Erratum to ‘KASL clinical practice guidelines for management of chronic hepatitis B’ [Clin Mol Hepatol 2022;28:276-331]
Article information
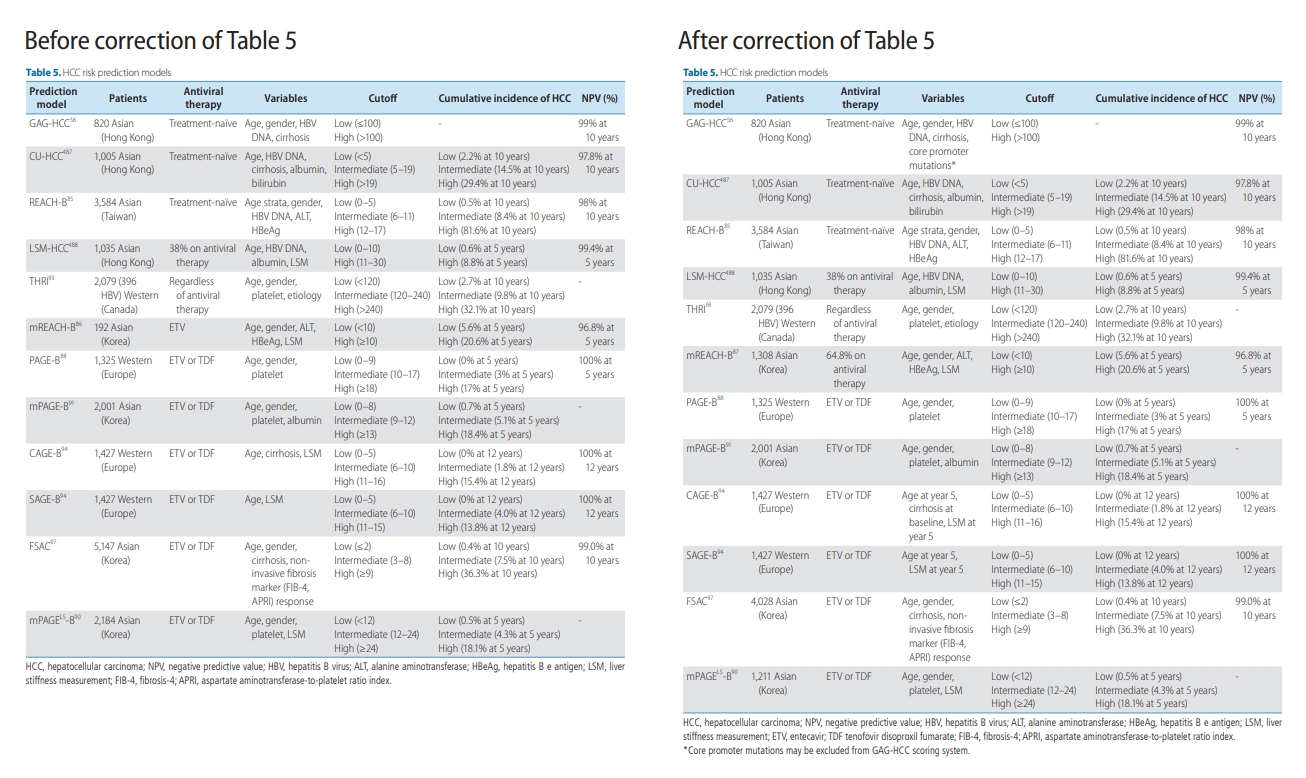
It has come to our attention that there are typographical errors in our article. The line 2 of page 283 ‘HBcAg’ should be read ‘HBeAg’. In line 8 of left column on page 286, ‘In general, greater than F2 fibrosis ..’ should read ‘In general, fibrosis stage F2 or greater ..’. In line 14 of left column on page 286, ‘inflammation greater than grade A2–A3’ should read ‘inflammatory activity of greater than or equal to A2’. The sentence on page 290 ‘.. 13.9% in 10 years, and they showed higher risk of death and liver-related complications than did treated cirrhotic patients.193,204’ should read ‘.. 13.9% in 5 years 193, and untreated inactive cirrhotic patients showed higher risk of death and non-HCC liver-related complications than antiviral-treated cirrhotic patients.204’ In Table 5, variables for CAGE-B and SAGE-B, the number of patients for FSAC and mPAGELS-B, and mREACH-B were corrected. We apologize for any inconvenience caused.