Hepatocellular carcinoma surveillance in patients with non-alcoholic fatty liver disease
Article information
Abstract
Non-alcoholic fatty liver disease (NAFLD) may progress to cirrhotic or non-cirrhotic hepatocellular carcinoma (HCC), and is currently recognized as the fastest growing cause of HCC worldwide. Accordingly, professional society guidelines recommend HCC surveillance in patients with cirrhosis from any etiology, and some may consider it beneficial in subgroups with non-cirrhotic NAFLD at higher risk for HCC. Notably, patients with NAFLD-related HCC are more likely to have HCC diagnosed at more advanced stages and have poorer outcomes when compared to other etiologies, and suboptimal effectiveness of HCC surveillance programs is a major culprit. In this review, we summarize the current guidelines for HCC surveillance and discuss its benefits versus potential harms for NAFLD patients. We also address the unique challenges of HCC surveillance in NAFLD, including higher proportion of NAFLD-related HCC without cirrhosis, poor recognition of at-risk patients, lack of consensus regarding the value of surveillance in non-cirrhotic NAFLD, subpar effectiveness of surveillance tools related to NAFLD phenotype, and preponderant surveillance underuse among NAFLD patients. Finally, we examine the effectiveness of currently used surveillance tools (i.e., ultrasound and alpha fetoprotein) and outline future perspectives including emerging risk stratification tools, imaging surveillance strategies (e.g., abbreviated magnetic resonance imaging protocols), blood-based biomarkers (e.g., GALAD and circulating tumor DNA panels), and interventions to improve surveillance adherence.
INTRODUCTION
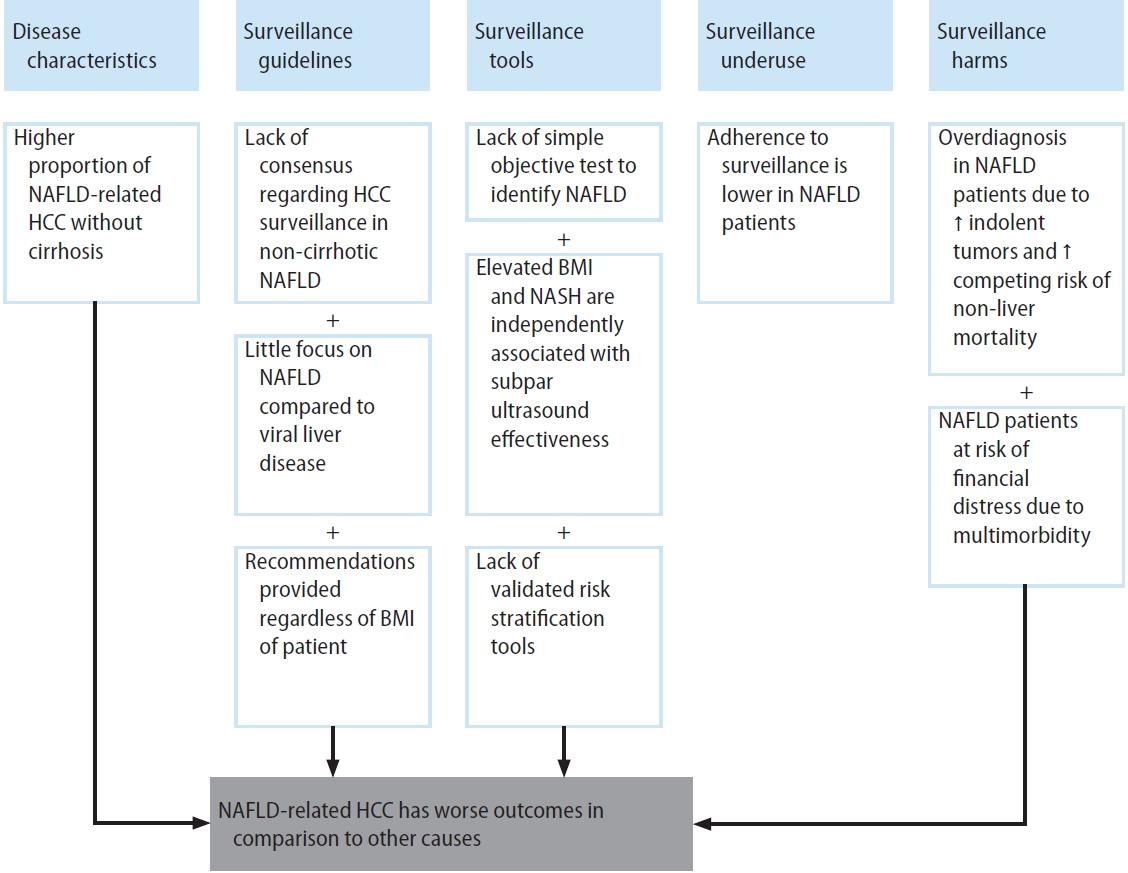
Non-alcoholic fatty liver disease (NAFLD) encompasses a spectrum of conditions including simple steatosis, which is usually benign in nature, and non-alcoholic steatohepatitis (NASH), which may progress to cirrhosis and hepatocellular carcinoma (HCC) [1,2]. NAFLD has become the fastest growing cause of HCC in Western countries over the past two decades [3-6]. When compared to other etiologies of chronic liver disease (CLD) such as hepatitis C virus and alcoholic liver disease, patients with NAFLD-related HCC are more likely to be diagnosed at later stages and have a worse prognosis [7,8]. There are several contributing factors including but not limited to suboptimal effectiveness of HCC surveillance programs [7-9]. HCC surveillance in patients with NAFLD is limited by unique challenges, including increased difficulty recognizing appropriate at-risk patients, a higher proportion of HCC occurring in the absence of cirrhosis compared to other etiologies, unsatisfactory effectiveness of surveillance tools, underuse of HCC surveillance, and higher competing risk of non-HCC-related mortality (Fig. 1) [10-12]. Herein, we will review the status of HCC surveillance in patients with NAFLD, explore areas of concern, and outline future perspectives.
GUIDELINES AND SUPPORTING EFFICACY DATA FOR HCC SURVEILLANCE
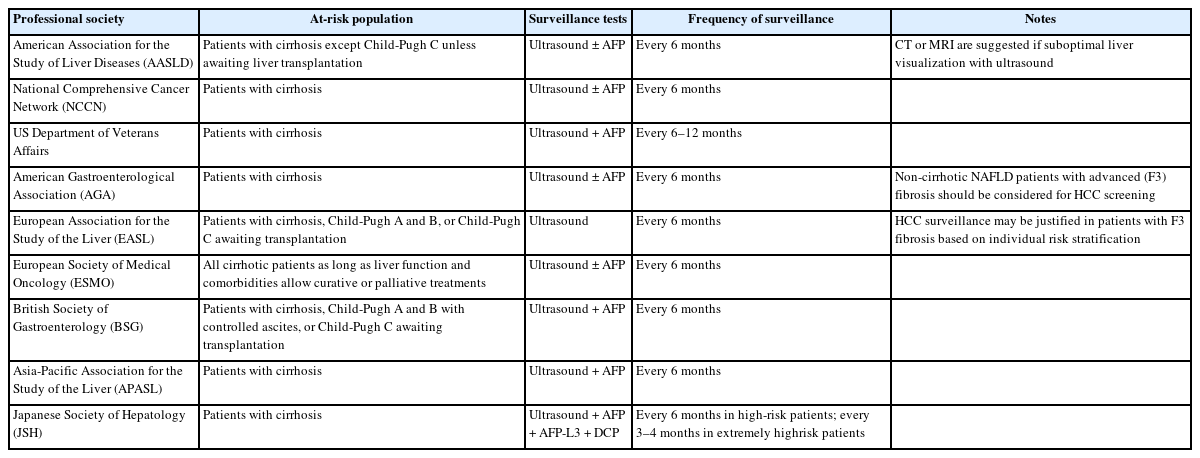
Multiple professional society guidelines including the American Association for the Study of Liver Disease (AASLD), European Association for the Study of the Liver (EASL), and Asian Pacific Association for the Study of the Liver (APASL) recommend HCC surveillance in at-risk individuals including subsets of chronic hepatitis B virus (HBV) infection and those with cirrhosis from any etiology (Table 1) [13-15].
The best data for HCC surveillance are derived from a large randomized controlled trial in patients with HBV infection, demonstrating a 37% reduction in HCC-related mortality [16]. However, it is unclear if these data apply to patients with cirrhosis, particularly those with NAFLD etiology, given differences in body habitus, liver heterogeneity, and hepatic steatosis that may impact surveillance effectiveness. When a randomized clinical trial of HCC surveillance was attempted in Australia, it had to be closed due to low enrollment due to poor patient and provider acceptance of the no-surveillance arm. We are therefore forced to rely on level II case-control and cohort studies, instead of level I randomized controlled trial data. Meta-analyses of these studies demonstrate a consistent association between HCC surveillance and improved clinical outcomes [17]. A recent systematic review and meta-analysis identified 59 relevant studies between January 2014 and July 2020, including a total of 145,396 patients [17]. HCC surveillance was associated with improved early-stage HCC detection (relative risk [RR], 1.86; 95% confidence interval [CI], 1.73–1.98), curative therapy receipt (RR, 1.83; 95% CI, 1.69–1.97), and reduced mortality (hazard ratio [HR], 0.67; 95% CI, 0.61–0.720) after adjusting lead-time bias [17].
Although clinical benefits were consistent in the subgroup of studies with >20% NAFLD patients, there were only two studies specifically examining the association between HCC surveillance and clinical outcomes in patients with NAFLD-related cirrhosis. Lo and colleagues reported a significant association with early-stage HCC detection (69.6% vs. 30%, P=0.001) whereas Aby et al. failed to find an association with curative treatment receipt (45.5% vs. 51.7%, P=0.70) [18,19]. The authors of the meta-analysis identified increased data in NAFLD as an area of need, particularly given unique challenges of HCC surveillance in NAFLD patients, as discussed below in detail.
There has been increasing recognition that benefits of HCC surveillance must be weighed against potential harms. Based on an established taxonomy, cancer screening harms are classified as physical, psychological, or financial harms [20]. Physical harms extend beyond complications and includes aspects such as pain or radiation exposure from surveillance and diagnostic testing for positive or indeterminate results. Psychological harm can occur through the whole screening process, including apprehension of a positive result, anxiety or depression caused by receipt of an abnormal result, overestimation of the likelihood of a diagnosis, and distress related to being labeled with a diagnosis [21]. Financial harm may also result from direct costs of screening and diagnostic evaluation as well as indirect costs such as co-pays and transportation as well as opportunity costs from missed work [22].
The above systematic review did not identify any studies examining financial or psychological harms, although the proportion of patients experiencing physical harms due to false positive or indeterminate results ranged from 8.8% to 27.5% across four applicable studies, with most harms being mild in severity. No studies rigorously evaluated etiologyspecific differences in surveillance harms, although existing data suggest similar risk in NAFLD patients than those with viral cirrhosis. Subsequently, a multi-center mixed-methods study highlighted patients with true and false positive results could be associated with increased psychological harms, including depression and anxiety. Patients also reported financial harms, including indirect costs from aspects such as transportation and parking and opportunity costs from missed work. Financial harms appeared to be higher in those with multiple comorbidities, which may be pertinent for patients with NAFLD; however, the study was underpowered to evaluate etiology-specific differences in risk of financial and psychological harms. Notably, these harms appeared to be milder than those observed in other cancer screening programs and did not result in decisional regret to undergo HCC surveillance.
Other potential harms, including overdiagnosis, have been well studied in other cancers but there are few data for HCC surveillance overall, including in those with NAFLD. Overdiagnosis may relate to several factors including misclassification of premalignant lesions as cancer, detection of indolent tumors, or high competing risk of mortality [23,24]. Although HCC has traditionally been regarded as a uniformly aggressive cancer, recent data suggest that one-fourth to one-third of tumors may be indolent with slower tumor growth patterns [25,26]. Across studies, patients with non-viral liver disease had more indolent growth patterns than those with viral etiologies, suggesting greater risk for length time bias and overdiagnosis. Further, overdiagnosis may be particularly relevant for patients with NAFLD given higher comorbidity burden [27].
Summary
HCC surveillance is recommended in patients with cirrhosis from any etiology. This practice is supported by cohort studies showing associations with increased early cancer detection and improved overall survival, although there are fewer data specifically in patients with NAFLD-related cirrhosis. HCC surveillance is associated with physical, financial, and psychological harms as well as risk of overdiagnosis, although existing data suggest these may be mild in severity. Continued data are needed to better define the overall value of HCC surveillance in patients with NAFLD cirrhosis.
IDENTIFICATION OF THE AT-RISK NAFLD POPULATION
HCC surveillance is currently recommended in all patients with cirrhosis from any etiology, including those with NAFLD-related cirrhosis. Cost-effectiveness analyses have suggested that HCC surveillance is cost-effective if the annual HCC incidence exceeds 0.8% in patients with compensated cirrhosis and exceeds 0.2% in patients without cirrhosis [28,29]. The annual incidence of HCC in patients with cirrhosis has traditionally been ~2–3% per year, although higher estimates have been reported in Asian cohorts with higher proportions of patients with active viral hepatitis. Several studies have shown that the annual incidence of HCC is lower in the setting of non-viral liver disease, with annual HCC incidence estimates ranging from 0.7% to 2.6% in patients with NAFLD cirrhosis [30,31] and from 0.01% to 0.13% in patients with non-cirrhotic NAFLD [32,33].
While there is general agreement about the application of surveillance programs in patients with NAFLD cirrhosis, there is a lack of consensus regarding the value of HCC surveillance in non-cirrhotic NAFLD. The AASLD guidelines restrict surveillance recommendations to those with cirrhosis [34,35], whereas a clinical practice update from the American Gastroenterological Association recommends surveillance in patients with F3 fibrosis [36] and EASL guidelines suggest HCC surveillance might be justified in F3 fibrosis patients based on individual risk stratification [14]. This debate has been contentious and noteworthy given growing literature demonstrating a substantial risk of developing HCC in the absence of cirrhosis in NAFLD patients compared to patients with other etiologies of liver disease [37]. Indeed, the proportion of NAFLD-related HCC patients without evidence of cirrhosis at diagnosis ranges from 46.2% to 54%, as compared to 2.8% to 22% of patients with HCC related to other etiologies [7,38]. A meta-analysis of existing literature reported a pooled proportion of 38.0% for non-cirrhotic HCC in NAFLD patients, compared to 14.2% for other liver disease etiologies, with an odds ratio of 2.61 (95% CI, 1.27–5.35) [39]. However, cohort studies suggest the annual incidence of HCC in non-cirrhotic NAFLD falls below the cost-effectiveness threshold. An analysis of the Veterans Affairs administrative database found an annual HCC incidence of only 0.008 per 100 person-years among 292,366 persons with non-cirrhotic NAFLD, although this group was heterogeneous regarding baseline fibrosis level. A subsequent prospective multicenter study involving 1,773 NAFLD patients included in the NASH clinical research network (1,237 patients with stage F0–F2; 369 stage F3; and 167 stage F4) found the incidence of HCC per 100 person-years was 0.04 for patients with F0–F2 fibrosis, compared to 0.34 for F3 fibrosis and 0.14 for F4 fibrosis [40]. A meta-analysis of 18 studies with 470,404 patients found a pooled annual incidence of 0.03 per 100 person-years (95% CI, 0.01–0.07) in non-cirrhotic NAFLD, compared to 3.78 per 100 person-years (95% CI, 2.47–5.78) in those with cirrhosis [41].
Overall, these data suggest that HCC surveillance is unlikely to be cost effective in non-cirrhotic NAFLD, outside of additional risk stratification tools. An in-depth discussion of HCC risk stratification in patients with NAFLD is beyond the scope of this review. However, in brief, several risk models incorporating clinical risk factors, genetic factors, and molecular factors have been proposed, with most not yet having been sufficiently validated for routine use in clinical practice [42-45]. If sufficiently validated, these risk models may facilitate a more individualized precision screening approach to targeted HCC surveillance to those at the highest risk [46,47]. While we await validated models to better risk stratify patients with non-cirrhotic NAFLD and identify subgroups who may benefit from HCC surveillance, consistently observed risk factors such as male sex, older age, and increasing number of metabolic syndrome components may help identify individuals with non-cirrhotic NAFLD who have higher HCC risk. Further, prior data clearly highlight the direct relationship between fibrosis stage and HCC risk, with F3 fibrosis posing significantly higher risk than F0-F2 fibrosis.
Summary
HCC surveillance in patients with NAFLD is currently restricted to those with cirrhosis. Surveillance is not cost-effective in broader non-cirrhotic patient populations based on current data, although it may be considered in individual patients with F3 fibrosis who are deemed to be at higher risk of developing HCC. There are several emerging risk stratification tools to accurately identify subgroups with non-cirrhotic NAFLD with sufficient risk to warrant surveillance, although these are currently insufficiently validated to be used in clinical practice.
SURVEILLANCE TOOLS IN PATIENTS WITH NAFLD
Ultrasound-based surveillance
Semi-annual ultrasound has been the standard of care strategy for HCC surveillance in at-risk groups for over 15 years [14,48]. Advantages of this strategy include widespread availability, low cost, non-invasiveness, and absence of patient exposure to ionizing radiations or contrast media [35]. Results from a meta-analysis showed that the pooled sensitivity and specificity of ultrasound alone for any-stage HCC detection in patients with cirrhosis were 84% and 91%, respectively, whereas the pooled sensitivity drops to 47% for those with early-stage HCC [49]. The suboptimal sensitivity of ultrasound for early-stage HCC contributes to a substantial number of patients diagnosed at later tumor stages, leading to worse overall survival [37].
The effectiveness of HCC detection by ultrasound is further limited in obese individuals and those with non-viral etiologies of liver disease. A retrospective study aimed at evaluating ultrasound accuracy for HCC diagnosis in obese patients demonstrated that sensitivity was 77% in patients with body mass index (BMI) <30 kg/m2 and 21% in patients with BMI ≥30 kg/m2 [50]. Ultrasound is also not as sensitive for early HCC detection in NAFLD patients when compared to other etiologies of CLD, mainly due to subcutaneous fat accumulation as well as liver fatty infiltration which both hamper visualization. A retrospective cohort study involving 941 patients with cirrhosis, in which ultrasounds were independently reviewed by three abdominal radiologists, demonstrated that obesity, non-viral etiologies of liver disease including NAFLD, and Child Pugh class B cirrhosis were all independently associated with worse ultrasound quality for evaluation of liver lesions [51]. A subsequent study using the ultrasound Liver Imaging Reporting and Data System (LI-RADS) visualization score validated these associations as well as demonstrated that obesity and non-viral liver disease etiologies were associated with persistent poor visualization over time [52]. These data are important given that poor ultrasound visualization is associated with worse test performance, with LI-RADS visualization score B (moderate limitations) being associated with increased odds of false positive results (odds ratio [OR], 1.60; 95% CI, 1.13–2.27) and LI-RADS visualization score C (severe limitations) being associated with significantly higher odds of false negative results (OR, 7.94; 95% CI, 1.23–51.2). Ultrasound sensitivity exceeded 75% for those with LI-RADS visualization scores of A or B, compared to only 27.3% in those with a visualization score of C. Results from another single-center study with 352 patients similarly found that ultrasound sensitivity was significantly lower in obese patients compared to non-obese patients (76% vs. 87%, respectively, P=0.01) and in NAFLD patients compared to those with other etiologies (59% vs. 84%, respectively, P=0.02) [53]. Overall, these data suggest that ultrasound visualization and other patient factors (e.g., presence of obesity, liver disease etiology, and Child Pugh class) may identify a subgroup of patients who would benefit from alternative surveillance modalities.
Role of alpha fetoprotein (AFP) in surveillance
AFP is the only biomarker to complete all five phases of biomarker validation [54]. Although it has insufficient accuracy to be used in isolation, there is accumulating evidence suggesting that adding AFP to ultrasound-based surveillance significantly improves test performance [12,22], with a sensitivity of approximately 97% for any-stage HCC detection and 63% for early-stage detection [49]. There was a small trade-off in specificity, decreasing to 84%, although this was felt to still exceed the accepted threshold for a false positive rate, and the overall diagnostic odds ratio was similar if not higher for the two tests in combination. Recent data have demonstrated decreasing trends in AFP levels among HCC patients, in parallel with a shift in epidemiology to increasing non-viral cases, suggesting that the optimal threshold for AFP in patients with NAFLD may be lower than the traditional cut-off of 20 ng/mL [55,56]. Further, there are data suggesting that longitudinal measurements of AFP, examining changes over time instead of single threshold assessments, may increase test performance, although there are fewer data for this approach in patients with NAFLD than viral etiologies [57,58].
Alternative imaging-based surveillance tools
While contrast-enhanced ultrasound can be used as a second-line diagnostic tool for HCC once a focal hepatic lesion is detected on conventional ultrasound, there are no strong data to demonstrate that this strategy would increase test performance for surveillance and early HCC detection. Further, logistical concerns such as need for repeat contrast injections may make this impractical for surveillance [15,35].
Other imaging modalities such as computed tomography (CT) scan or magnetic resonance imaging (MRI) are increasingly being considered for HCC surveillance. Results from a prospective cohort study (the Prospective Intra-individual Cohort Study to Compare Gadoxetic Acid [Primovist®]-Enhanced Magnetic Resonance Image and Ultrasonography for the Surveillance of Early Stage Hepatocellular Carcinoma in Patients at High-Risk study, NCT01446666) suggested that MRI-based screening had a significantly higher sensitivity and specificity than ultrasound for early-stage HCC in highrisk patients with cirrhosis [59]. The trial performed concurrent ultrasound and MRI in 407 patients for 1.5 years, over which time 43 were diagnosed with HCC. MRI had significantly higher sensitivity for early-stage HCC detection (85.7% vs. 26.2%) as well as higher specificity (97.0% vs. 94.4%). However, the study was largely limited to patients with HBV-related cirrhosis, and these results have yet to be validated in broader patient populations including those with NAFLD cirrhosis. Other potential barriers including radiologic capacity and patient concerns such as claustrophobia may limit uptake so would need to be considered when estimating effectiveness of an MRI-based strategy. A cost-effectiveness analysis suggested an MRI-based strategy could be cost-effective in patients with annual incidence rate of HCC is >1.81% [59], but not those with lower annual incidence, such as those with NAFLD cirrhosis [2,30,31]. Instead, it may be best reserved for patient subgroups, such as those with inadequate ultrasound visualization [36].
To address the potential concerns about cost-effectiveness, several investigators have proposed abbreviated MRI (AMRI) protocols, in which selected sequences are performed and in-scanner time is reduced from approximately 45 minutes to 15 minutes. Potential protocols include non-contrast MRI protocols, dynamic contrast-enhanced protocols, and hepatobiliary phase AMRI, with each demonstrating promising performance in case-control studies. A meta-analysis of studies examining AMRI performance reported sensitivities of 69% and 86% for HCC lesions <2 cm and those ≥2 cm, and AMRI having higher sensitivity than that of ultrasound (82% vs. 53%) [60]. A post-hoc analysis of the PRIUS study simulating AMRI by selecting MRI sequences similarly reported that AMRI had significantly higher sensitivity than that of ultrasound (86.0% vs. 27.9%, P<0.001), albeit with a higher false positive rate (4.4% vs. 3.7%) [61].
Another study, in which low dose two-phase CT (arterial phase and 3-minute delayed phase) and ultrasound were concurrently performed in a cohort of 139 patients with cirrhosis, similarly found that two-phase CT had significantly higher sensitivity for early-stage HCC (83.3% vs. 29.2%, P<0.001) and specificity (95.6% vs. 87.7%, P=0.03) compared to ultrasound-based surveillance. However, concerns regarding contrast exposure and cumulative radiation exposure may limit broader uptake as a surveillance modality [35].
Biomarker-based surveillance tools
Growing evidence suggests that novel biomarkers could play a role to improve HCC surveillance in NAFLD patients. GALAD, consisting of Gender, Age, AFP-L3, AFP, and des-carboxy-prothrombin (DCP), is a promising biomarker panel with extensive phase II biomarker data, including in patients with NAFLD [12,62,63]. The largest study to evaluate GALAD is a multi-center case-control study examining GALAD in 6834 patients with CLD with (n=2,430) and without (n=4,404) HCC [64]. In this study, GALAD achieved a sensitivity and specificity of 60.6–80.2% and 88.6–95.8% for early HCC detection, respectively. However, this study included majority patients with viral hepatitis so unclear if these results would apply to those with NAFLD. A single-center cohort analysis suggested performance may be further improved by combining GALAD with ultrasound (GALADUS score), which resulted in an area under the receiver operating characteristic curve (AUC) of 0.98 [62]. In a subsequent case-control study including NAFLD-related patients with and without HCC, the diagnostic performance of GALAD proved to be excellent for HCC detection [65]. Indeed, GALAD accurately detected HCC at any tumor stage with a significantly better performance than each individual biomarker, including AFP, AFP-L3, or DCP (AUC: 0.96 vs. 0.88, 0.86, and 0.87, respectively; P<0.001 for each). GALAD performance was independent of cirrhosis, as similar AUCs were obtained for patients with and without cirrhosis (AUC: 0.93 and 0.98, respectively). However, recent phase III data suggested lower diagnostic performance when evaluated in cohort analyses [66].
There are other novel candidate biomarkers that could be of added value for HCC surveillance including methylated circulating tumor DNA (ct-DNA), microRNAs (miRNAs), long non-coding RNAs, exosomes, epigenetics, and lipidomics. Indeed, methylated ct-DNA released from cancer cells could be harvested in “liquid biopsies” and used as potential non-invasive biomarkers to detect HCC at an early stage [67]. In an international case-control study, the performance of a novel multitarget HCC blood test (mt-HBT) incorporating DNA methylation biomarkers (HOXA1, TSPYL5, and B3GALT6), AFP, and patient sex was clinically validated in an independent sample including 156 HCC patients [68]. In this study, mt-HBT detected early-stage tumors with 82% sensitivity, which was significantly higher than AFP (40%; P<0.001) and GALAD (71%; P=0.03). Notably, early-stage sensitivity was stable across subgroups, including sensitivities of 85% and 77% in patients with BMI values <30 kg/m2 and those with BMI ≥30 kg/m2, respectively as well as across all examined liver disease etiologies, making mt-HBT a potentially valuable tool for surveillance in patients with NAFLD. A recent network meta-analysis suggested similar efficacy of mt-HBT compared to ultrasound and AFP for early-stage HCC detection [69], although the authors noted the strength of data differed for the two modalities. In another multicenter validation study HelioLiver Test, another ct-DNA biomarker panel, yielded a sensitivity of 76% (95% CI, 60–87%) for early-stage HCC, significantly higher than AFP and GALAD [70]. Both ct-DNA assays are undergoing prospective evaluation at this time, so we anticipate validation in the near future.
There are several other biomarkers that have early phase II biomarker data, although a comprehensive review of these biomarkers is beyond the scope of this review. For example, HCC patients have elevated levels of liver-specific miRNAs including miR-106b-3p, miR-101-3p and miR-1246 when compared to healthy subjects, suggesting the potential utility of these biomarkers for early HCC detection in high-risk patients [71]. Specific hydroxymethylated genes are also associated with HCC in the absence of elevated AFP, suggesting a role of these epigenetically modified genes as potential biomarkers [72]. Similarly, Lewinska and colleagues identified a serum lipidome that was able to accurately distinguish NAFLD patients with HCC from controls without HCC [73].
Although these blood-based biomarkers have promising early data, most have only been evaluated in phase II case-control studies but not validated in phase III or phase IV cohort studies [54]. Phase II studies are subject to selection bias and spectrum bias, potentially overestimating biomarker performance, highlighting the importance of subsequent validation. Further, much of the data for these biomarkers has been derived in patients with viral hepatitis, highlighting a need for increased data in emerging patient populations, including those after sustained virological response or NAFLD.
Summary
Ultrasound alone has insufficient sensitivity for early detection of HCC, which can be improved by using in combination with AFP. Emerging imaging surveillance strategies (e.g., MRI) and blood-based biomarkers (e.g., GALAD and ct-DNA panels) have promising early data suggesting high accuracy, although these require further validation prior to routine use in clinical practice.
SURVEILLANCE INTERVAL
Most guidelines recommend HCC surveillance in at-risk individuals every 6 months [22], as it has a better sensitivity than a 6–12 months interval [74], and a similar sensitivity but higher specificity and lower cost than a 3 months interval [75]. There are no data suggesting that HCC surveillance intervals should be tailored to liver disease etiology.
Summary
HCC surveillance should be performed at semi-annual intervals in at-risk patients, including those with NAFLD cirrhosis.
SURVEILLANCE UNDERUSE
Adherence to the HCC surveillance programs is often suboptimal [37]. A systematic review showed that the HCC surveillance was performed in only 24% of patients with cirrhosis [76]. There was geographic variation in surveillance receipt, with the lowest receipt among studies from the United States, compared to those from Europe and Asia (17.8% vs. 43.2% and 34.6%, respectively; P<0.001). Subgroup analyses also demonstrated higher surveillance use among subspecialty care studies, compared to center-based and population-based studies (73.7% vs. 29.5% and 9.8%, respectively). Most notably, surveillance underuse is particularly concerning among NAFLD patients, as this one of the most consistent correlates for surveillance underuse across studies. In fact, studies suggest up to half of NAFLD-related HCC cases are not detected through surveillance [7].
There are many patient- and provider-level barriers to HCC surveillance, contributing to HCC surveillance underuse [77-79]. Provider-level barriers to surveillance include time constraints in clinic, inadequate knowledge about guidelines, and difficulty identifying at-risk patients [80]. As discussed above, identification of at-risk patients with NAFLD can be particularly problematic for providers. Patient-reported barriers include challenges with the scheduling process, transportation difficulties, and cost of testing [81,82]. These data highlight the need for interventions targeting the surveillance process at multiple levels to increase optimal adherence [77]. Surveillance adherence can be improved through a variety of interventions including patient or provider education, electronic medical record reminder systems, automated recall systems via radiology, or population health programs using mailed outreach [83-87]. Most studies suggest similar efficacy of interventions across patient subgroups, including liver disease etiology, although few have performed rigorous moderator analyses.
Summary
HCC surveillance is underused in clinical practice, including in patients with NAFLD, related to patient and provider-reported barriers. Several multi-level interventions are efficacious for increasing surveillance utilization.
CONCLUSION AND FUTURE PERSPECTIVE
NAFLD is the now fastest growing cause of HCC worldwide, so it is critical to understand practices that can maximize survival for patients with NAFLD-related HCC (Fig. 2). In that vein, surveillance has been associated with significantly improved early tumor detection and survival. However, effectiveness of surveillance in clinical practice among patients with NAFLD has been limited by poor recognition of at-risk patients, suboptimal test effectiveness for early tumor detection, and surveillance underuse. Emerging risk stratification tools, imaging and blood-based surveillance strategies, and interventions to increase surveillance implementation all offer hope for improvements.

Current and future perspectives for HCC surveillance in NAFLD patients. NAFLD, non-alcoholic fatty liver disease; AFP, alpha fetoprotein; CT, computed tomography; MRI, magnetic resonance imaging; GALADUS, GALAD with ultrasound; ct-DNA, circulating tumor DNA; miRNA, microRNA; lncRNA, long non-coding RNA.
Notes
Authors’ contribution
Drafting of the manuscript (Seif El Dahan); Critical revision of the manuscript for important intellectual content (all authors); Obtained funding (Singal); Study supervision (Singal).
Conflicts of Interest
Amit Singal has served as a consultant or on advisory boards for Bayer, Wako Diagnostics, Exact Sciences, Roche, Glycotest, and GRAIL. None of the authors have any relevant conflicts of interest.
Acknowledgements
This study was conducted with support from NIH U01 CA230694 and R01 CA212008. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The funding agencies had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation of the manuscript.
Abbreviations
AASLD
American Association for the Study of Liver Disease
AFP
alpha fetoprotein
AMRI
abbreviated magnetic resonance imaging
APASL
Asian Pacific Association for the Study of the Liver
AUC
area under the receiver operating characteristic curve
BMI
body mass index
CI
confidence interval
CLD
chronic liver disease
ct-DNA
circulating tumor DNA
CT
computed tomography
DCP
des-carboxy-prothrombin
EASL
European Association for the Study of the Liver
GALADUS
GALAD with ultrasound
HBV
hepatitis B virus
HCC
hepatocellular carcinoma
HR
hazard ratio
LI-RADS
Liver Imaging Reporting and Data System
miRNA
microRNA
MRI
magnetic resonance imaging
mt-HBT
multitarget HCC blood test
NAFLD
non-alcoholic fatty liver disease
NASH
non-alcoholic steatohepatitis
OR
odds ratio
RR
relative risk