Prediction models of hepatocellular carcinoma recurrence after liver transplantation: A comprehensive review
Article information
Abstract
Liver transplantation (LT) is one of the most effective treatments for hepatocellular carcinoma (HCC). Although LT eliminates HCC and greatly reduces recurrence, some patients experience recurrence after LT. Criteria and models for screening patients with a high probability of HCC recurrence after LT, starting with the Milan criteria, have been published. These models have changed over time, but a standard has not been established. We summarized HCC prediction models after LT by focusing on the application of radiologic, serologic, and pathologic factors and recent trends. This review will look at studies that are based on living donor LT and deceased donor LT, as well as studies that downstaging procedures have been performed preoperatively. This ultimately aims to help make decisions for evaluating the HCC state and selecting candidates for LT according to the circumstances of each transplantation center.
INTRODUCTION
Liver transplantation (LT) has been widely used for endstage liver diseases, such as acute or chronic liver failure, as well as for patients with hepatocellular carcinoma (HCC). Approximately 25% of LTs are performed for underlying HCC in Western countries, with an increasing proportion of nonalcoholic steatohepatitis (NASH) cases, while hepatitis C virus (HCV) is decreasing [1,2]. With a high prevalence of hepatitis B virus (HBV), HCC also accounts for a high proportion of LTs in Asia (17–42%), especially in China [3,4]. LT can completely remove HCC from the liver by replacing the underlying liver, which is the basis of HCC development.
However, LT for patients with HCC still bears the risk of recurrence, leading to patient death, especially when the HCC is at an advanced stage [5,6]. To evaluate the risk of HCC recurrence after LT, the Milan criteria (MC), which employs simple guidelines using tumor size and numbers to perform LT, were introduced in 1996 and adopted by the United Network for Organ Sharing (UNOS) in 2002 [7]. Although the application of MC showed good post-LT recurrence-free survival, the indication for this criterion is relatively narrow for the majority of HCC patients; thus, an extended guideline for LT was published by the University of California, San Francisco (UCSF), in 2001 [8].
Although these criteria significantly contributed to clinical practice at the beginning with high prognostic power and led to the revival of LT, they are restricted, leaving out some patients who can benefit from LT. In addition, these criteria are based only on the radiological aspect and do not reflect various factors that can affect HCC recurrence. Practically, preoperative imaging evaluations differ in the accuracy of actual discrimination of HCC according to examination methods such as magnetic resonance imaging (MRI) or computed tomography (CT), as well as according to the individuality of the interpreter [9]. Although the Liver Imaging Reporting and Data System was developed to standardize the interpretation of liver lesions and showed great effects, a recent meta-analysis of 2,056 patients showed a sensitivity of only 67% and specificity of 92% compared to those of pathologic reports, suggesting low accuracy in detecting HCC before performing LT [10]. Therefore, the addition of several approaches, including multiple non-radiologic factors, such as serologic or pathologic factors, into the criteria for LT in patients with HCC, has been proposed (Fig. 1). This article aims to review previously published prediction models for HCC recurrence after LT and aid in the application of these models in clinical practice.

Prediction models based on recruited factors. UCSF, University of California, San Francisco; AMC, Asan Medical Center; AFP, alpha-fetoprotein; SMC, Samsung Medical Center; RETREAT, Risk Estimation of Tumor Recurrence After Transplant; LiTES-HCC, Liver Transplant Expected Survival-hepatocellular carcinoma.
PREDICTION MODELS BASED ONLY ON RADIOLOGIC FACTORS
The main concept behind the selection criteria for LT in patients with HCC is to exclude patients with HCC with vascular invasion or poor differentiation, which may lead to HCC recurrence and patient death [11]. The tumor size and number are closely related to these two factors [12].
Mazzaferro et al. [7] at the National Cancer Institute in Milan, presented the MC, the most monumental criterion for LT for patients with HCC in 1996, after analyzing the explant pathology of 48 patients. The criteria included single tumor up to 5 cm in diameter or two or three tumors, each smaller than 3 cm in diameter. Patients who met these criteria showed excellent 4-year overall survival and recurrence-free survival after transplantation (85.0% and 92.0%, respectively), whereas those who did not meet the criteria showed poor survival (50.0% and 59.0%, respectively). After this publication, several centers following the MC also showed favorable outcomes and the MC became the standard guideline for patients with HCC. The same LT center also reviewed 90 articles and applied a meta-analysis of 25 articles analyzing the outcomes of patients within MC, showing similar favorable survival rates after LT (5-year patient survival of at least 70%) [13].
The UCSF criteria, which were published in 2001, presented a requirement slightly different from that of MC based on the explant pathology of 70 patients (Table 1) [8]: a solitary tumor ≤6.5 cm, or ≤3 nodules with the largest lesion being ≤4.5 cm and a total tumor diameter of ≤8 cm. These extended criteria led to a favorable 5-year patient survival rate of 75.2%, which enabled the LT center to recruit more candidates whose HCC status was beyond the MC. Mazzaferro et al. [14] also presented simple criteria in 2009 (up-to-seven) designed for patients who did not meet the MC, including those with cumulative tumor number and diameter (cm) of the largest tumor ≤7. This study was based on 1,156 patients from 20 countries in Europe, the USA, and Brazil who showed a 5-year patient survival of 71.2% among those who met the criteria.
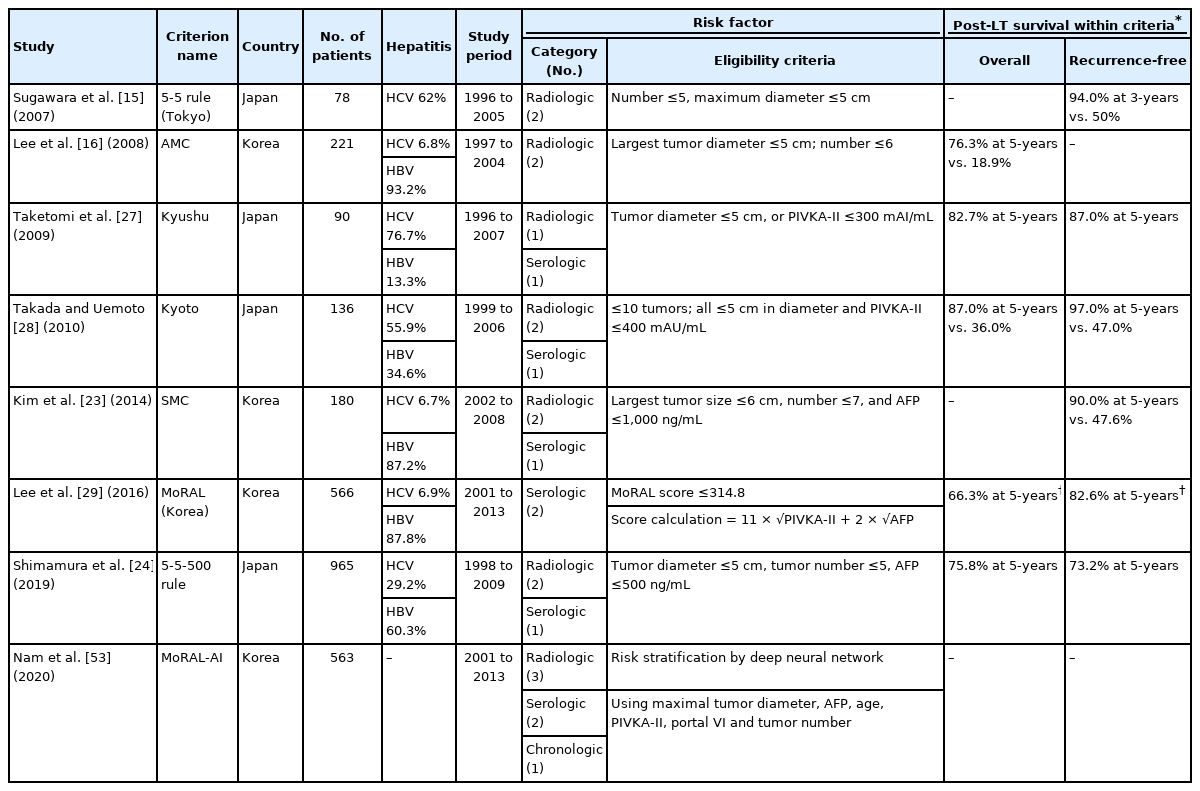
The above criteria were developed in the West and were analyzed and presented in a deceased donor liver transplantation (DDLT) setting. The need for standard criteria for living donor liver transplantation (LDLT) was raised, and the 5-5 rule and Asan Medical Center criteria were introduced in Japan and Korea in 2007 and 2008, respectively (Table 2). According to these criteria, the maximum tumor size must be less than 5 cm, and the maximum number of tumors should be 5 or 6 for each criterion. Each study reported favorable recurrence-free survival in patients who met the criteria [15,16].
The above studies were based on analyzed pathological data; however, the criteria depended on preoperative radiologic images. These early models contributed significantly to good outcomes after LT, and thereafter, efforts to include a large number of candidates using serological markers began to emerge.
PREDICTION MODELS APPLYING RADIOLOGICAL AND SEROLOGIC FACTORS
Alpha-fetoprotein (AFP) is a marker for HCC differentiation and vascular invasion [11]. In a study that developed the UCSF criteria, AFP levels >1,000 ng/mL were shown to be related to poor outcomes [8].
In 2012, the Liver Transplantation French Group proposed an AFP scores using radiologic features and AFP levels [17]. By scoring factors according to criteria such as tumor numbers greater than 4, tumor sizes 3–6 cm or >6 cm, and AFP levels of 100–1,000 ng/mL or greater, patients with AFP scores greater than two showed poorer 5-year survival and higher 5-year recurrence rates (47.5% and 50.6%, respectively) than patients with AFP scores of 0–2 (67.8% and 8.8%, respectively) in the validation cohort of 435 patients (Table 1). This model was additionally validated in several different populations, including Asian and LDLT populations, showing its favorable usefulness in predicting HCC recurrence after LT [18-21].
The transplantation center of the University of Milan, which proposed the MC, recently suggested a new criteria, the Metroticket 2.0 criteria, which include AFP levels as selection factors [22]. This study included 1,018 patients from three centers in Italy as the training cohort and 341 patients in China as the validation cohort; the demographic characteristics, including the hepatitis etiology, were relatively different between the two groups; HCV dominated in Italy and HBV dominated in China. These criteria suggested the limitation of radiological features according to the AFP level: if the AFP level is lower than 200 ng/mL, the sum of the tumor number and size should be ≤7; if the AFP level is between 200 and 400 ng/mL, the sum of the tumor number and size should be ≤5; and if the AFP level is ≥400 and <1,000, the sum of the tumor number and size should be ≤4. Patients who met these criteria showed excellent 5-year patient survival and recurrence-free survival in the training (79.7% and 89.6%, respectively) and validation cohorts (80.8% and 86.4%, respectively).
In the LDLT era, the Samsung Medical Center criteria were presented in Korea by applying both the tumor size, number, and AFP levels (largest tumor size ≤6 cm, number ≤7, and AFP level ≤1,000 ng/mL). In addition, more restrictive criteria, the 5-5-500 rule (tumor diameter ≤5 cm, tumor number ≤5, and AFP ≤500 ng/mL), were presented in Japan, and both showed comparable results [23,24].
While the role of prothrombin-induced by vitamin K absence-II (PIVKA-II) in the detection of HCC has been growing, attempts to use this marker for LT selection have also been undertaken. Some reports have shown that PIVKA-II is better than AFP for detecting advanced HCC with poor differentiation and microvascular invasion [25,26]. The Kyushu criteria and the Kyoto criteria were announced at around the same time for LDLT patients in Japan, with PIVKA-II levels within 300 mAU/mL and 400 mAU/mL, respectively, included as the criteria (Table 2). Unlike the Kyoto criteria, in which all three conditions of tumor size (≤5 cm), number (≤10 tumors), and PIVKA level (≤400 mAU/mL) must be met, the Kyushu criteria suggest that LT is feasible if only one of the two conditions (tumor diameter ≤5 cm or PIVKA-II levels ≤300 mAU/mL) is satisfied, presenting very broad criteria for LT indication [27,28].
These criteria combining radiological factors with AFP levels contribute to expanding LT candidates beyond the MC and are easily applied with simple calculations of tumor size and number and AFP levels; thus, they are currently widely used [11].
In addition, a model to predict tumor recurrence after LDLT (MoRAL, Korea) was developed in Korea, which included only two serologic factors, AFP and PIVKA-II, as LT selection criteria (MoRAL score = 11 × √PIVKA-II + 2 × √AFP) [29]. The basis for this is that while diffuse or infiltrative HCC is difficult to identify using radiologic methods, AFP, which reflects maximal tumor size, and PIVKA-II, which reflects the number and type of a tumor, are objective and reproducible values. Patients with a MoRAL score within 314.8 showed a 5-year recurrence rate of 75.9%. When stratified by the MC and a MoRAL score cut-off of 314.8, the recurrence-free survival of patients decreased in the following order: low MoRAL score within MC > low MoRAL score beyond MC > high MoRAL score within MC > high MoRAL score beyond MC. Even in patients whose radiologic findings were beyond the MC, the overall survival and recurrence-free survival rates were relatively high when the MoRAL scores were ≤314.8 (82.6% and 66.3%, respectively). The authors suggest that the use of MoRAL scores along with MC is valuable for the selection of LT candidates.
PREDICTIVE MODELS RECRUITING PATHOLOGICAL FACTORS
Along with radiological, serological factors, and pathological factors are known to be powerful predictive factors for HCC recurrence after LT as they directly show microvascular invasion and tumor differentiation. It is a standard procedure to confirm explant pathology after LT; however, pathological diagnosis before LT is not routinely performed. In any case, pre-LT biopsy is used for a more accurate prediction of HCC recurrence. One study assessed only moderate or well-differentiated HCC cases confirmed by pre-LT biopsy; these patients received LT and had favorable 5-year survival of 75% and recurrence-free survival of 92% [30]. From a similar point of view, the expanded Toronto criteria suggested that pre-LT biopsy should be performed for patients beyond the MC to exclude poorly differentiated tumor types. They also suggested that the center should not limit patients within MC who show no vascular invasion or systemic symptoms by size and number of tumors [31]. These criteria may be a good way to expand the pool for LT candidates; however, preoperative liver biopsy may be difficult to perform in some patients, and there is also the risk of cancer seeding [32]. In addition, sonography-guided needle biopsy of the liver has a relatively lower accuracy of tumor differentiation than explant pathology [33].
Explant pathology may aid in predicting HCC recurrence after LT effectively, aiding in the determination of the HCC evaluation interval, thus reducing unnecessary radiation exposure and costs [34]. The Risk Estimation of Tumor Recurrence After Transplant (RETREAT) scoring system, which uses microvascular invasion, the sum of tumor sizes, and the number of explant pathologies for its score calculation, was published in 2017 based on a training cohort of 721 patients and a validation cohort of 341 patients in the USA [35]. With scores ranging from 0 to 8, the study showed a very high 5-year recurrence rate of 75.2% in patients with scores of 5 or greater, while only 2.9% of the patients scored 0. The authors suggested applying different HCC surveillance intervals and adjusting the levels of immunosuppressants and mammalian target of rapamycin (mTOR) inhibitors, which have antineoplastic effects, based on patients’ RETREAT scores.
Halazun et al. [36] in the USA presented a HCC prediction score system using both pre- and post-operative factors such as AFP, neutrophil-to-lymphocyte ratio (NLR), radiological tumor size, and explant tumor specificity in both the pre-LT and post-LT periods. This new Model Of Recurrence After Liver transplantation (MORAL, USA) score uses a similar abbreviation to the HCC prediction model (MoRAL, Korea), which is based on LDLT patients and uses only AFP and PIVKA as predictive factors. In this score, preoperative AFP levels >200 ng/mL, NLRs ≥5, and largest tumor size >3 cm (pre-MORAL score) were significant risk factors in the pre-LT period, while tumor grade 4, vascular invasion, size >3 cm, and number >3 on explant pathology (post-MORAL score) may aid prediction during the post-LT period. The low-risk group (score 0–2) had a 5-years recurrence-free survival rate of 98.5%, while the very-high-risk group (score >10) had a 1-year recurrence-free survival rate of only 17.9%. They also suggested the combo-MORAL score, a combined version of both pre- and post-MORAL scores with high prediction power and a c-statistic of 0.91. Notably, in this scoring system, an NLR ≥5 was regarded as one of the most important factors affecting HCC recurrence, along with a tumor grade of 4 (adding 6+ score).
A recent study published a method for predicting patient survival using the Liver Transplant Expected Survival-HCC (LiTES-HCC) score based on 6,502 DDLT patients registered in the USA’s Organ Procurement and Transplantation Network/UNOS [37]. In this study, 11 HCC-related and non-related factors were combined into the score calculation: age, bilirubin, chronic kidney disease, INR, diabetes, etiology of liver disease, change in tumor diameter, AFP change from waiting to transplant time, pre-transplant location, ventilation, interaction between diabetes mellitus and age, and interaction between chronic kidney disease and NASH. The 5-year survival rates in the highest and lowest score groups were 86.3% and 67.0%, respectively, and the 10-year survival rates were 72.7% and 47.7%, respectively. HCC recurrence was not confirmed in the UNOS data; therefore, this study has limitations in that it did not show recurrence-free survival data, and it only included patients with DDLT who had been waiting for ≥6 months. However, an attempt to predict survival by considering numerous HCC-related and non-related factors would be highly significant. In addition, the survival curve could be obtained by inputting patient data into the website (https://amantero.shinyapps.io/LiTES/), which may be helpful in determining LT.
DOWNSTAGING PROCEDURE AND TUMOR RESPONSE OF HCC BEFORE LT
The technique of locoregional therapy (LRT), which is traditionally performed for inoperable HCC patients with advanced liver cirrhosis, has recently been advancing. This includes traditional trans-arterial chemoembolization, radiofrequency ablation, the newest 3D-conformal radiation therapy, trans-arterial radioembolization, and stereotactic body radiotherapy, and helps reduce tumor activity [38,39]. Recently, the LT paradigm has been changed to make it suitable for LT by downstaging HCC beyond the MC or for more advanced HCC.
The goal of downstaging is different in each study; however, the first goal is often to meet the MC (MC-IN) [40,41]. The European Association for the Study of the Liver recommends LT only for patient with MC-IN after downstaging. The UCSF group presents slightly different criteria that can benefit from LRT before LT through the UCSF downstaging protocol and criteria for defining the ‘success of downstaging’ (Table 3) [42]. According to these criteria, the UNOS T2 criteria are recommended as a goal for downstaging before DDLT, and it is recommended that LDLT be performed only when the already known UCSF criteria have been met. A study that included three centers in California and used the same UCSF downstaging protocol showed comparable outcomes of downstaging followed by LT. Both studies showed similar 5-year survival rates (77.8–80.0%) and 5-year recurrence-free survival rates (87.0–90.8%). However, the success rate of downstaging was 58.0–65.3%, and the risk of downstaging failure was particularly high in those with AFP ≥1,000 ng/mL or Child B or C [41].
In addition to absolute criteria such as MC-IN, tumor responses, such as complete response (CR) or partial response (PR) according to the modified Response Evaluation Criteria in Solid Tumors (mRECIST), are good indicators for HCC prediction, while progressive disease (PD) is associated with poor outcomes (Supplementary Table 1) [43]. The study by the European Hepatocellular Cancer Liver Transplant Study group considered two factors as high-risk factors for HCC recurrence after LT: 1) AFP slope >15 ng/mL/month and 2) tumor progression even after LRT. Regardless of whether the MC criteria were met, patients’ survival rates were very poor if there was even one risk factor present. In addition, MC-IN patients had slightly better survival if there were no risk factors present [44]. This study also showed that tumor recurrence after LT is higher when the AFP change over time is >15 ng/mL/month compared with AFP value at a specific time point. Since it is difficult for individuals waiting for DDLT to have their AFP levels confirmed at the time of their operation, this AFP trend can be greatly significant for transplantation decisions.
Both the quality of the response and duration of surveillance are important considerations. One study that included 6,160 patients from the UNOS database showed that the survival rate after LT was better in a cohort with a long waiting time (WT), which is thought to be because it is difficult to confirm early recurrence of HCC when the interval to LT is short [45]. Some downstaging studies mainly maintained 3- or 6-month intervals and showed reasonable results [42]. Therefore, the guidelines of the European Association for the Study of the Liver (2018) and American Association for the Study of Liver Disease (2018) recommend a minimum of 3 to 6 months of surveillance [46,47].
Taking all the above into consideration, Lai et al. [48] presented the time-radiological-response-alpha-fetoprotein-inflammation (TRAIN) score in 2016 by combining various factors, including tumor response, AFP change, NLR, and WT after LRT. The score equation is as follows: TRAIN score = 0.988 (if mRECIST-PD) + 0.838 (if AFP slope ≥15.0 ng/mL/month) + 0.452 (if NLR ≥5.0) – 0.03 × WT (month). With the greatest effects observed from the mRECIST-PD or high AFP slopes, even after LRT, this study revealed the poorest patient survival and recurrence-free survival when the score was ≥1.0. This score is significant in that the criteria are applied by actively using time-dependent variables in situations in which many patients wait without receiving LT immediately. In addition, the score calculation is quite simple.
Mazzaferro et al. [49] recently performed a randomized controlled trial (XXL criteria) of a downstaging procedure followed by LT in patients with HCC who were beyond the MC at nine Italian transplantation centers. The inclusion criteria for the downstaging procedure were: age between 18 and 65 years, expected 5-year survival after LT of at least 50%, and liver function of Child-Pugh A-B7. Patients with mRECIST-PR or CR after a 3-month observation period were randomly assigned to either the LT therapy group or the non-LT group with only LRT (among 74 initially enrolled patients, 29 dropped out, 23 were assigned to the LT group, and 22 were assigned to the control group). The 5-year recurrence-free survival and overall survival rates were much higher in the LT group than in the control group (76.8% vs. 18.3% and 77.5% vs. 31.2%, respectively; P=0.035). This result showed that patients with PR or CR within the 3-month interval after LRT may receive a huge survival benefit from transplantation
Macro-vascular invasion was not included in the aforementioned criteria for LT. Traditionally, cases with macro-vascular invasion are not suitable for LT, and the European Association for the Study of the Liver guidelines (2018) specified it as an absolute contraindication. However, recent cases of HCC with vascular invasion, including portal vein tumor thrombosis, showed favorable outcomes with a downstaging procedure followed by LT [50]. However, there are few studies on this topic, and more trials are required for LT in HCC with vascular invasion.
OTHER APPROACHES
Some approaches that use artificial intelligence (AI), including deep learning models, exist in the field of HCC. One study including 48,151 electronic healthcare records of HCV patients, developed recurrent neural network models for predicting HCC occurrence at 3 years of follow-up [51]. Saillard et al. [52] used the pathological slides of 194 patients with resected HCC to develop a deep learning model for predicting HCC recurrence and validated the model with 328 patients. Recently, in the transplantation era, three large LT centers in Korea have developed an HCC recurrence prediction model using a deep neural network, denoted as MoRAL-AI. This model was developed using radiologic, serologic, and biologic factors (maximum tumor diameter, AFP, age, PIVKA-II, portal vein invasion, and tumor number) in a derivation cohort of 349 LDLT patients and was validated with 214 LDLT patients, which showed a better discrimination function (c-index, 0.75) than previously developed models [53]. Notably, the relative significance of portal vein tumor thrombosis for HCC recurrence was not higher than that of tumor diameter, AFP, age, and PIVKA-II. The rate of preoperative portal vein invasion was 14.7%, and the center used the peeling-off technique in these cases during transplantation [54].
DISCUSSION
Since the introduction of MC, various other models have been proposed to guide LT in patients with HCC. The models that mainly assessed only radiologic factors in the beginning, gradually added serological factors and considered pathologic factors before and after surgery; they tend to consider the tumor response to LRT and wait for downstaging [11]. The limits of tumor number and size vary in range, sometimes according to serological factors. AFP levels with cut-off values of 200–500 ng/mL are widely used, and values >1,000 ng/mL are regarded as extremely critical. PIVKA-II levels are used less frequently, with a cut-off value of 300 or 400 mAU/mL, and this factor was used in combination with AFP levels in the MoRAL (Korea) model. A preoperative NLR ≥5 was regarded as a very high-risk factor for HCC recurrence in the MORAL (USA) model. The pathological factors considered mainly focused on microvascular invasion and tumor differentiation, with precise calculation of the size and number of explant livers. Validation studies were attempted for each of the models both internally and externally, and one systematic review article mentioned that the AFP model was well validated without considerable differences in five studies across the West and East [34].
HCC models should be considered depending on whether LDLT or DDLT is used. Donor recruitment is mainly influenced by sociocultural or administrative backgrounds, rather than by differences in tumor biology itself. LDLT is practiced at a high rate in Asia, particularly Korea, Japan, and India [55]. The choice of DDLT or LDLT affects the interval between the first HCC detection and the operation. As mentioned by the author who presented the TRAIN score, the longer the WT, the greater the tendency of increase in dropouts due to HCC metastasis. Conversely, the UNOS survey reported that the shorter the WT, the lower the number of dropouts, but with the possibility of HCC recurrence bound to increase [49,56]. In Asian countries with a high LDLT rate, WT may be shorter than that in Western countries, and the recurrence rate may instead increase.
Hepatitis, either by HCV or HBV, is known to contribute to HCC recurrence after LT [57,58]. However, several of the models in the above studies were developed in the West, and most of them had higher rates of HCV infection than those in the East. An important point to consider for HCV is that with the development of direct-acting antiviral agents (DAAs), HCV treatment has become more effective. Approved as a treatment for HCV in 2013, DAA may improve the cure rates for HCV and affect HCC prevention. However, the effect of DAAs on the reduction of HCC recurrence after LT remains controversial. One multicenter study of 875 HCV-HCC recipients showed no association between DAA and HCC recurrence, while another study with 171 HCV-HCC recipients showed a trend of decreased HCC recurrence in patients receiving DAAs (hazard ratio, 0.38; P=0.07) [59,60]. These studies had the limitation of a relatively short follow-up period; therefore, more studies are needed to confirm the relationship between DAAs and HCC recurrence. Conversely, as HBV is predominant in the East, the potential differences in the effects of HBV and HCV on recurrence after LT should be considered. Considering the differences between these two etiologies, a study on the LiTES-HCC score was conducted in 6,502 patients (HCV rate: 43.0%) after excluding patients with HCV who had received LT before DAA administration. In addition, by conducting training and validation sets in the West (Italy) and East (China), which have different ratios of HBV and HCV infections, Metroticket 2.0 showed usefulness in both regions.
However, the basis for applying these criteria depending on the region, is unclear. Rather, the criteria should be applied while considering the situation of deceased donor poor, the preference and rate of LDLT, the WT of HCC patients, and the number of downstaging procedures in each center and country. In the case of DDLT, the Model for End-stage Liver Disease (MELD) score of patients should also be considered for the possibility of receiving a graft. The MELD score was a topic that came out while studying the 90-day survival rate of cirrhosis patients who initially received a transjugular intrahepatic portosystemic shunt [61]. This has been used as a criterion for DDLT selection by UNOS since 2002, reducing WT and death rate, and has been applied not only in the USA but also in many countries around the world [62]. The original MELD score was determined based on bilirubin, INR, creatinine, and whether dialysis was performed. Since 2016, the MELD-Na+ score, including the sodium level, has been used in the USA, and research has shown that the prognostic ability is better for HCC patients than the original MELD [63].
However, the sodium level has a severe limitation in that it fluctuates greatly depending on the patient’s volume state and diuretics use. In addition, there are some opinions that the HCC factors should be incorporated into the MELD score. Guerrini et al. [64] suggested the HCC-MELD score which consists of AFP level, tumor size, and MELD score. Abdel-Wahab et al. [65] proposed the MELD-insulin-like growth factor-1 (IGF-1) score, which is based on studies showing that lower baseline IGF-1 is related to worse survival outcomes in HCC patients. Although these new-MELD scores showed better predictive power and may be useful for priority assignment, more studies are needed to validate their reproducibility. There are also administrative and ethical issues to consider in the application of MELD and new HCC-associated MELD scores separately for non-HCC and HCC patients.
While radiologic examination methods for diagnosing HCC mainly use CT or MRI, some studies have shown the usefulness of positron emission tomography (PET)/CT in deciding the treatment plan for HCC [66,67]. This depends on the phenomenon that well-differentiated HCC shows PET-negativity due to similar fluoro-deoxyglucose metabolism as a normal liver, whereas poorly differentiated HCC has a further decrease in glucose-6-phosphatase activity, leading to a high PET-positivity similar to metastasis. One study showed that the 5-year recurrence rate was 19% in the subgroup of patients with AFP <115 ng/mL and PET-negativity even though they were beyond the MC which was very low compared to the observed 53% in the group with high AFP levels or PET-positivity [68]. Additionally, molecular or genetic analyses may be helpful in predicting HCC recurrence after LT. Several studies have shown that the results of miRNA analysis and post-LT outcomes are related; however, these techniques are still too technically difficult to perform before LT [69-71]. The number of patients included in these studies is not sufficient, and more studies are needed to confirm the use of PET or molecular analysis for HCC prediction after LT.
Additionally, technical advances, such as AI, which lead to higher sensitivity and specificity in detecting HCC via radiological imaging, should be considered. Recent studies have shown that deep learning methods that analyze CT or MRI may help detect HCC more accurately [72]. In these methods, the criteria used for LT selection based on existing radiologic factors will have a greater impact, or new radiologic factors that predict poor prognosis beyond tumor size or number may be applied to the criteria. For example, a recent study analyzing CT and MRI as deep learning methods presented a model that can detect a relatively high prediction rate (area under curve, 0.78–0.85) of microvascular invasion [73].
Independent of the criteria selection, a combination of immunosuppressants and mTOR inhibitors may be considered to reduce HCC recurrence after LT [74]. mTOR inhibitors such as sirolimus are known to have anticancer effects, whereas calcineurin inhibitors can promote cancer growth [75,76]. The recently published SiLVER (Sirolimus in Liver Transplant Recipients with HCC study) trial was a randomized control trial involving 508 patients, which aimed to analyze the effect of sirolimus on HCC recurrence. Overall survival and disease-free survival were significantly better when sirolimus was used for ≥3 months after LT, and these results were even more pronounced in patients with AFP levels <10 ng/mL [77].
CONCLUSION
The application of the MC has led to good LT rates, and several expanded criteria for selecting and treating people who do not meet MC have been published. Serological factors such as AFP and PIVKA-II levels and their change patterns can also be considered, and tumor differentiation in pathologic reports can also be considered if possible. It would be desirable to consider both downstaging and tumor response after LRT, especially in regions where WTs are long after registering for DDLT. Even after transplantation, there are models that can help determine the use of immunosuppressants and surveillance interval according to the tumor characteristics of the explant pathology. In addition, as LRT develops, LT is likely to be increasingly attempted after downstaging in far more advanced HCC patients, including patients with portal vein tumor thrombosis, in the future. It is expected that more sophisticated models that can save many patients will be developed based on diagnostic methods and AI technology.
Notes
Authors’ contributions
Sang Jin Kim: Design of the work, Collect the articles, Write the manuscript; Jong Man Kim: Supervision of design, Critical revision of the article
Conflicts of Interest
The authors have no conflicts to disclose.
Abbreviations
AFP
alpha-fetoprotein
AI
artificial intelligence
CR
complete response
CT
computed tomography
DAA
direct-acting antiviral agent
DDLT
deceased donor liver transplantation
HBV
hepatitis B virus
HCC
hepatocellular carcinoma
HCV
hepatitis C virus
IGF-1
insulin-like growth factor-1
LDLT
living donor liver transplantation
LiTES-HCC
Liver Transplant Expected Survival-HCC
LRT
locoregional therapy
LT
liver transplantation
MC
Milan criteria
MELD
Model for End-stage Liver Disease
mRECIST
modified Response Evaluation Criteria in Solid Tumors
MRI
magnetic resonance imaging
mTOR
mammalian target of rapamycin
NASH
nonalcoholic steatohepatitis
NLR
neutrophil-to-lymphocyte ratio
PD
progressive disease
PET
positron emission tomography
PIVKA-II
prothrombin-induced by vitamin K absence-II
PR
partial response
RETREAT
Risk Estimation of Tumor Recurrence After Transplant
SiLVER
Sirolimus in Liver Transplant Recipients with HCC
TIPS
transjugular intrahepatic portosystemic shunt
TRAIN
time-radiological-response-alpha-fetoprotein-inflammation
UCSF
University of California
UNOS
United Network for Organ Sharing
WT
waiting time
SUPPLEMENTAL MATERIAL
Supplementary material is available at Clinical and Molecular Hepatology website (http://www.e-cmh.org).
mRECIST criteria