in BDL rats, and N-Lac significantly decreased BNP levels in both heart and serum from cirrhotic animals but not sham controls. These BNP results along with the improvement of blood pressure in cirrhotic rats all support the notion that the N-lac improves cardiac function in cirrhotic cardiomyopathy. Therefore our results imply that galectin-3 inhibition may be a useful therapeutic target for cirrhotic cardiomyopathy.
In conclusion, galectin-3 was significantly increased in the cirrhotic heart, and significantly inhibited contractility via TNFα. Inhibition of galectin-3 decreased the cardiac content of TNFα and reversed the depressed contractility in the cirrhotic heart. Galectin-3 appears to play a pathogenic role in cirrhotic cardiomyopathy and may be a potential new therapeutic strategy for cirrhotic cardiomyopathy.
Notes
Authors’ contributions
Ki Tae Yoon, Hongqun Liu, Jing Zhang and Sojung Han are involved in model creation, data collection and analyses, manuscript drafting. Samuel S. Lee desigend this study. All authors approved the final version.
Conflicts of Interest
The authors have no conflicts to disclose.
Acknowledgements
We thank Dr. Yongxiang Chen for the help in immunohistochemistry.
SUPPLEMENTAL MATERIAL
Supplementary material is available at Clinical and Molecular Hepatology website (http://www.e-cmh.org).
Representative haematoxylin and eosin staining of livers (×200). There was significant structural damage in BDL liver. Short term treatment by N-Lac had no significant change on the liver pathology in BDL rats. (A) Sham. (B) Sham+N-Lac. (C) BDL. (D) BDL+N-Lac. BDL, bile duct ligation; Sham, sham operated; N-Lac, N-acetyllactosamine.
epresentative Masson staining of livers (×100). There was significant fibrosis in BDL liver. Short term treatment by N-Lac had no significant attenuation of the fibrosis. (A) Sham. (B) Sham+N-Lac. (C) BDL. (D) BDL+N-Lac. BDL, bile duct ligation; Sham, sham operated; N-Lac, N-acetyllactosamine.
Abbreviations
BDL
bile duct ligation
BNP
brain natriuretic peptide
ELISA
enzyme-linked immunosorbent assay
GAPDH
glyceraldehyde 3-phosphate dehydrogenase
HE
haematoxylin and eosin
HFpEF
heart failure with preserved ejection fraction
HRP
horseradish peroxidase conjugated
HRP
horseradish peroxidase
N-Lac
N-acetyllactosamine
TBS-T
Tris-buffered saline buffer
TNFα
tumor necrosis factor alpha
References
Article information Continued
Notes
Study Highlights
• Galectin-3 inhibits cardiac contractility.
• Galectin-3 is increased in patients and rat models with cirrhosis.
• Galectin-3 inhibition improved cardiac contractility in cirrhotic hearts.
• Galectin-3 is a potential therapeutic target in cirrhotic cardiomyopathy.