| Clin Mol Hepatol > Volume 28(4); 2022 > Article |
|
Drug discovery in nonalcoholic steatohepatitis (NASH) is moving fast. Drugs that are currently under development aim to target two primary endpoints: NASH resolution and fibrosis reduction [1]. Disease mechanisms and pathogenic-related pathways are the main focus of novel compounds [2], which are still in early phase studies.
Drug repurposing focuses on identifying and developing new uses for existing drugs. This approach can offer an alternative for overcoming the obstacles of novel drug discovery, including time-consuming assessment of pharmacokinetics and pharmacodynamics, as well as toxicity profiling evaluation [3]. For example, repurposed drugs are already approved by major regulatory agencies, including the United States Food and Drug Administration (FDA) and or the European Medicines Agency.
In this issue, Lee et al. [4] aimed to evaluate the effects of auranofin on hepatic steatosis, inflammation, and fibrosis using in vivo and in vitro models of NASH. The authorsā experimental observations showed that auranofin reduced the expression of inflammation-related targets, such as nuclear factor kappa B (NF-ĪŗB) and NF-ĪŗB inhibitor alpha, and fibro-genesis-related pathways in LX-2 cells [4]. Likewise, auranofin reduced palmitic acid-induced inflammation and adipogenesis in HepG2 cells [4]. Moreover, using two different animal models, the authors found that auranofin attenuated liver fibrosis in the bile duct-ligation mouse model and reduced hepatic steatosis and fibrosis in the Western diet-induced NASH model of nonalcoholic fatty liver disease (NAFLD) [4].
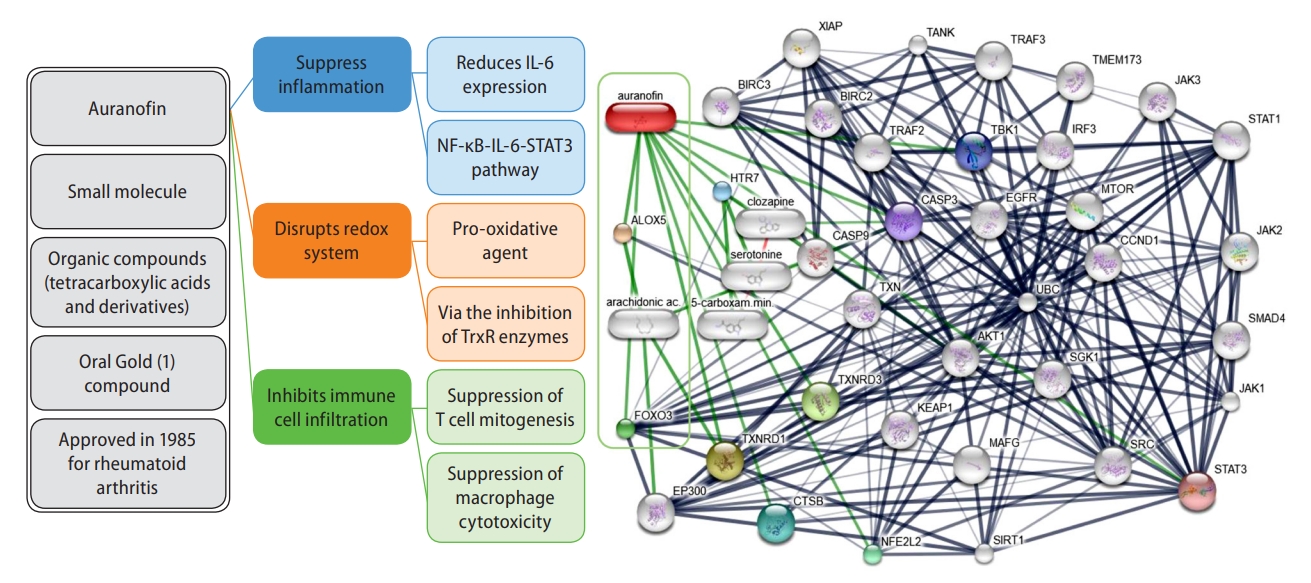
These exciting results prompt several reflections. First, auranofin, a gold-small molecule complex used as a second-line drug to treat rheumatoid arthritis (Fig. 1), targets key metabolic-related pathways. Previous experimental evidence showed that auranofin improved lipid accumulation and hepatic inflammation in in intro models by inhibiting NLRP3-related inflammasome [5]. In addition, auranofin decreased body weight, epididymal fat weight, serum aspartate aminotransferase, and several metabolic parameters, including serum glucose, triglycerides, cholesterol, and low-density lipoprotein cholesterol levels, in an animal model of NAFLD [5]. Second, auranofin has anti-inflammatory properties. The primary action mechanism of auranofin is the inhibition of reduction/oxidation enzymes, such as thioredoxin reductase (TrxR) (Fig. 1). Indeed, auranofin has helped in preventing immune cell infiltration to the site of inflammation in patients with rheumatoid arthritis, as the drug indirectly inhibits the secretion of pro-inflammatory cytokines, such as interleukin (IL)-8 and IL-6, from macrophages and monocytes, by inhibiting the NF-ĪŗB-STAT3-signaling pathway [6]. For this reason, auranofin has been tested for treating several chronic conditions, including cancer, neurodegenerative diseases such as Alzheimerās or Parkinsonās, viral infections such as acquired immune deficiency syndrome, and even parasitic and bacterial infections [7]. More recently, auranofin has been tested for the treatment of COVID-19 due to its antiviral, anti-inflammatory, and anti-reactive oxygen species properties, which might stop the cytokine storm associated with the severe form of the disease [8]. Conversely, auranofin may induce cell death by enhancing free radical production [9]. Third, the aforementioned study results showed that auranofin could reduce inflammation and fibrogenesis in human NASH by targeting key molecular disease pathways.
Figure 1 shows the interaction network of compounds, genes, and proteins associated with auranofin. The most significant chemical-protein interaction shown in green lines denotes an attractive connection with arachidonate 5-lipoxygenase (ALOX5) and arachidonic acid, two relevant actors in NAFLD biology [10,11]. Another meaningful chemical-protein interaction is with E1A binding protein p300, which functions as histone acetyltransferase-regulating gene transcription via chromatin remodeling. This pathway might partly explain the epigenetic mechanisms associated with the pathogenesis of NAFLD [12]. Some other know transcription factors and proteins, including STAT1 and STAT3, BIRC3 and BIRC2, TRAF3, caspases, and MTOR, some of which are critical mediators of inflammation and fibrogenesis in NAFLD [13,14]. among others, are highlighted in Figure 1.
Finally, taken together, there is plausible evidence for the potential role of auranofin in ameliorating molecular drivers of NASH and NASH fibrosis, suggesting that this gold compound might be repurposed for the treatment of NAFLD/NASH.
The United States FDA approved an auranofin-gold (I)-containing oral pill in 1985 as a primary treatment against active, progressive, or destructive forms of inflammatory arthritis [15]. However, this drug was replaced by modern compounds, which, in part, was clinically justified by the large number of adverse effects associated with the long-term use of the drug. Some of the most problematic adverse events were thrombocytopenia and bone marrow suppression, which is the reason why this drug received a black box warning from the FDA. Despite that, auranofin, with a chemical name of (1-Thio-beta-D-glucopyranosato) (triethylphosphine) gold 2,3,4,6-tetraacetate, is being tested in many phase I clinical trials for diverse conditions, such as human immunodeficiency virus infection, glioblastoma multiforme, lung adenocarcinoma, amebiasis, small cell, non-small cell, and squamous cell lung carcinoma, as well as in phase II trials for the treatment of pain, chronic lymphocytic leukemia, ovarian carcinoma, and chronic lymphocytic leukemia (ClinicalTrials.gov).
As a result, the potential effects and safety profile of auranofin in human NAFLD must be proven.
FOOTNOTES
FigureĀ 1.
Auranofin chemical-protein interaction network. The prediction was performed by the web resource STITCH version 5.0, available at http://stitch.embl.de, which is a database of known and predicted interactions between chemicals and proteins. The interactions include direct (physical) and indirect (functional) associations, which stem from computational prediction, knowledge transfer between organisms, and interactions aggregated from other (primary) databases. The prediction was restricted to human evidence. Thicker lines represent stronger associations; for example, protein-protein interactions are shown in grey, chemical-protein interactions in green, and interactions between chemicals in red.
REFERENCES
1. Dufour JF, Anstee QM, Bugianesi E, Harrison S, Loomba R, Paradis V, et al. Current therapies and new developments in NASH. Gut 2022;71:2123-2134.



2. Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med 2018;24:908-922.




3. Sookoian S, Pirola CJ. Repurposing drugs to target nonalcoholic steatohepatitis. World J Gastroenterol 2019;25:1783-1796.



4. Lee SM, Koh DH, Jun DW, Roh YJ, Kang HT, Oh JH. Auranofin attenuates hepatic steatosis and fibrosis in non-alcoholic fatty liver disease via NRF2 and NF-ĪŗB signaling pathways. Clin Mol Hepatol 2022;28:827-840.




5. Hwangbo H, Kim MY, Ji SY, Kim SY, Lee H, Kim GY, et al. Auranofin attenuates non-alcoholic fatty liver disease by suppressing lipid accumulation and NLRP3 inflammasome-mediated hepatic inflammation in vivo and in vitro. Antioxidants (Basel) 2020;9:1040.



6. Han S, Kim K, Kim H, Kwon J, Lee YH, Lee CK, et al. Auranofin inhibits overproduction of pro-inflammatory cytokines, cyclooxygenase expression and PGE2 production in macrophages. Arch Pharm Res 2008;31:67-74.



7. Roder C, Thomson MJ. Auranofin: repurposing an old drug for a golden new age. Drugs R D 2015;15:13-20.




8. Rothan HA, Stone S, Natekar J, Kumari P, Arora K, Kumar M. The FDA-approved gold drug auranofin inhibits novel coronavirus (SARS-COV-2) replication and attenuates inflammation in human cells. Virology 2020;547:7-11.



9. Fan C, Zheng W, Fu X, Li X, Wong YS, Chen T. Enhancement of auranofin-induced lung cancer cell apoptosis by selenocystine, a natural inhibitor of TrxR1 in vitro and in vivo. Cell Death Dis 2014;5:e1191.




10. Sookoian S, Pirola CJ. PNPLA3, the triacylglycerol synthesis/hydrolysis/storage dilemma, and nonalcoholic fatty liver disease. World J Gastroenterol 2012;18:6018-6026.



11. Sookoian S, Pirola CJ. PNPLA3, the history of an orphan gene of the potato tuber protein family that found an organ: the liver. Hepatology 2014;59:2068-2071.


12. Pirola CJ, Sookoian S. Epigenetics factors in nonalcoholic fatty liver disease. Expert Rev Gastroenterol Hepatol 2022;16:521-536.


13. Sookoian S, CastaƱo G, Gianotti TF, Gemma C, Rosselli MS, Pirola CJ. Genetic variants in STAT3 are associated with nonalcoholic fatty liver disease. Cytokine 2008;44:201-206.


- TOOLS
-
METRICS

-
- 0 Crossref
- 0 Scopus
- 2,572 View
- 68 Download
- ORCID iDs
-
Carlos J. Pirola

https://orcid.org/0000-0001-8234-4058Silvia Sookoian

https://orcid.org/0000-0001-5929-5470 - Related articles



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print



