| Clin Mol Hepatol > Volume 28(4); 2022 > Article |
|
ABSTRACT
Background/Aims
Due to increases in obesity and type 2 diabetes, the prevalence of nonalcoholic fatty liver disease (NAFLD) has also been increasing. Current forecast models may not include non-obese NAFLD. Here, we used the Bayesian approach to forecast the prevalence of NAFLD through the year 2040.
Methods
Prevalence data from 245 articles involving 2,699,627 persons were used with a hierarchical Bayesian approach to forecast the prevalence of NAFLD through 2040. Subgroup analyses were performed for age, gender, presence of metabolic syndrome, region, and smoking status. Sensitivity analysis was conducted for clinical setting and study quality.
Results
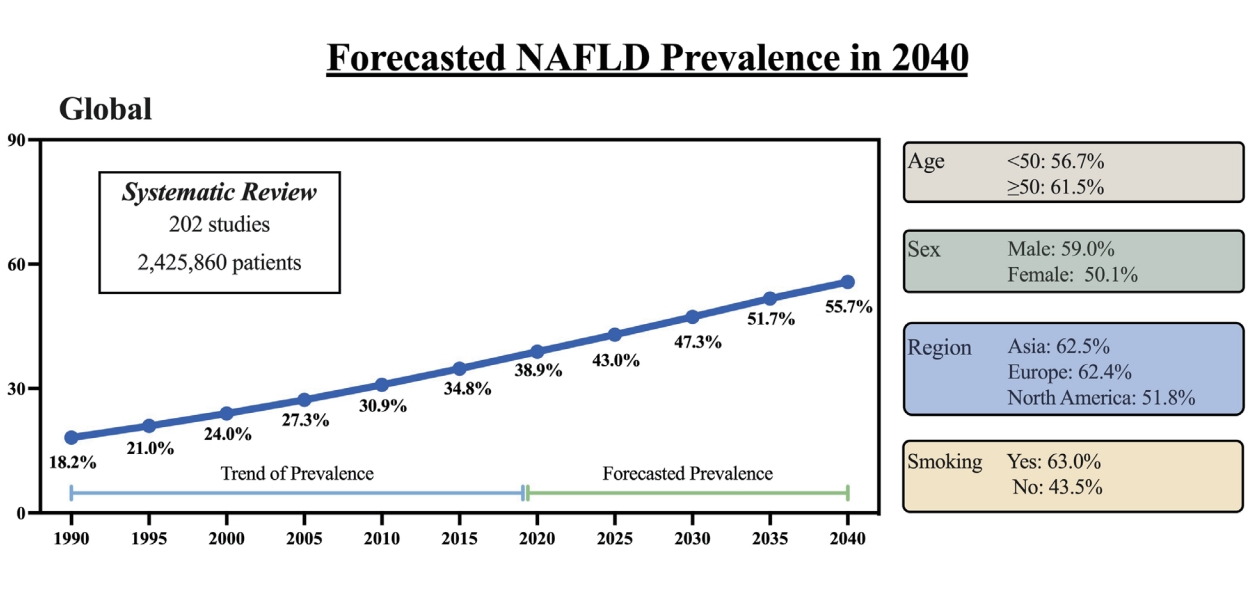
The forecasted 2040 prevalence was 55.7%, a three-fold increase since 1990 and a 43.2% increase from the 2020 prevalence of 38.9%. The estimated average yearly increase since 2020 was 2.16%. For those aged <50 years and Ōēź50 years, the 2040 prevalence were not significantly different (56.7% vs. 61.5%, P=0.52). There was a significant difference in 2040 prevalence by sex (males: 60% vs. 50%) but the trend was steeper for females (annual percentage change: 2.5% vs. 1.5%, P=0.025). There was no difference in trends overtime by region (P=0.48). The increase rate was significantly higher in those without metabolic syndrome (3.8% vs. 0.84%, P=0.003) and smokers (1.4% vs. 1.1%, P=0.011). There was no difference by clinical/community setting (P=0.491) or study quality (P=0.85).
Conclusion
By 2040, over half the adult population is forecasted to have NAFLD. The largest increases are expected to occur in women, smokers, and those without metabolic syndrome. Intensified efforts are needed to raise awareness of NAFLD and to determine long-term solutions addressing the driving factors of the disease.
Graphical Abstract
Nonalcoholic fatty liver disease (NAFLD) is a metabolically related liver disease that is strongly associated with the metabolic conditions of insulin resistance, type 2 diabetes, and obesity [1]. These three conditions alter the storage of fat in the liver which can lead to hepatic steatosis. In NAFLD, steatosis occurs in over 5% of hepatocytes without excessive alcohol use, viral hepatitis, or steatogenic drugs. Currently, the global prevalence of NAFLD in adults is 25ŌĆō30%, though the prevalence rates vary by country, with the highest rates reported in the Middle East and North Africa regions and South America, followed by Asia, North America, Europe, and then Africa [2-4]. NAFLD is also prevalent in children, with the prevalence rate estimated at 7.40% in the general pediatric population [2].
NAFLD can be a progressive disease leading to nonalcoholic steatohepatitis (NASH), fibrosis, cirrhosis, hepatocellular carcinoma (HCC), and liver transplantation; and NAFLD patients are at an increased risk of mortality, especially cardiovascular mortality [2,5-7]. Although only approximately 20% of those with simple fatty liver (NAFL) will progress in their disease state, a large number of the general population affected can lead to a large increase in the utilization of health-care resources [6]. In fact, economic studies on NAFLD have shown that people with NAFLD have higher health-care costs and increased years of disability [8-10]. Those with NAFLD have also reported worse quality of life, especially in their ability to be physically active [11].
While research continues on how best to diagnose NAFLD and NASH, as well as other treatments outside life-style modification, gaining an appreciation of the forecasted growth of NAFLD if the trends of obesity and type 2 diabetes mellitus continue to grow is important for policy makers and healthcare providers [12].
A few recent studies have forecasted NAFLD prevalence up to year 2030 [13-17]. These studies have suggested that NAFLD appears to be increasing at the same rate as the epidemics of obesity and diabetes, such that if the prevalence of obesity and diabetes level off through 2030, there will be only a modest increase in the total number of NAFLD cases (increases of 0ŌĆō30%). Currently, Europe and Germany are among countries with the highest prevalence of NAFLD; however, by 2030, Italy is expected to have a NAFLD prevalence of 29.5%, outpacing Germany, while France is expected to have the lowest prevalence of NAFLD in Europe. In Asia, China is expected to have the largest increase in the prevalence of NAFLD due to its rapid urbanization, and is forecasted to reach a NAFLD prevalence of 28.4% by 2030 [13-17]. However, using data on NAFLD prevalence from 167 studies and almost 1.5 million people, a systematic review and meta-analysis of NAFLD prevalence for China published in 2020 already found a NAFLD prevalence of 29.9%, which was still increasing [18].
In addition, most of these studies were either country-specific and/or based on population obesity prevalence, rather than published data for past trends of NAFLD prevalence. Markov modeling was also used as the basis for the analysis, which has inherent biases in that people can only be in one stage at a time, many simulations must be run to account for the multiple stages of disease, and the inputs are based on transition probabilities that are only in their infancy of development at this time [19]. Therefore, to overcome these limitations, the present study forecasted future prevalence using the Bayesian method based on the published data of past NAFLD prevalence trends.
We utilized the prevalence data from 245 articles involving 2,699,627 persons who were identified through a systematic literature search review of PubMed, Embase, and Cochrane Library from inception to March 2020. The details of the systematic review and characteristics of these 245 articles were previously described in a prior published study that evaluated the current and past trends of NAFLD prevalence [2]. Briefly, NAFLD was diagnosed via liver ultrasound in the vast majority of the studies (202 studies, 82.4%) and via other imaging methods, such computed tomography (seven studies), magnetic resonance imaging (three studies), controlled attenuation parameter (four studies), fatty liver index (22 studies), liver biopsy (one study), or a combination of methods (five studies). Only data from ultrasound-based studies were used for forecasting analysis, except for sub-analysis by geographic region due to limited data for some regions, as further described below [2].
Aggregated subgroup data are available for forecasting analysis of the following subgroups. By age, there were 101 studies providing data for 1,615,429 patients aged <50 years and 72 studies providing data for 403,237 patients aged 50 years or older. By sex, there were 148 studies providing stratified data for 899,753 male persons and 789,004 female persons. By geographic region, there were six studies (23,952 patients) from North America, 11 studies (15,062 patients) from Europe, 182 studies (2,385,999 patients) from Asia, two studies from Africa, and three studies from South America. Therefore, forecasting analyses were only performed for North America, Europe, and Asia. Additionally, 37 studies provided stratified data for 54,736 patients with metabolic syndrome (metabolic syndrome) and 198,432 patients who did not have metabolic syndrome, and 62 studies provided data for 230,188 smokers and 741,399 non-smokers. Lastly, there were 97 clinical center studies (143,480 patients), 105 community-based studies (1,434,840 patients), 127 studies (1,241,714 patients) with quality scores Ōēż7 (based on a modified Newcastle Ottawa scale [5], and 79 studies (1,184,146 patients) with quality scores >7 to provide data for sensitivity analyses by study setting and study quality.
We utilized a hierarchical Bayesian approach to best describe and fit the prevalence of NAFLD. A random effects parameter was used to address heterogeneity across the studies with NAFLD prevalence studies with NAFLD prevalence as included in a prior study [2]. The overall individual study prevalence of NAFLD was considered as the proportion of subjects reported as having NAFLD divided by the total number of reported subjects. When using Bayesian models, prevalence may be considered a random variable with an unknown parameter. Therefore, prevalence estimates of NAFLD were transformed using logit transformation. Logit transformation follows a normal distribution: logit(p[i]) = ╬╝i and ╬╝i~normal (Mu, precision ╬╝). The base model to pool the overall prevalence of age-related NAFLD was as follows: ╬╝i = ╬▓0 + ╬▓ ├Ś [study year ŌĆō mean-year], where mean-year was centered by the overall mean year across all studies. Lastly, a random effects parameter due to study heterogeneity was included using the conjugate gamma distribution: gamma (0.01, 0.01). The gamma distribution was utilized for unknown quantities for values between 0 and infinity. All coefficients and the intercept in the model were specified with non-informative normal priorsŌĆöi.e., ├¤~normal (0, 1├Ś10-6), Mu~normal (0, 1├Ś10-6). The Just Another Gibbs Sampler (R2JAGS) and Markov-chain Monte Carlo procedure (MCMCpack) within RStudio (R 3.6.3; Boston, MA, USA) were used to estimate posterior distributions. Convergence estimation was assessed by Gelman-Rubin convergence statistics.
In addition to the overall global NAFLD prevalence forecast up to year 2040, we performed forecasting for subgroups for which data were available. In total, stratified analyses were performed for age (<50 vs. Ōēź50 years), sex (male vs. female), geographic region (North America, Europe, and Asia), according to the presence of metabolic syndrome (with metabolic syndrome vs. no metabolic syndrome), and smoking status (smoking history vs. no smoking).
Figure 1 depicts the overall forecast for NAFLD prevalence through 2040. As shown, the prevalence of NAFLD in 1990 was 18.2% with a forecasted prevalence rate in 2040 to be 55.7%, a 3-fold increase from 1990 and a 43.2% increase from the 2020 prevalence of 38.9%. The estimated average yearly increase since 2020 is 2.16% (Table 1).
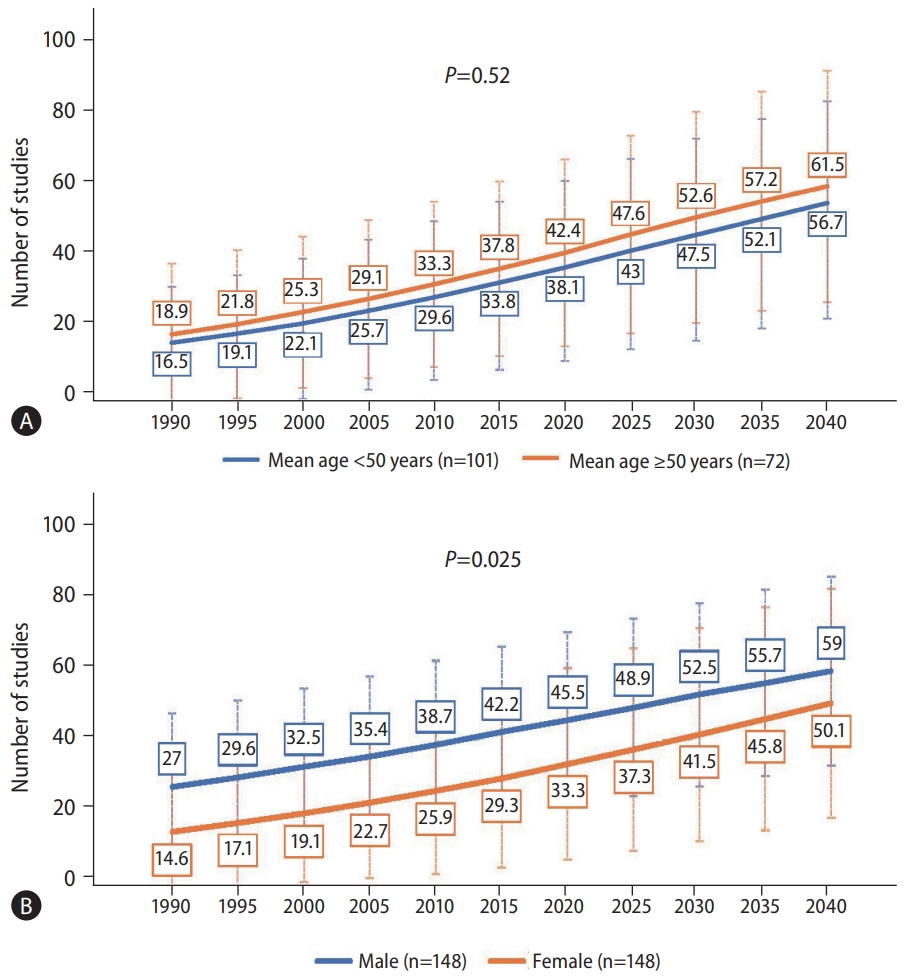
We found that the increasing trends for the prevalence of NAFLD for people aged <50 years and those Ōēź50 years were similar (Fig. 2A, P=0.52). For those aged <50 years, the prevalence of NAFLD was expected to be 56.7% by 2040, while for those aged Ōēź50 years, the prevalence was expected to be greater than 60%. The average annual percentage change in NAFLD prevalence since 2020 were 2.44% and 3.46% for people aged <50 years and Ōēź50 years, respectively (Table 1).
On the other hand, there was a significant difference in the trends for the increase in prevalence of NAFLD by sex, whereby the females had a larger spike in their trend noted to begin around the year 2025 (Fig. 2B, P=0.025), though the prevalence increased over time for both males and females. Males were forecasted to have a NAFLD prevalence of almost 60% by 2040, and a yearly increase of 1.48% from 2020 when it was 45.5%. Females NAFLD prevalence in 2040 was forecasted to be approximately 50%, and a yearly increase of 2.52% from 2020 when it was approximately 33.3% (Table 1).
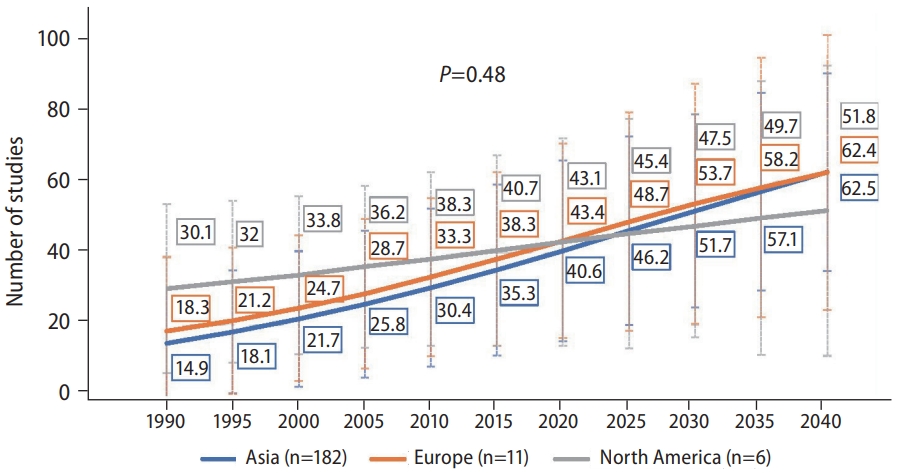
When we investigated the NAFLD forecast by region, we found no difference in increasing trends overtime by region (Fig. 3, P=0.48). The prevalence of NAFLD in Europe was forecasted to increase from approximately 18% in 1990, 43.4% in 2020, to over 60% by 2040, with Asia having an almost identical increase, with both regions having an average yearly increase of 2.19% and 2.70%, respectively (Table 1). In North America, the prevalence increased from approximately 30% in 1990, 43.1% in 2020, to 50% by 2040, with an average yearly increase of 1.01% since 2020.
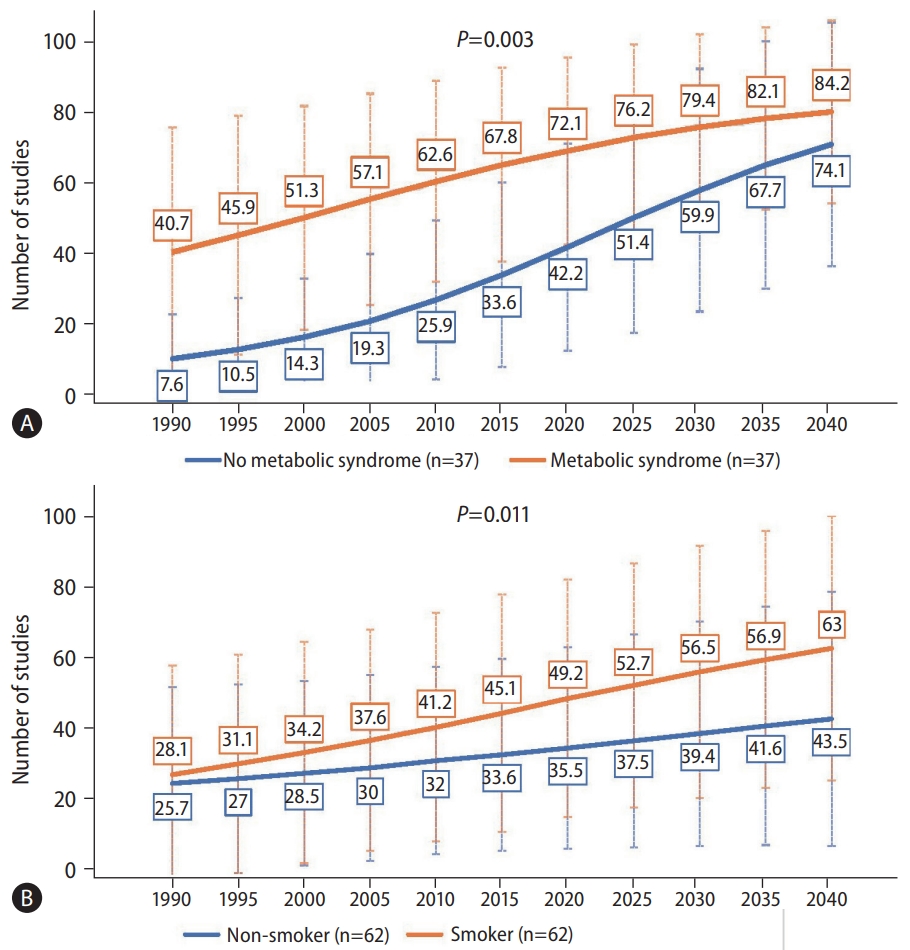
There was also a significant difference in the increasing trends of NAFLD between those with and without metabolic syndrome (Fig. 4A, P=0.003). However, the rate of increase was significantly higher for those without metabolic syndrome compared to those with metabolic syndrome, with the average annual increase of 3.78% and 0.84%, respectively (Table 1).
There was also a significant difference in the increasing trend of NAFLD prevalence by smoking status (Fig. 4B, P=0.011). Those who were smokers saw an increase of NAFLD prevalence from 28.1% in 1990, 49.2% in 2020, to 63% by 2040, whereas those who were non-smokers saw an increase from 25.7% in 1990, 35.5% in 2020, to 43.5% in 2040. The average rate of change for smokers was 1.40%, while that for non-smokers was only 1.13% (Table 1).
There was no difference in the increasing trend of NAFLD prevalence by clinical center or community setting (P=0.491) or the quality of the studies used to generate the forecast data (P=0.85) (Supplementary Fig. 1).
In this study, we used past data and trends to forecast the prevalence of NAFLD by the year 2040. This method of forecasting was different than what those used and reported in recent studies, where investigators used Markov modeling, and we also performed the forecast based on published NAFLD prevalence data rather than surrogate data on obesity [13-17]. Although we are aware that the increasing burdens of obesity and diabetes are driving the increase in NAFLD, not all patients with NAFLD have diabetes or obesity [20-22]; therefore, our modeling methods took this information into consideration by using NAFLD historic data that includes trend data of patients with NAFLD regardless of the presence of obesity, rather than relying mainly on the trends of obesity. We found that if the current increase in NAFLD continues at the same pace, over half the adult population will have NAFLD by 2040.
The projection from the current study is much higher than previous estimates, which forecasted the prevalence of NAFLD to be only up 33% by 2030 [13-17]. In fact, our analysis found that the estimated NAFLD prevalence of 38.9% for 2020 was almost identical to two recent prevalence estimates of 37.3% and 37.6% from two recent meta-analyses [2,23], and all of these estimates were higher than the highest forecasted estimate of 33% reported in an earlier study [13-17]; therefore, our methodology appears robust. Also, considering the risk of adverse outcomes such as cirrhosis, liver transplantation, and mortality, the burden of NAFLD can become enormous if it continues to increase at our projected rate of 2.16% a year, unless aggressive action is taken to reverse this trend. In fact, NASH is now poised to become the leading indicator for liver transplantation in the next few years in addition to NASH HCC, which is already one of the leading indicators for liver transplantation among those with HCC [24,25].
The present study has several unique findings which may help policy makers in making decisions as to where interventions may be most warranted and to increase the awareness of NAFLD [26-29]. When we forecasted NAFLD prevalence by age, there was no difference the in prevalence rates in 2040 between those aged <50 years or Ōēź50 years. Both age groups were expected to reach prevalence rates of almost 50%, suggesting that NAFLD should no longer be considered as a disease that is only found in older individuals. In fact, a growing number of studies are showing that the prevalence of NAFLD is increasing in children as well [5,30,31], with the global NAFLD prevalence among children forecasted to reach 30.7% by 2040 [5]. Therefore, treatment interventions will need to be appropriate for all age groups, and early intervention at an early age would be crucial to bring down the prevalence rate among the adult population.
Our analysis by sex also provided interesting results. Both males and females were expected to have an increase in their 2040 NAFLD prevalence forecast, but the females were expected to have a higher rate increase. Although NAFLD is currently more prevalent in males, NASH has been found to be more prevalent in females [32,33]. In fact, NASH is the leading indicator for liver transplant among women [34]. These findings are worrisome, as they could suggest that females may be at higher risk for adverse outcomes in the coming years. Future research is needed to determine the significance of this finding.
We found those without metabolic syndrome will have a significantly higher rate of increase compared to those with metabolic syndrome. This finding may seem counterintuitive since NAFLD is a metabolic based liver disease, but as our understanding of NALFD increases, investigators have recently suggested that the main driver for NAFLD development is the presence of insulin resistance and the related liver lipotoxicity [34,35]. In addition, it appears that visceral obesity is a better indicator of the status of obesity rather than body mass index, and that waist circumference or abdominal adiposity correlated with the risk of mortality rather than metabolic syndrome itself [36].
In this study, there was no difference in NAFLD prevalence over time by country or region. All studied areas were expected to have increased prevalence by 2040. However, we did note that smokers were expected to have a significantly higher rate of NAFLD than those non-smokers in 2040. As such, Asia and Eastern Europe may need to watch this trend more closely, as they are known to have some of the highest rates of smoking, whereas smoking rate has been decreasing in the United States and other western nations [37,38].
The strength of the study is the use of Bayesian statistical modeling, large sample size, and widespread geographic coverage to forecast a more accurate assessment of NAFLD trends. Forecasting, however, does not address the impact of those trends nor do forecasting models take into consideration any changes in factors accounting for observed trends. For example, if the observed trajectory of the causative factors of NAFLD were to change, we would be unable to forecast this change. Moreover, although the advances in weight-reduction and antidiabetic medications may reduce the obesity and metabolic disease burden that may reduce the prevalence and incidence of NAFLD, these new and often expensive medications are likely not accessible to people in most regions of the world and many disadvantaged populations residing in high-income countries. At the same time, the current COVID-19 pandemic has caused severe disruption in access to healthcare and rising cost of living that may worsen the current obesity and metabolic disease epidemics, which can contribute to even higher NAFLD prevalence. In fact, population-based data from the United States have shown increased mortality during the COVID-19 pandemic as compared to the predicted level based on past trends for several chronic liver disease, including NAFLD, and especially in ethnic minorities [39,40]. However, the information provided in our analysis provides a worst-case scenario which may help policy makers in developing policies to raise awareness of NAFLD, while simultaneously providing direction and funding for reversing the upward trajectory for both obesity and type 2 diabetes mellitus. This is particularly relevant as NAFLD is increasingly being recognized as a metabolic liver disease with a proposed name change to metabolic fatty liver disease (MAFLD) [41], and therapeutic strategies should include not only liver specific agents, such as anti-fibrotics, but also systemic metabolic modifying agents, such as antidiabetic and antilipid medications [42,43].
In this forecasting study, we project that the global NAFLD prevalence in the year 2040 will be 55.2%, with the highest prevalence of over 60% forecasted for Asia and Europe. In addition, women, smokers, and those without metabolic syndrome are forecasted to have the largest increases in NAFLD prevalence. Given that approximately 20% of those with NAFLD can progress to more advanced disease and that the presence of NAFLD increases the risk of mortality, efforts may need to intensify to not only raise awareness of NAFLD but also to determine long-term solutions to the driving factors of NAFLD, such as obesity, insulin resistance, and type 2 diabetes mellitus.
FOOTNOTES
AuthorsŌĆÖ contributions
Study design: MHL, YHY, MHN; Data analysis: MHL, SB, BZ, YHY, MHN; Data collection: MHL; Drafting of manuscript: MHL, YHY, LH, MHN; Data interpretation, review and revision of manuscript: all authors.
Conflicts of Interest
MHN: Research funding: Pfizer, Enanta, Gilead, CurveBio, Exact Sciences, Helio Health, Glycotest, National Cancer Institute, B.K. Kee Foundation, Vir Biotech; Consulting: Gilead, Intercept, GSK, Exact Science, Novartis, Janssen, Bayer
RC: Research funding: Gilead, Siemens Healthineer
All other authors do not have any conflict of interest to declare.
SUPPLEMENTAL MATERIAL
Supplementary material is available at Clinical and Molecular Hepatology website (http://www.e-cmh.org).
Supplementary┬ĀFigure┬Ā1.
Sensitivity analyses of forecasted nonalcoholic fatty liver disease prevalence (A) by type of study setting and (B) study quality. P-values compare the rate of increase between subgroups via linear regression.
Figure┬Ā2.
Forecast of nonalcoholic fatty liver disease prevalence by (A) age and (B) sex. P-values compare the rate of increase between subgroups via linear regression.
Figure┬Ā3.
Forecast of nonalcoholic fatty liver disease (NAFLD) prevalence* for North America, Europe, and Asia. P-values compare the rate of increase between subgroups via linear regression. *Based on past trend of ultrasound diagnosed prevalence of NAFLD. North America subgroup included studies that used FLI to diagnose NAFLD. Data were insufficient to perform forecasting for Africa and South America.
Figure┬Ā4.
Forecast of nonalcoholic fatty liver disease prevalence by the presence of (A) metabolic syndrome or (B) cigarette smoking. P-values compare the rate of increase between subgroups via linear regression.
Table┬Ā1.
Annual rate of change in the prevalence of NAFLD between 2020 to 2040, overall and in subgroups
| Patient group | Annual rate of change in NAFLD prevalence (%) | 95% CI (%) |
|---|---|---|
| Overall | 2.16 | 1.60ŌĆō2.71 |
| Age (years) | ||
| ŌĆāŌēż50 | 2.44 | 1.57ŌĆō3.31 |
| ŌĆā>50 | 3.46 | 1.05ŌĆō3.46 |
| Sex | ||
| ŌĆāMale | 1.48 | 1.08ŌĆō1.89 |
| ŌĆāFemale | 2.52 | 1.56ŌĆō3.49 |
| Geographic location* | ||
| ŌĆāNorth America | 1.01 | 0.00ŌĆō3.02 |
| ŌĆāEurope | 2.19 | 0.12ŌĆō4.25 |
| ŌĆāAsia | 2.70 | 2.23ŌĆō3.17 |
| Metabolic syndrome | ||
| ŌĆāNo | 3.78 | 2.90ŌĆō4.66 |
| ŌĆāYes | 0.84 | 0.74ŌĆō0.94 |
| Cigarette smoking | ||
| ŌĆāNo | 1.13 | 0.00ŌĆō2.71 |
| ŌĆāYes | 1.40 | 0.83ŌĆō1.97 |
REFERENCES
1. Cusi K, Isaacs S, Barb D, Basu R, Caprio S, Garvey WT, et al. American Association of Clinical Endocrinology clinical practice guideline for the diagnosis and management of nonalcoholic fatty liver disease in primary care and endocrinology clinical settings: co-sponsored by the American Association for the Study of Liver Diseases (AASLD). Endocr Pract 2022;28:528-562.

2. Le MH, Yeo YH, Li X, Li J, Zou B, Wu Y, et al. 2019 global NAFLD prevalence: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2021 Dec 7;doi: 10.1016/j.cgh.2021.12.002.

3. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-metaanalytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73-84.


4. Golabi P, Paik JM, AlQahtani S, Younossi Y, Tuncer G, Younossi ZM. Burden of non-alcoholic fatty liver disease in Asia, the Middle East and North Africa: data from global burden of disease 2009-2019. J Hepatol 2021;75:795-809.


5. Li J, Ha A, Rui F, Zou B, Yang H, Xue Q, et al. Meta-analysis: global prevalence, trend and forecasting of non-alcoholic fatty liver disease in children and adolescents, 2000-2021. Aliment Pharmacol Ther 2022;56:396-406.



6. Younossi ZM, Henry L. Fatty liver through the ages: nonalcoholic steatohepatitis. Endocr Pract 2022;28:204-213.


7. Manne V, Handa P, Kowdley KV. Pathophysiology of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Clin Liver Dis 2018;22:23-37.


8. Younossi ZM, Blissett D, Blissett R, Henry L, Stepanova M, Younossi Y, et al. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology 2016;64:1577-1586.



9. Paik JM, Golabi P, Younossi Y, Saleh N, Nhyira A, Younossi ZM. The growing burden of disability related to chronic liver disease in the United States: data from the Global Burden of Disease Study 2007-2017. Hepatol Commun 2021;5:749-759.




10. Paik JM, Golabi P, Younossi Y, Srishord M, Mishra A, Younossi ZM. The growing burden of disability related to nonalcoholic fatty liver disease: data from the Global Burden of Disease 2007-2017. Hepatol Commun 2020;4:1769-1780.




11. Younossi ZM, Stepanova M, Noureddin M, Kowdley KV, Strasser SI, Kohli A, et al. Improvements of fibrosis and disease activity are associated with improvement of patient-reported outcomes in patients with advanced fibrosis due to nonalcoholic steatohepatitis. Hepatol Commun 2021;5:1201-1211.




12. Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 2018;15:11-20.



13. Estes C, Anstee QM, Arias-Loste MT, Bantel H, Bellentani S, Caballeria J, et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016-2030. J Hepatol 2018;69:896-904.


14. Alswat K, Aljumah AA, Sanai FM, Abaalkhail F, Alghamdi M, Al Hamoudi WK, et al. Nonalcoholic fatty liver disease burden - Saudi Arabia and United Arab Emirates, 2017-2030. Saudi J Gastroenterol 2018;24:211-219.



15. Goossens N, Bellentani S, Cerny A, Dufour JF, Jornayvaz FR, Mertens J, et al. Nonalcoholic fatty liver disease burden - Switzerland 2018-2030. Swiss Med Wkly 2019;149:w20152.


16. Swain MG, Ramji A, Patel K, Sebastiani G, Shaheen AA, Tam E, et al. Burden of nonalcoholic fatty liver disease in Canada, 2019-2030: a modelling study. CMAJ Open 2020;8:E429-E436.



17. Adams LA, Roberts SK, Strasser SI, Mahady SE, Powell E, Estes C, et al. Nonalcoholic fatty liver disease burden: Australia, 2019-2030. J Gastroenterol Hepatol 2020;35:1628-1635.




18. Wu Y, Zheng Q, Zou B, Yeo YH, Li X, Li J, et al. The epidemiology of NAFLD in Mainland China with analysis by adjusted gross regional domestic product: a meta-analysis. Hepatol Int 2020;14:259-269.



20. Ye Q, Zou B, Yeo YH, Li J, Huang DQ, Wu Y, et al. Global prevalence, incidence, and outcomes of non-obese or lean non-alcoholic fatty liver disease: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2020;5:739-752.


21. Golabi P, Paik J, Fukui N, Locklear CT, de Avilla L, Younossi ZM. Patients with lean nonalcoholic fatty liver disease are metabolically abnormal and have a higher risk for mortality. Clin Diabetes 2019;37:65-72.




22. Younossi ZM, Stepanova M, Negro F, Hallaji S, Younossi Y, Lam B, et al. Nonalcoholic fatty liver disease in lean individuals in the United States. Medicine (Baltimore) 2012;91:319-327.


23. Riazi K, Azhari H, Charette JH, Underwood FE, King JA, Afshar EE, et al. The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2022;7:851-861.


24. Golabi P, Bush H, Stepanova M, Locklear CT, Jacobson IM, Mishra A, et al. Liver transplantation (LT) for cryptogenic cirrhosis (CC) and nonalcoholic steatohepatitis (NASH) cirrhosis: data from the scientific registry of transplant recipients (SRTR): 1994 to 2016. Medicine (Baltimore) 2018;97:e11518.


25. Younossi ZM, Stepanova M, Ong J, Trimble G, AlQahtani S, Younossi I, et al. Nonalcoholic steatohepatitis is the most rapidly increasing indication for liver transplantation in the United States. Clin Gastroenterol Hepatol 2021;19:580-589 e5.


26. Le MH, Yeo YH, Cheung R, Wong VW, Nguyen MH. Ethnic influence on nonalcoholic fatty liver disease prevalence and lack of disease awareness in the United States, 2011-2016. J Intern Med 2020;287:711-722.



27. Younossi Z, Stepanova M, Ong JP, Jacobson IM, Bugianesi E, Duseja A, et al. Nonalcoholic steatohepatitis is the fastest growing cause of hepatocellular carcinoma in liver transplant candidates. Clin Gastroenterol Hepatol 2019;17:748-755 e3.


28. Younossi ZM, Ong JP, Takahashi H, Yilmaz Y, Eguc Hi Y, El Kassas M, et al. A global survey of physicians knowledge about nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2022;20:e1456-e1468.


29. Alqahtani SA, Paik JM, Biswas R, Arshad T, Henry L, Younossi ZM. Poor awareness of liver disease among adults with NAFLD in the United States. Hepatol Commun 2021;5:1833-1847.




30. Bush H, Golabi P, Younossi ZM. Pediatric non-alcoholic fatty liver disease. Children (Basel) 2017;4:48.



31. Arshad T, Paik JM, Biswas R, Alqahtani SA, Henry L, Younossi ZM. Nonalcoholic fatty liver disease prevalence trends among adolescents and young adults in the United States, 2007-2016. Hepatol Commun 2021;5:1676-1688.




32. Arshad T, Golabi P, Paik J, Mishra A, Younossi ZM. Prevalence of nonalcoholic fatty liver disease in the female population. Hepatol Commun 2018;3:74-83.




33. Wang J, Wu AH, Stanczyk FZ, Porcel J, Noureddin M, Terrault NA, et al. Associations between reproductive and hormone-related factors and risk of nonalcoholic fatty liver disease in a multiethnic population. Clin Gastroenterol Hepatol 2021;19:1258-1266 e1.



34. Noureddin M, Vipani A, Bresee C, Todo T, Kim IK, Alkhouri N, et al. NASH leading cause of liver transplant in women: updated analysis of indications for liver transplant and ethnic and gender variances. Am J Gastroenterol 2018;113:1649-1659.




35. Stefan N, Cusi K. A global view of the interplay between nonalcoholic fatty liver disease and diabetes. Lancet Diabetes Endocrinol 2022;10:284-296.


36. Khan RS, Bril F, Cusi K, Newsome PN. Modulation of insulin resistance in nonalcoholic fatty liver disease. Hepatology 2019;70:711-724.



37. Golabi P, Paik JM, Arshad T, Younossi Y, Mishra A, Younossi ZM. Mortality of NAFLD according to the body composition and presence of metabolic abnormalities. Hepatol Commun 2020;4:1136-1148.




38. World Health Organization (WHO). Current smokers. WHO web site. <http://gamapserver.who.int/gho/interactive_charts/tobacco/use/atlas.html>. Accessed 31 Jul 2022.
39. Gao X, Lv F, He X, Zhao Y, Liu Y, Zu J, et al. Impact of the COVID-19 pandemic on liver disease-related mortality rates in the United States. J Hepatol 2022 Aug 18;doi: 10.1016/j.jhep.2022.07.028.



40. Yeo YH, He X, Lv F, Zhao Y, Liu Y, Yang JD, et al. Trends of cirrhosis-related mortality in the United States during the COVID-19 pandemic: a cohort study. J Clin Trans Hepatol 2022;Accepted.
41. Ng CH, Huang DQ, Nguyen MH. Nonalcoholic fatty liver disease versus metabolic-associated fatty liver disease: prevalence, outcomes and implications of a change in name. Clin Mol Hepatol 2022;28:790-801.
- TOOLS




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Supplement
Supplement Print
Print





