| Clin Mol Hepatol > Volume 28(4); 2022 > Article |
|
ABSTRACT
Background/Aims
Methods
Results
FOOTNOTES
SUPPLEMENTAL MATERIAL
Supplementary┬ĀFigure┬Ā1.
Supplementary┬ĀFigure┬Ā2.
Supplementary┬ĀFigure┬Ā3.
Supplementary┬ĀFigure┬Ā4.
Supplementary┬ĀFigure┬Ā5.
Supplementary┬ĀFigure┬Ā6.
Supplementary┬ĀFigure┬Ā7.
Supplementary┬ĀFigure┬Ā8.
Supplementary┬ĀFigure┬Ā9.
Supplementary┬ĀFigure┬Ā10.
Supplementary┬ĀFigure┬Ā11.
Supplementary┬ĀFigure┬Ā12.
Supplementary┬ĀFigure┬Ā13.
Supplementary┬ĀFigure┬Ā14.
Supplementary┬ĀFigure┬Ā15.
Supplementary┬ĀFigure┬Ā16.
Supplementary┬ĀFigure┬Ā17.
Supplementary┬ĀTable┬Ā1.
Supplementary┬ĀTable┬Ā2.
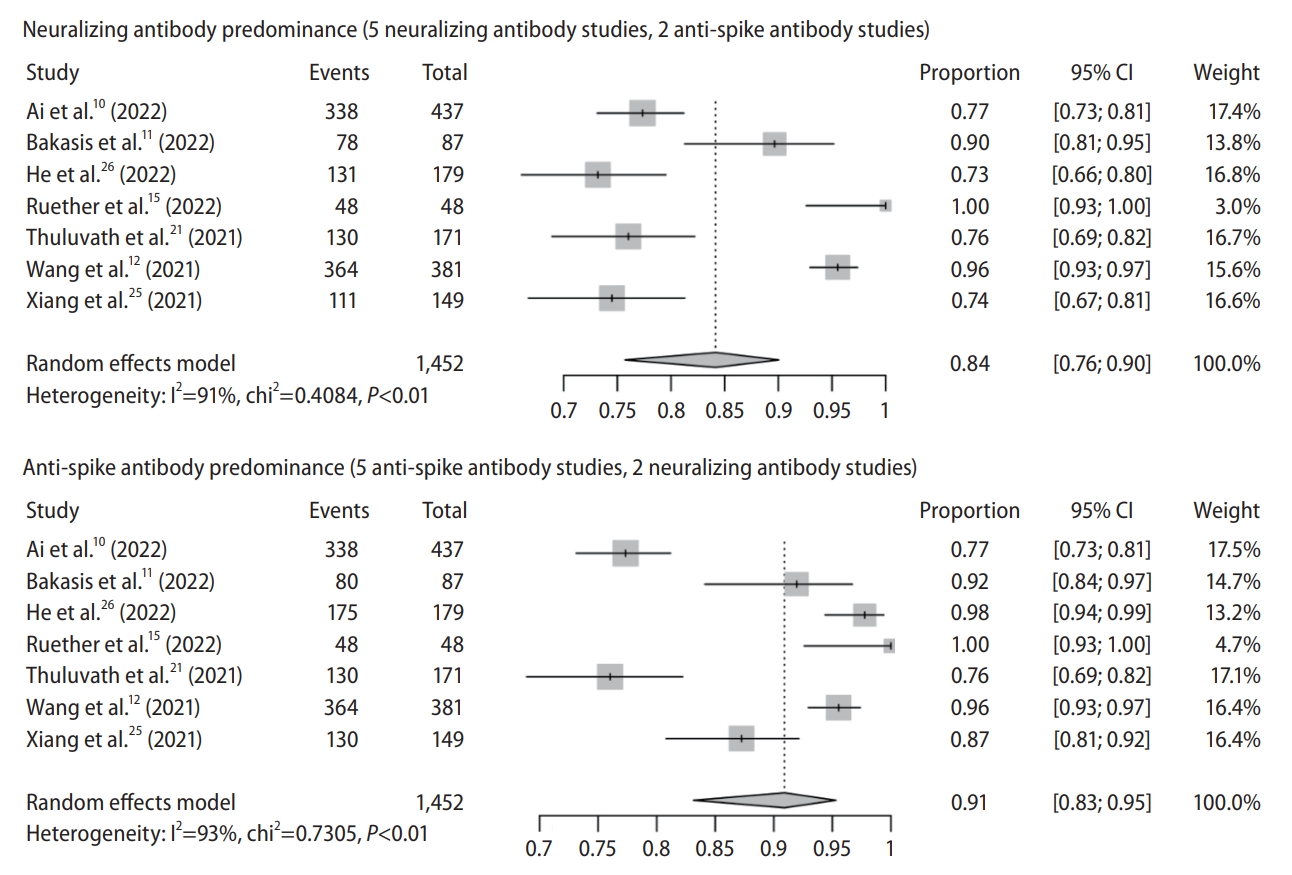
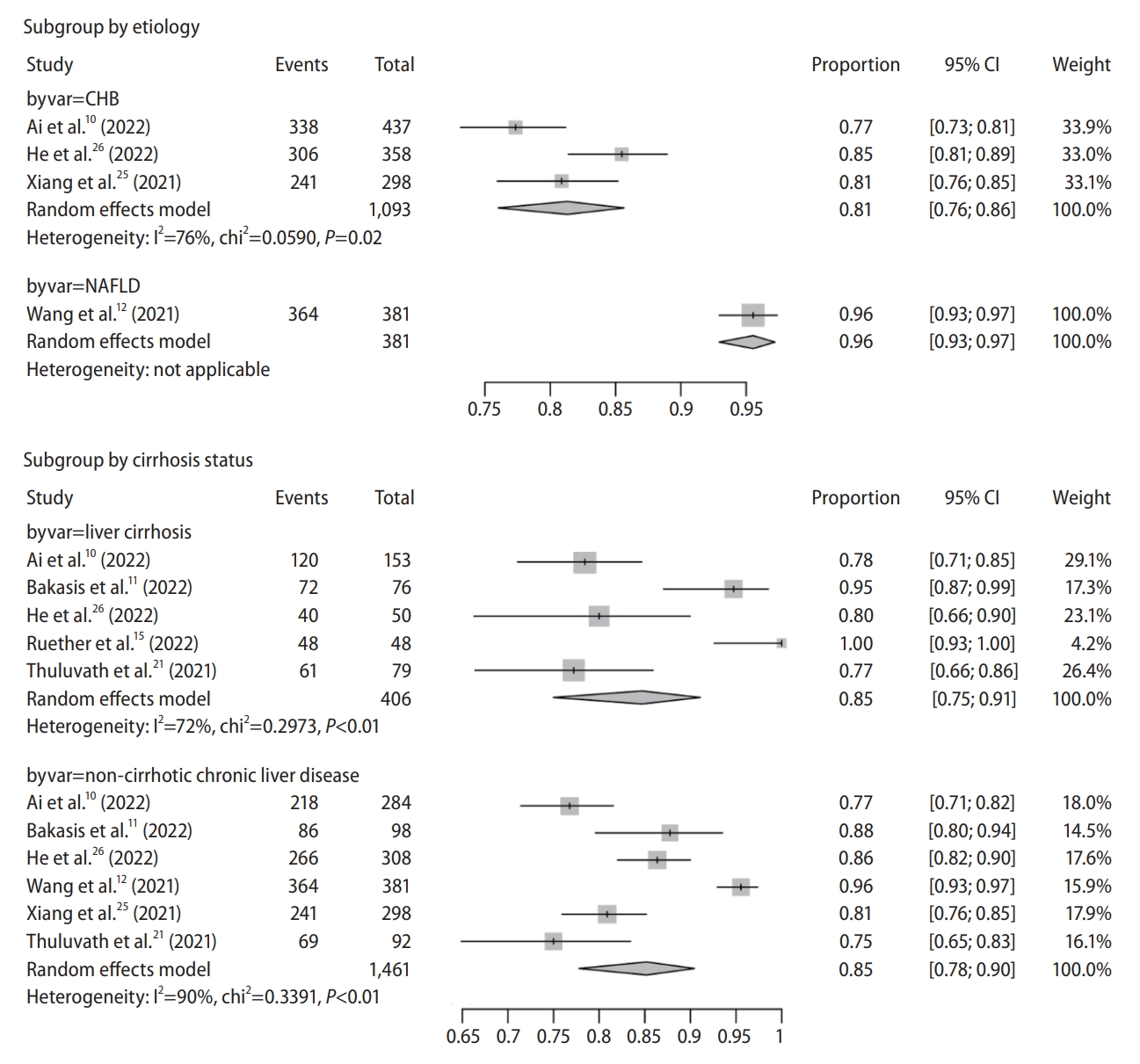
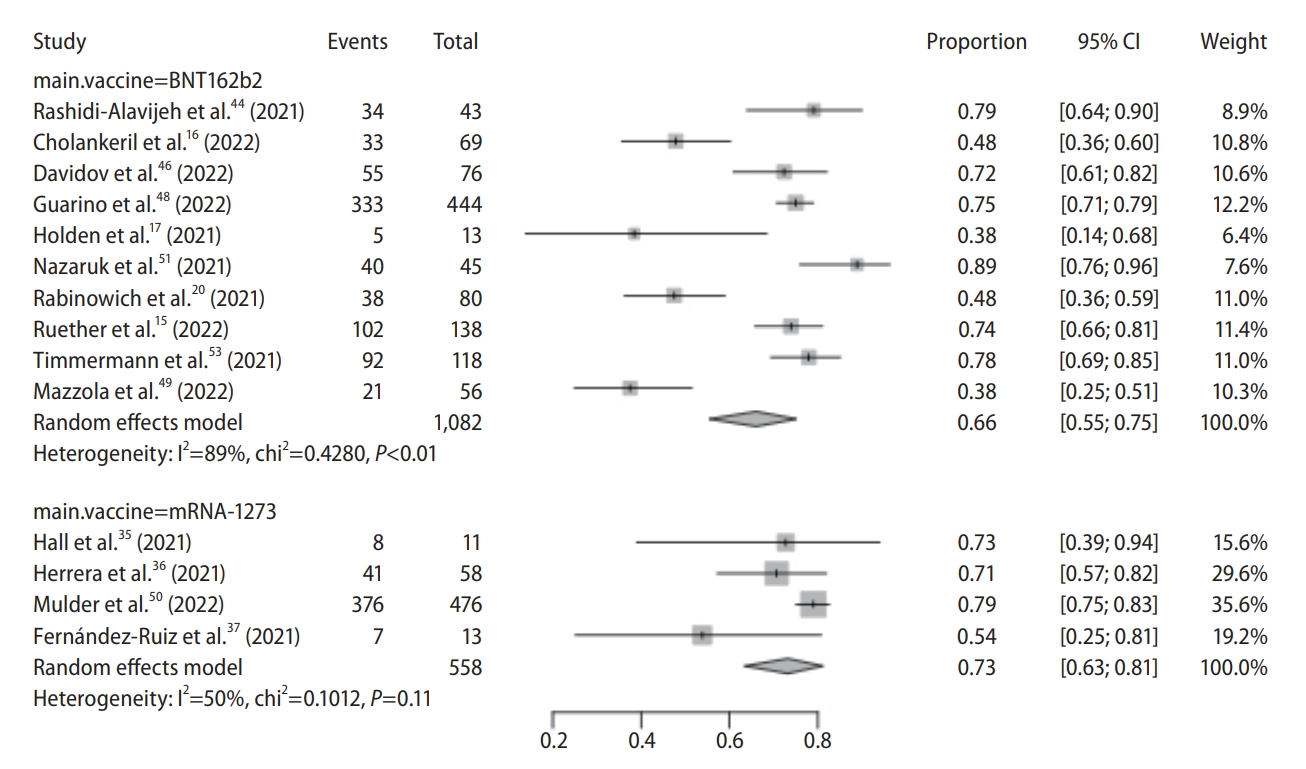
Figure┬Ā2.

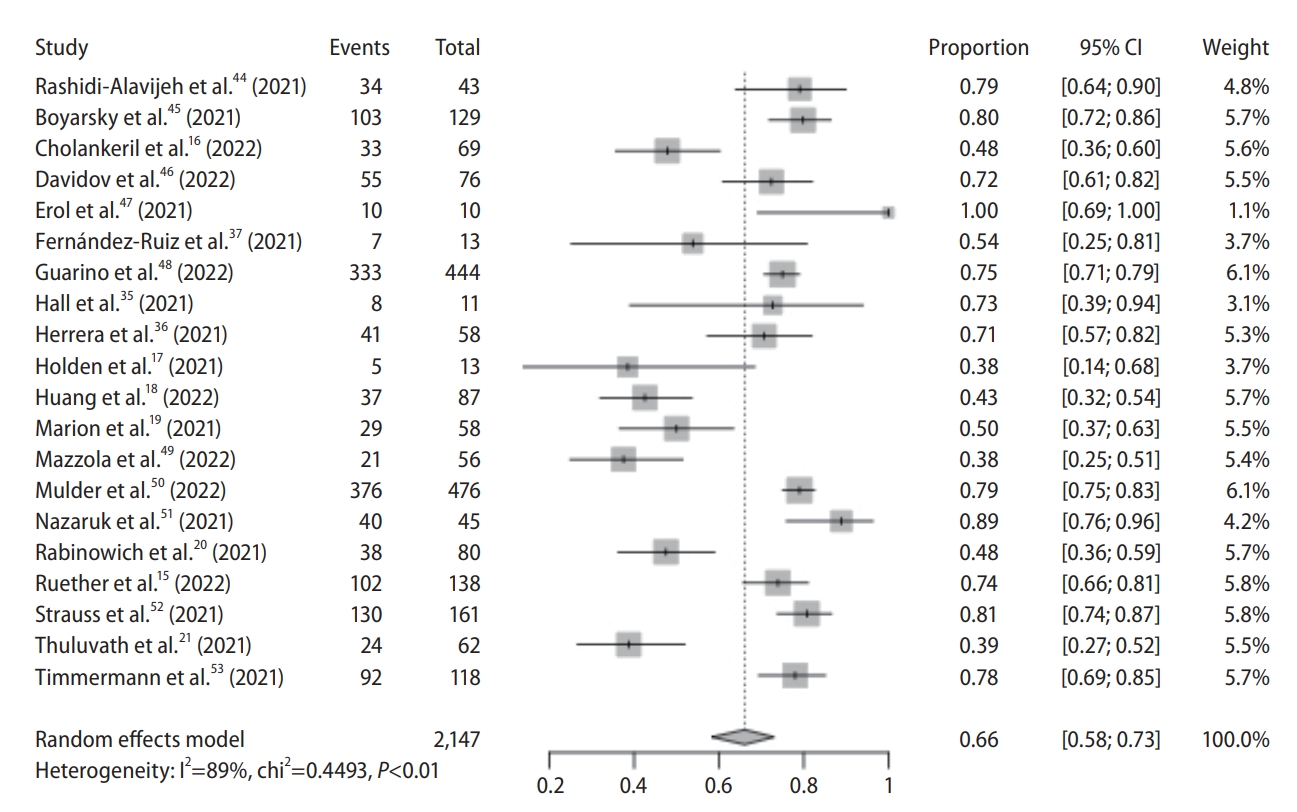
Figure┬Ā3.
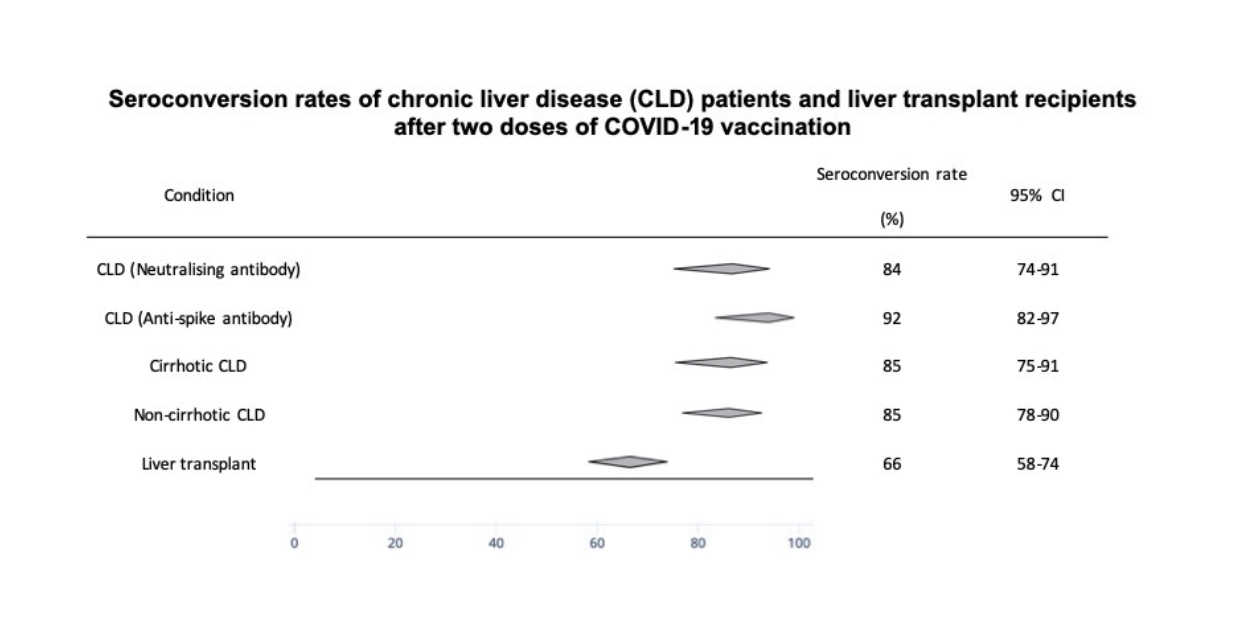
Figure┬Ā5.
Table┬Ā1.
| Study | Country | Study duration | Participants | Age (years) | Male | Liver disease | Immunosup-pressants used | Vaccine type | Antibody type | Time interval of antibody measurement from second dose of vaccination | Method of antibody test | Cut-off of antibody level regarded as seropositive | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chronic liver disease | |||||||||||||
| ŌĆā | Ai et al. [10] | China | January to August 2021 | 437 | Median (IQR): 47.0 (38.0ŌĆō56.0) | 278 (63.6%) | Non-cirrhotic chronic liver disease: HBV, 260 (91.5%); HCV, 8 (2.82%); NAFLD, 9 (3.17%); ALD, 1 (0.35%); AIH/PBC/PSC, 1 (0.35%); others, 5 (1.76%) | NA | CoronaVac: NA | Neutralising Ab | Ōēź14 days | CLIA | >10.0 AU/mL |
| BBIBP-CorV: NA | |||||||||||||
| Cirrhosis: HBV, 124 (81.0%); HCV, 12 (7.84%); NAFLD. 3 (1.96%); AIH/PBC/PSC, 7 (4.58%); others, 7 (4.58%) | WIBP-CorV: NA | ||||||||||||
| Bakasis et al. [11] | Greece | March to May 2021 | 87 | Median (range): cirrhosis, 67.0 (27ŌĆō86); noncirrhotic liver disease, 65.0 (35ŌĆō81) | 43 (49.4%) | Non-cirrhotic chronic liver disease: HBV, 23 (46.9%); HCV, 1 (2.04%); NAFLD, 7 (14.3%); AIH, 6 (12.2%); PBC, 11 (22.4%); PSC, 1 (2.04%) | MTX: 5 (5.75%) | BNT162b2: 81 (93.1%) | Antispike Ab, neutralising Ab | Cirrhosis: 24 days (IQR, 14ŌĆō57) | ELISA | Anti-spike Ab: >1.1 OD ratio | |
| AZA: 7 (8.05%) | |||||||||||||
| RTX: 4 (4.60%) | |||||||||||||
| Cirrhosis: HBV, 7 (18.4%); HCV, 1 (2.63%); NAFLD, 9 (23.7%); ALD, 6 (15.8%); AIH, 8 (21.1%); PBC, 1 (2.63%); PSC, 3 (7.89%); HSC, 1 (2.63%); BCS, 1 (2.63%); DILI, 1 (2.63%) | MMF: 6 (6.90%) | mRNA-1273: 6 (6.90%) | Non-cirrhotic liver disease: 25 days (IQR, 15ŌĆō45) | Neutralising Ab: >30% inhibitory concentration | |||||||||
| TNFI: 4 (4.60%) | |||||||||||||
| S: 14 (16.1%) | |||||||||||||
| He et al. [26] | China | July to August 2021 | 362 | Median (range): 45.0 (19.0ŌĆō78.0) | 223 (61.6%) | HBV, 362 (100%); cirrhosis, 48 (13.3%) | NA | CoronaVac: NA | Antispike Ab, antispike RBD Ab, neutralising Ab | Ōēź21 days (range, 21ŌĆō105) | ELISA | Anti-spike Ab, antispike RBD Ab: Ōēź2.1 OD ratio | |
| BBIBP-CorV: NA | Neutralising Ab: Ōēź20% inhibitory concentration | ||||||||||||
| Ruether et al. [15] | Germany | NA | 48 | Mean┬▒SD: 53.8┬▒9.5 | 108 (58.1%) | Cirrhosis: ALD, 23 (47.9%); VH, 3 (6.30%); AIH, 11 (22.9%); NAFLD, 4 (8.30%); CC, 5 (10.4%); ALF, 1 (2.10%); others, 1 (2.10%); HCC, 5 (10.4%)* | NA | mRNA/mRNA: 44 (91.6%) | Antispike RBD Ab, antispike trimer Ab | Median (IQR): 28 days (21ŌĆō41) | CLIA | Anti-spike RBD Ab: >100 U/mL | |
| BNT162b2: 38 (79.2%) | ECLIA | ||||||||||||
| mRNA-1273: 6 (12.4%) | Anti-spike trimer Ab: >100 BAU/mL | ||||||||||||
| AZD1222/AZD1222: 1 (2.08%) | |||||||||||||
| AZD1222/mRNA: 3 (6.25%) | |||||||||||||
| Thuluvath et al. [21] | USA | NA | 171 | Mean┬▒SD: noncirrhotic chronic liver disease, 60.4┬▒13.9; liver cirrhosis, 63.8┬▒11.1 | Noncirrhotic chronic liver disease: 37 (40.2%) | Non-cirrhotic chronic liver disease: AIH/PBC/PSC, 36 (39.1%); ALD, 2 (2.17%); HBV/HCV, 20 (21.7%); NAFLD, 36 (39.1%); others, 8 (8.70%) | AZA: 25 (14.6%) | BNT162b2: 80 (46.8%) | Antispike Ab | Mean┬▒SD: non-cirrhotic chronic liver disease, 40.8┬▒19.6; liver cirrhosis, 40.9┬▒23.9 | ECLIA | >250 U/mL | |
| S: 19 (11.1%) | mRNA-1273: 77 (45.0%) | ||||||||||||
| Liver cirrhosis: 40 (50.6%) | Cirrhosis: AIH/PBC/PSC, 17 (21.5%); ALD, 17 (21.5%); HBV/HCV, 17 (21.5%); NAFLD, 33 (41.8%); others, 8 (10.1%) | TAC: 0 (0.00%) | |||||||||||
| Others: 11 (6.43%) | JNJ-78436735: 14 (8.19%) | ||||||||||||
| Wang et al. [12] | China | October 2020 to March 2021 | 381 | Median (IQR): 39.0 (33.0ŌĆō48.0) | 179 (47.0%) | NAFLD: 381 (100%) | NA | BBIBPCorV: 381 (100%) | Neutralising Ab | 14 days | CLIA | NA | |
| Xiang et al. [25] | China | March to September 2021 | 149 | Median (IQR): 41.0 (33.0ŌĆō49.0) | 108 (72.5%) | HBV: 284 (100%) | NA | CoronaVac: NA | Antispike RBD Ab, neutralising Ab | Median (IQR): 33 days (24ŌĆō48) | CLIA | Anti-spike RBD Ab: 1 AU/mL | |
| BBIBP-CorV: NA | |||||||||||||
| WIBP-CorV: NA | Neutralising Ab: 0.05 AU/mL | ||||||||||||
| Liver transplant | |||||||||||||
| Rashidi-Alavijeh et al. [44] | Germany | February to March 2021 | 43 | Median (IQR): 47.0 (36.0ŌĆō54.0) | 26 (60.5%) | HCC: 10 (23.3%); PSC: 7 (16.3%); AC: 6 (14.0%); HCV: 3 (6.98%); ALF: 3 (6.98%); WD: 3 (6.98%); CC: 2 (4.65%); AAD: 2 (4.65%); others: 7 (16.3%) | TAC+EVE: 22 (51.2%) | BNT162b2: 43 (100%) | Antispike Ab | 15 days (IQR: 12ŌĆō24) | CLIA | Ōēź13.0 AU/mL | |
| TAC+MMF: 11 (25.6%) | |||||||||||||
| TAC: 7 (16.3%) | |||||||||||||
| CSA: 2 (4.65%) | |||||||||||||
| EVE: 1 (2.33%) | |||||||||||||
| Boyarsky et al. [45] | USA | December 2020 to March 2021 | 129 | NA | NA | NA | NA | BNT162b2: NA | Antispike Ab | Median (IQR): 29 days (28ŌĆō31) | ECLIA | Ōēź0.8 U/mL | |
| mRNA-1273: NA | |||||||||||||
| Cholankeril et al. [16] | USA | January to February 2021 | 69 | Median (IQR): 63.0 (51ŌĆō68) | 48 (69.6%) | ALD: 24 (34.8%); NAFLD: 13 (18.8%); HCC: 21 (30.4%) | TAC: 64 (92.8%) | BNT162b2: 69 (100%) | Antispike Ab | 30ŌĆō75 days | ELISA | Titer Ōēź1 | |
| MMF: 23 (33.3%) | |||||||||||||
| S: 22 (31.9%) | |||||||||||||
| Davidov et al. [46] | Israel | January to May 2021 | 76 | Mean┬▒SD: 59.0┬▒15 | 43 (56.6%) | HBV: 7 (9.30%); HCV: 19 (25.3%); NAFLD: 13 (17.3%); PSC: 11 (14.7%); PBC: 3 (4.00%); others: 23 (30.3%) | CNI (TAC/CSA): 40 (52.6%) | BNT162b2: 76 (100%) | Antispike RBD Ab | Mean┬▒SD: 38┬▒24 days | ELISA | Titer Ōēź1.1 | |
| CNI+MMF: 12 (15.8%) | |||||||||||||
| CNI+EVE: 10 (13.2%) | |||||||||||||
| CNI+S: 9 (11.8%) | |||||||||||||
| CNI+MMF+S: 4 (5.26%) | |||||||||||||
| SRL: 1 (1.32%) | |||||||||||||
| Erol et al. [47] | Turkey | April to June 2021 | 10 | NA | NA | NA | NA | BNT162b2: 4 (40.0%) | Antispike Ab | 28ŌĆō42 days | CLIA | Ōēź50 AU/mL | |
| CoronaVac: 6 (60.0%) | |||||||||||||
| Fern├Īndez-Ruiz et al. [37] | Spain | April to June 2021 | 13 | NA | NA | NA | NA | mRNA-1273: 13 (100%) | Antispike Ab | 14 days | ELISA | OD Ōēź1.1 | |
| Guarino et al. [48] | Italy | May to August 2021 | 444 | Median (IQR): seronegative, 65.6 (59.4-71.0); seropositive, 65.2 (56.9-70.1) | 332 (74.8%) | VH: 342 (77.0%); ALD: 34 (7.66%); NAFLD: 8 (1.80%); AIH: 18 (4.05%); others: 43 (9.68%) | TAC/CSA: 357 (80.4%) | BNT162b2: 444 (100%) | Antispike Ab | Median (IQR): 1st collection, 28 days (28ŌĆō31); 2nd collection, 88 days (86ŌĆō91) | CLIA | >25 AU/mL | |
| MMF: 151 (34.0%) | |||||||||||||
| EVE/SRL: 118 (26.6%) | |||||||||||||
| Hall et al. [35] | Canada | March to April 2021 | 11 | NA | NA | NA | NA | mRNA-1273: 11 (100%) | Antispike RBD Ab, neutralising Ab | 28ŌĆō42 days | ECLIA | Anti-spike RBD Ab: Ōēź0.8 U/mL | |
| ELISA | Neutralising Ab: >30% inhibitory concentration | ||||||||||||
| Herrera et al. [36] | Spain | NA | 58 | Median (range): 61.5 (18.0ŌĆō88.0) | 40 (69.0%) | NA | CNI: 53 (91.4%) | mRNA-1273: 58 (100%) | Antispike RBD Ab | Ōēź28 days | CLIA | NA | |
| MMF: 15 (25.9%) | |||||||||||||
| S: 13 (22.4%) | |||||||||||||
| mTORI: 13 (22.4%) | |||||||||||||
| Holden et al. [17] | Denmark | From January 2021 | 13 | NA | NA | NA | NA | BNT162b2: NA | Antispike Ab | Median (IQR): 5.6 weeks (5.1ŌĆō6.3) | ELISA | Titer Ōēź1.1 | |
| Huang et al. [18] | USA | January to April 2021 | 87 | NA | NA | NA | NA | BNT162b2: NA | Antispike Ab | Ōēź14 days | ELISA | Titer >1:50 | |
| mRNA-1273: NA | |||||||||||||
| Marion et al. [19] | France | January to April 2021 | 58 | NA | NA | NA | NA | BNT162b2: NA | Antispike Ab | 28 days | ELISA | NA | |
| mRNA-1273: NA | |||||||||||||
| Mazzola et al. [49] | France | January to April 2021 | 58 | Median (IQR): 64.0 (58.0ŌĆō68.2) | 43 (74.1%) | NA | S: 15 (25.9%) | BNT162b2: 58 (100%) | Antispike Ab | 28 days | CLIA | Ōēź50 AU/mL | |
| CNI: 45 (77.6%) | |||||||||||||
| MMF: 33 (56.9%) | |||||||||||||
| mTORI: 13 (22.4%) | |||||||||||||
| Mulder et al. [50] | Netherlands | March to July 2021 | 476 | Median (IQR): BNT162b2, 71.0 (59.0ŌĆō79.0); mRNA-1273, 59.0 (49.0ŌĆō66.0); AZD1222, 63.0 (60.0ŌĆō64.0) | 286 (60.1%) | PSC: 101 (21.2%); HCC: 103 (21.6%); ALF: 46 (9.66%); PBC/SSC/CBD: 37 (7.77%); NAFLD/ALD: 42 (8.82%); CC: 22 (4.62%); VH: 24 (5.04%); DC: 20 (4.20%); others: 50 (10.5%); retransplant: 31 (6.51%) | TAC: 243 (51.1%) | BNT162b2: 25 (5.25%) | Antispike Ab | Median (IQR): BNT162b2, 31 (29.0ŌĆō40.0); mRNA-1273, 43.0 (33.0ŌĆō56.3); AZD1222, 31.0 (26.0ŌĆō38.0) | CLIA | NA | |
| MMF: 27 (5.67%) | |||||||||||||
| CSA: 5 (1.05%) | mRNA-1273: 430 (90.3%) | ||||||||||||
| SRL: 2 (0.42%) | |||||||||||||
| EVE: 1 (0.21%) | AZD1222: 21 (4.41%) | ||||||||||||
| AZA: 1 (0.21%) | |||||||||||||
| S: 39 (8.19%) | |||||||||||||
| TAC+MMF: 113 (23.7%) | |||||||||||||
| TAC+S: 27 (5.67%) | |||||||||||||
| TAC+SRL: 16 (3.36%) | |||||||||||||
| CSA+EVE: 6 (1.26%) | |||||||||||||
| TAC+AZA: 7 (1.46%) | |||||||||||||
| TAC+EVE: 5 (1.05%) | |||||||||||||
| MMF+EVE: 3 (0.63%) | |||||||||||||
| MMF+SRL: 2 (0.42%) | |||||||||||||
| AZA+S: 1 (0.21%) | |||||||||||||
| CSA+AZA: 1 (0.21%) | |||||||||||||
| MMF+S: 1 (0.21%) | |||||||||||||
| TAC+MMF+S: 8 (1.68%) | |||||||||||||
| TAC+SRL+S: 1 (0.21%) | |||||||||||||
| Nazaruk et al. [51] | Poland | January to June 2021 | 55 | Mean┬▒SD: 58.4┬▒13.3 | 44 (80.0%) | NA | S: 20 (36.4%) | BNT162b2: 55 (100%) | Antispike Ab | 28ŌĆō56 days | CLIA | >50 AU/mL | |
| MMF: 16 (29.1%) | |||||||||||||
| AZA: 5 (9.10%) | |||||||||||||
| CSA: 11 (20.0%) | |||||||||||||
| TAC: 43 (78.2%) | |||||||||||||
| SRL: 2 (3.60%) | |||||||||||||
| EVR: 2 (3.60%) | |||||||||||||
| CNI/MMF: 24 (43.6%) | |||||||||||||
| CNI+S/MMF/AZA/mTORI: 18 (32.7%) | |||||||||||||
| CNI/mTORI+S+MMF/AZA: 13 (23.6%) | |||||||||||||
| Rabinowich et al. [20] | Israel | From December 2020 | 80 | Mean┬▒SD: 60.1┬▒13.3 | 56 (70.0%) | HBV: 13 (16.3%); HCV: 26 (32.5%); NAFLD: 16 (20.0%); ALD: 3 (3.75%); AIH: 6 (7.50%); PBC: 3 (3.75%); PSC: 7 (8.75%); ALF: 2 (2.50%); CC: 3 (3.75%); WD: 1 (1.25%); SSC: 1 (1.25%); CHF: 1 (1.25%); HCC: 26 (32.5%)* | S: 24 (30.0%) | BNT162b2: 80 (100%) | Antispike Ab | 10ŌĆō20 days | CLIA | Ōēź15 AU/mL | |
| TAC: 65 (81.3%) | |||||||||||||
| CSA: 10 (12.5%) | |||||||||||||
| EVE: 18 (22.5%) | |||||||||||||
| AZA: 4 (5.00%) | |||||||||||||
| MMF: 40 (50.0%) | |||||||||||||
| Ruether et al. [15] | Germany | NA | 138 | Mean┬▒SD: 55.0┬▒13.2 | 79 (57.2%) | ALD: 28 (20.3%); VH: 17 (12.3%); AIH: 40 (29.0%); NAFLD: 7 (5.10%); PLD: 5 (3.60%); CC: 13 (9.4%); ALF: 5 (3.60%); others: 23 (16.7%); HCC: 25 (18.1%)* | S: 43 (31.2%) | mRNA/mRNA: 121 (87.7%) | Antispike RBD Ab, antispike trimer Ab | Median (IQR): 29 days (25ŌĆō39) | CLIA | Anti-spike RBD Ab: >100 U/mL | |
| TAC: 95 (68.8%) | ECLIA | ||||||||||||
| CSA: 33 (23.9%) | BNT162b2: 110 (79.7%) | Anti-spike trimer Ab: >100 BAU/mL | |||||||||||
| CNI: 33 (23.9%) | |||||||||||||
| CNI+S: 19 (13.8%) | mRNA-1273: 11 (7.97%) | ||||||||||||
| CNI+mTORI: 17 (12.3%) | |||||||||||||
| CNI+MMF: 48 (34.8%) | AZD1222/AZD1222: 6 (4.35%) | ||||||||||||
| CNI+AZA: 9 (6.50%) | |||||||||||||
| Biologics: 8 (5.8%) | AZD1222/mRNA: 11 (7.97%) | ||||||||||||
| Strauss et al. [52] | USA | January to April 2021 | 161 | Median (IQR): 64.0 (48.0ŌĆō69.0) | 69 (42.9%) | NA | TAC: 81 (50.3%) | BNT162b2: 85 (52.8%) | Antispike Ab, antispike RBD Ab | Median (IQR): 30 days (28ŌĆō31) | ECLIA | >250 U/mL | |
| MMF: 35 (21.7%) | |||||||||||||
| S: 22 (13.7%) | mRNA-1273: 76 (47.2%) | ||||||||||||
| SRL: 11 (6.83%) | |||||||||||||
| CSA: 8 (4.97%) | |||||||||||||
| AZA: 6 (3.73%) | |||||||||||||
| ERL: 3 (1.86%) | |||||||||||||
| Thuluvath et al. [21] | USA | NA | 62 | Mean┬▒SD: 65.7┬▒8.7 | 41 (66.1%) | AIH/PBC/PSC: 8 (12.9%); ALD: 13 (21.0%); HBV/HCV: 26 (41.9%); NAFLD: 15 (24.2%); others: 16 (25.8%) | AZA: 2 (3.23%) | BNT162b2: 24 (38.7%) | Antispike Ab | Mean┬▒SD: 38.9┬▒19.6 days | ECLIA | >250 U/mL | |
| S: 8 (12.9%) | |||||||||||||
| TAC: 41 (66.1%) | mRNA-1273: 33 (53.2%) | ||||||||||||
| Others: 29 (46.8%) | JNJ-78436735: 5 (8.06%) | ||||||||||||
| Timmermann et al. [53] | Germany | May to July 2021 | 118 | Mean (range): 66.1 (28.0ŌĆō89.0) | 75 (63.6%) | ALD: 25 (21.1%); VH: 28 (23.7%); LT: 26 (22.0%); AIH: 18 (15.3%); CC: 4 (3.4%); other: 17 (14.4%) | TAC: 42 (35.6%) | BNT162b2: 114 (96.6%) | Antispike Ab | Mean (range): 44.6 days (21ŌĆō132) | ELISA | NA | |
| MMF: 16 (13.6%) | |||||||||||||
| TAC+MMF: 24 (20.3%) | mRNA-1273: 3 (2.54%) | ||||||||||||
| TAC+EVE: 15 (12.7%) | |||||||||||||
| EVR: 1 (0.85%) | JNJ-78436735: 1 (0.85%) | ||||||||||||
| CSA+MMF: 3 (2.54%) | |||||||||||||
| CSA: 2 (1.69%) | |||||||||||||
| TAC+AZA: 1 (0.85%) | |||||||||||||
IQR, interquartile range; HBV, hepatitis B virus; HCV, hepatitis C virus; NAFLD, non-alcoholic fatty liver disease; ALD, alcoholic liver disease; AIH, autoimmune hepatitis; PBC, primary biliary cholangitis; PSC, primary sclerosing cholangitis; NA, not available; Ab, anti-body; CLIA, chemiluminescence immunoassays; HSC, hepatic sarcoidosis; BCS, Budd-Chiari syndrome; DILI, drug induced liver injury; MTX, methotrexate; AZA, azathioprine; RTX, rituximab; MMF, mycophenolate mofetil; TNFI, tumour necrosis factor inhibitor; S, steroid; OD, optical density; RBD, receptor binding domain; ELISA, enzyme-linked immunosorbent assay; SD, standard deviation; VH, viral hepatitis; CC, cryptogenic cirrhosis; ALF, acute liver failure; HCC, hepatocellular carcinoma; ECLIA, electrochemiluminescence immunoassay analyzer; TAC, tacrolimus; AC, alcoholic cirrhosis; WD, WilsonŌĆÖs disease; AAD, ╬▒-1 antitrypsin deficiency; EVE, everolimus; CSA, cyclosporin A; CNI, calcineurin inhibitors; SRL, sirolimus; mTORI, mTOR inhibitors; SSC, secondary sclerosing cholangitis; CBD, congenital biliary disease; DC, dysmetabolic cirrhosis; CHF, congenital hepatic fibrosis; PLD, pediatric liver disease; LT, liver tumour.
Abbreviations
REFERENCES
- TOOLS
-
 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link XML Download
XML Download Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC-
 Download Citation
Download Citation
 Supplement1
Supplement1 Supplement2
Supplement2 Supplement3
Supplement3 Supplement4
Supplement4 Supplement5
Supplement5 Supplement6
Supplement6 Supplement7
Supplement7 Supplement8
Supplement8 Supplement9
Supplement9 Supplement10
Supplement10 Supplement11
Supplement11 Supplement12
Supplement12 Supplement13
Supplement13 Supplement14
Supplement14 Supplement15
Supplement15 Supplement16
Supplement16 Supplement17
Supplement17 Supplement18
Supplement18 Supplement19
Supplement19 Supplement20
Supplement20 Print
Print-
Share :



-
METRICS

- ORCID iDs
-
Wai Kay Seto

https://orcid.org/0000-0002-9012-313XMan Fung Yuen

https://orcid.org/0000-0001-7985-7725 - Related articles