Screening strategy for non-alcoholic fatty liver disease
Article information
Abstract
Non-alcoholic fatty liver disease (NAFLD) is the most common chronic liver disease, affecting approximately 25% of the general population worldwide, and is forecasted to increase global health burden in the 21st century. With the advancement of non-invasive tests for assessing and monitoring of steatosis and fibrosis, NAFLD screening is now feasible, and is increasingly highlighted in international guidelines related to hepatology, endocrinology, and pediatrics. Identifying high-risk populations (e.g., diabetes mellitus, obesity, metabolic syndrome) based on risk factors and metabolic characteristics for non-invasive screening is crucial and may aid in designing screening strategies to be more precise and effective. Many screening modalities are currently available, from serum-based methods to ultrasonography, transient elastography, and magnetic resonance imaging, although the diagnostic performance, cost, and accessibility of different methods may impact the actual implementation. A two-step assessment with serum-based fibrosis-4 index followed by imaging test vibration-controlled transient elastography can be an option to stratify the risk of liverrelated complications in NAFLD. There is a need for fibrosis surveillance, as well as investigating the cost-effectiveness of different screening algorithms and engaging primary care for first-stage triage screening.
INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD) is the most common chronic liver disease that places an increasing burden on global health in the 21st century, and is known to affect approximately 25% of the general population worldwide [1]. NAFLD includes two pathologically distinct conditions: non-alcoholic fatty liver and non-alcoholic steatohepatitis (NASH); the latter covers a wide spectrum of disease severity, including inflammation, hepatocyte injury (hepatocellular ballooning), and fibrosis at different stages [2,3]. Without appropriate management, it can progress to cirrhosis and liver-related complications, including hepatocellular carcinoma (HCC) and liver failure [4]. Compared to the general population, individuals with NAFLD have an increased risk of overall mortality, with common causes of death, besides liver-related ones, being cardiovascular disease and malignancy [5-8]. A modelling study forecasted the total NAFLD population of eight major countries to increase by 18.3% from 2016 onward, reaching a prevalence of 28.4% by 2030 [9]. Most individuals with NAFLD remain undiagnosed and, worryingly, the prevalence of advanced fibrosis and cirrhosis is projected to double by 2030 [9]. Despite the high population prevalence of NAFLD, recognition and management of the condition varies, with improvements still required in investigations at the primary care level and in the staging of fibrosis [10].
The need for NAFLD screening in the community has been questioned given the high associated direct and indirect costs, the low predictive value of non-invasive tests, the risks of liver biopsy, and the lack of effective treatment for NAFLD [11]. However, the progressive form of NAFLD (i.e., NASH), particularly when associated with advanced fibrosis, should be identified in patients at risk (age >50 years, type 2 diabetes mellitus or metabolic syndrome) [12], due to its prognostic implications. Although familial clustering occurs, based on current evidence, family screening is not generally advisable [12]. There is also a lack of validated cost-utility studies on the effectiveness of screening.
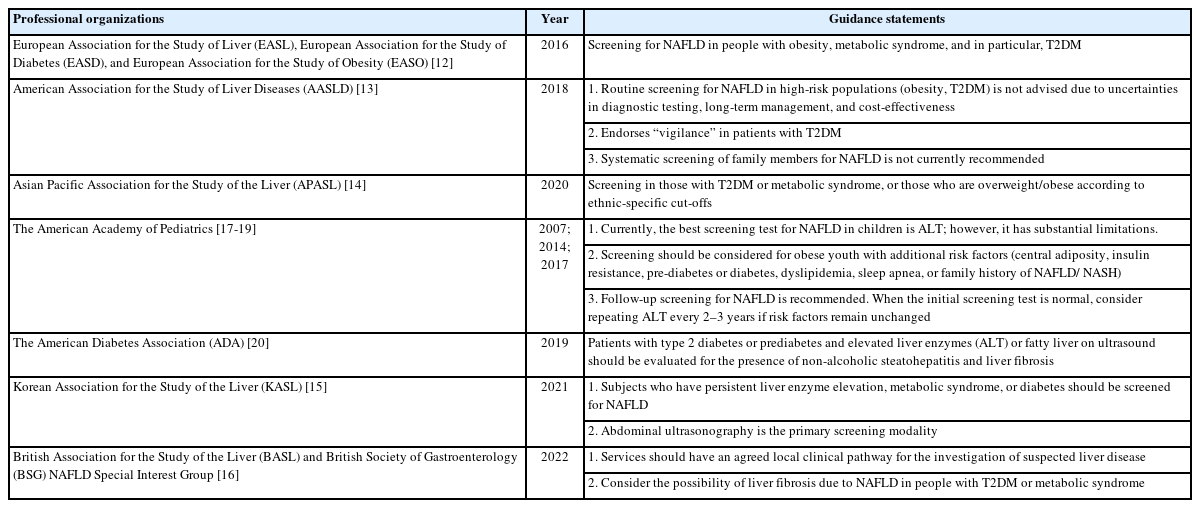
Currently, there is no consensus on the recommended population requiring screening for NAFLD. The American Association for the Study of Liver Diseases (AASLD) recommends against routine screening in any population, regardless of body mass index (BMI) [13], but also endorses “vigilance” in patients with type 2 diabetes mellitus (T2DM). The guidelines issued by the European Association for the Study of Liver (EASL), European Association for the Study of Diabetes (EASD), and European Association for the Study of Obesity (EASO) recommend screening in individuals with obesity or metabolic syndrome [12]; the recommendations from the Asian Pacific Association for the Study of the Liver (APASL) [14] and the Korean Association for the Study of the Liver (KASL) [15] are similar. There are also variations in the recommendations from British [16], diabetic and pediatric professional associations (Table 1) [17-20].
In this review, we aimed to highlight the high-risk populations in which NAFLD screening may prove beneficial, summarize recent non-invasive tests for the screening for NAFLD, and discuss the importance of fibrosis surveillance.
SCREENING FOR NAFLD IN HIGH-RISK POPULATIONS: A PROMISING STRATEGY TO MITIGATE THE FUTURE BURDEN OF LIVER DISEASE
Screening should ideally be performed via an organized program that has the capacity to identify target populations, and perform thorough evaluation, monitoring, and treatment [21]. Screening should preferably be the main purpose of the program; if risk factors of NAFLD require management, patients should be referred to appropriate healthcare providers (Table 2).
DIABETES MELLITUS
NAFLD is found in 50–60% of T2DM patients and up to 45% of type 1 diabetes mellitus (T1DM) patients [22], which raises an important question: Should we screen for NAFLD in the diabetic population?
Disease progression is more aggressive in T2DM patients with underlying hepatic necroinflammation and fibrosis. Mechanistically, lipotoxicity-induced mitochondrial dysfunction and activation of inflammatory pathways, rather than steatosis, cause progressive liver damage [23]. Among patients with T2DM, NASH is a leading cause of end-stage liver disease and a risk factor for cardiovascular disease [24]. Similar to diabetic retinopathy and nephropathy, NASH is increasingly being recognized as a complication of T2DM [25], which may imply the condition should be considered for incorporation into diabetic complication screening programs. Since T2DM patients are at high risk of developing NASH, concomitant NAFLD can be present even when liver transaminases are normal [26].
Several studies have reported the results of screening for liver fibrosis in the general population or individuals with T2DM using non-invasive methods (mainly by transient elastography). A population-based study from Hong Kong [27] investigated liver fat and fibrosis using proton-magnetic resonance spectroscopy (1H-MRS) and transient elastography in 922 healthy individuals recruited by random selection. The prevalence of NAFLD (defined by an intrahepatic triglyceride content >5%) was 27.3%, and the prevalence of advanced fibrosis (liver stiffness >9.6 kPa) was 3.7%. In another study involving 1,918 T2DM patients [28], the prevalence of increased liver stiffness (>9.6 kPa, suggestive of stage ≥F3) was 18%. Among approximately one-third of patients who underwent a liver biopsy, 56% had steatohepatitis, 21% had advanced fibrosis, and 29% had cirrhosis. A prospective study demonstrated the feasibility of using two accurate, precise, and validated non-invasive image-based biomarkers: magnetic resonance imaging-estimated proton density fat fraction (MRI-PDFF) to quantify liver fat, and magnetic resonance elastography (MRE) to detect advanced fibrosis in T2DM patients in a primary care setting [29], with a 65% prevalence of NAFLD and a 7.1% prevalence of advanced fibrosis found in the study population.
Altogether, these results confirmed the increased prevalence of advanced fibrosis among individuals with T2DM, thereby justifying the potential benefits of screening for NAFLD among T2DM patients, although the use of magnetic resonance (MR)-based technologies would raise issues related to cost and accessibility.
OBESITY AND THE ENTITY OF LEAN NAFLD
It has been well-documented that obesity is associated with an increased risk of NAFLD. Increased BMI and waist circumference, a measure of visceral adiposity, are positively related to the presence of NAFLD [30] and predict advanced disease, particularly in the elderly [31]. Common obesity comorbidities, such as sleep apnea [32], also contribute to the disease burden of NAFLD. The majority (>95%) of patients with morbid obesity undergoing bariatric surgery would have underlying NAFLD [33,34], of which the prevalence of advanced fibrosis is estimated at 10% [35]. Since obesity can limit successful liver stiffness measurements, the XL probe (lower ultrasound frequency of 2.5 MHz; can reach deeper liver tissue 35–75 mm from the skin surface) has been shown to be effective in liver stiffness measurement in obese patients with increased success rates of measurements, compared to the standard M probe [36,37].
In addition, patients with BMI <25 kg/m2 but with visceral fat accumulation or dysfunctional adipose tissue can exhibit NAFLD with or without elevation in liver aminotransferases [38,39]; these individuals are usually described as “lean NAFLD.” The populations of lean NAFLD vary worldwide, comprising 17.3% of the NAFLD cohort in the United States [40], but with higher proportions of 50% and 75% in Japan [41] and India, respectively [42]. However, the concept of lean NAFLD is somewhat misleading and simplistic, as it draws a line at 25 kg/m2 (or 23 kg/m2 for Asian people). The definition of “lean” is based on BMI, but it does not consider how the weight is distributed in the body (fat vs. muscle, intra-abdominal fat vs. subcutaneous fat). Thus, lean NAFLD refers to the presence of NAFLD in lean people who often have some abdominal fat accumulation or other subtle metabolic abnormalities [43]. Caucasian lean subjects with NAFLD represent a wide spectrum of NAFLD, which can develop into advanced liver disease, metabolic comorbidities, cardiovascular disease, as well as liver-related mortality [43]. These findings illustrate the oversimplified concept of lean NAFLD.
The indications for screening of NAFLD in lean individuals are not well-defined; NAFLD may be easily missed since such patients do not fit the classic phenotype of obesity [44]. The fibrosis-4 (FIB-4) index and NAFLD fibrosis score (NFS), while well-validated, are generally more useful in excluding fibrosis than identifying it. A recent study found NFS and FIB-4 to be less accurate in discriminating the severity of disease in lean NAFLD patients [45]. Meanwhile, both non-obese and lean groups had substantial long-term liver and non-liver comorbidities. A retrospective study from 1999–2016 indicated that non-obese NAFLD individuals had higher 15-year cumulative all-cause mortality (51.7%) compared to obese NAFLD (27.2%) and non-NAFLD (20.7%) individuals in the United States [46]. These findings suggest that obesity should not be the sole criterion for NAFLD screening [47].
METABOLIC SYNDROME
A third condition in which screening may be considered is metabolic syndrome, which comprises multiple metabolic and cardiovascular risk factors, primarily increased waist circumference, and a mixed combination of dyslipidemia, hypertension, and diabetes/prediabetes [48]. NAFLD parallels the prevalence of metabolic syndrome and its components, which also increases the risk of advanced disease. The link between metabolic syndrome and NAFLD is complex and bidirectional. Evidence indicated that NAFLD diagnosed via ultrasonography was associated with an increased risk of incident metabolic syndrome with a pooled relative risk of 3.22 [49]. suggesting that a vicious cycle of worsening disease states is likely to exist.
A cohort study over a 6-year follow-up period has observed 3,913 new cases of NAFLD in 15,791 Han Chinese individuals, and the risk of incident NAFLD was markedly higher in those with metabolic syndrome [50]. The hazard ratios for incident NAFLD increased when three features of metabolic syndrome were present as compared to individuals who exhibited no metabolic syndrome components. Advanced fibrosis was observed in 10.4% of health checkup examinees by FIB-4 index and shear wave elastography in health checkup examinations [51]. Furthermore, metabolic syndrome with mild-to-moderate alcohol consumption was associated with advanced fibrosis [51].
The EASL-EASD-EASO Clinical Practice Guidelines 2016 indicated that all individuals with steatosis should be screened for features of metabolic syndrome, independent of liver enzymes [12]. For patients with newly-presenting metabolic syndrome, screening for NAFLD by liver enzymes and/or ultrasound should be routine [12]. Since all components of metabolic syndrome correlate with liver fat level, regardless of BMI, the presence of metabolic syndrome in any particular patient should prompt an assessment of the risk of NAFLD, and vice versa, the presence of NAFLD should prompt an examination of all components of metabolic syndrome. A thorough evaluation of each element of the metabolic syndrome is required as part of the metabolic workup.
METABOLIC DYSFUNCTION-ASSOCIATED FATTY LIVER DISEASE IN CONCOMITANT LIVER DISEASE
The diagnosis of NAFLD conventionally requires the exclusion of other chronic liver diseases, including excess alcohol use and viral hepatitis [13]. Steatosis of metabolic origin can occur in chronic hepatitis B, chronic hepatitis C, and alcoholic liver disease. In fact, the distinction between “alcoholic” and “non-alcoholic” may not be clear-cut, with overlap and heterogeneity between the two conditions. One example would be a high-alcohol-producing bacteria-Klebsiella pneumoniae, which resides in the gut microbiota of >60% Chinese NAFLD patients, and produces high levels of ethanol which accelerates the development of steatosis regardless of alcoholic intake [52].
In order to establish defined “positive” clinical criteria, an international panel of experts have detailed the rationale for an update of the nomenclature describing the liver disease associated with metabolic dysfunction, known as metabolic dysfunction-associated fatty liver disease (MAFLD) [53]. According to the recent international consensus statement, the diagnosis of MAFLD is based on the detection of liver steatosis combined with the coexistence of at least one of three positive criteria, which include overweight or obesity, T2DM, or clinical evidence of metabolic dysfunction, such as an increased waist circumference and an abnormal lipid or glycemic profile [54]. The diagnosis can be established irrespective of any presence of concomitant chronic liver disease. Concomitant MAFLD has been shown to be associated with adverse outcomes in both chronic hepatitis B virus (HBV) infection [55] and alcoholic liver disease [56]. Concomitant presence of diabetes, obesity, and metabolic screening should prompt screening, although it remains uncertain if screening may be beneficial for additional sub-groups.
AGE, SEX, AND ETHNICITY
An important risk factor for NAFLD development is increasing age, demonstrated by a NAFLD prevalence of over 50% in elderly Taiwanese (mean age: 70.3 years) [57], as well as over 60% of middle-aged (age >45 years) Southeast Asians [58]. Another important factor is sex, with NAFLD more common in men than in women, although NAFLD risk increases in women after menopause, suggesting that estrogen has a protective role [59]. Moreover, the impact of ethnicity cannot be ignored. As evidenced by a population‐based cohort in the United States, NAFLD prevalence differs significantly between ethnicities, being more common in non-Hispanic whites (28.4%) compared to Asian Americans (18.3%) [60]. Consistently, in another population study of 4,538 people, NAFLD prevalence was the lowest in non-Hispanic Blacks (18.0%) and Asians (18.1%), and the highest amongst Mexican Americans (48.4%). Within the NAFLD group, advanced fibrosis was the highest in non-Hispanic Blacks (28.5%) and the lowest amongst non-Hispanic Asians (2.7%) [61].
NAFLD is underdiagnosed in children due to a lack of recognition, screening, or appreciation of associated complications by healthcare providers. One study showed that less than one-third of children with obesity were screened for NAFLD through laboratory testing at clinic visits [62]. Children may not be recognized as being obese at clinic visits, and age-appropriate norms for BMI may go unacknowledged. Similar to adults, children with features of metabolic syndrome, such as obesity, hypertension, insulin resistance, and dyslipidemia, are at higher risk for NAFLD [63]. NAFLD may also be incidentally discovered in children while undergoing imaging. The 2017 North American Society of Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) guideline [16] recommends that screening for NAFLD should be considered for all obese youths starting at the age of 9–11 years with additional risk factors (central adiposity, insulin resistance, pre-diabetes or diabetes, dyslipidemia, sleep apnea, or family history of NAFLD/NASH) by alanine aminotransferase (ALT) levels, but recommends against using routine ultrasonography owing to low sensitivity. However, the 2018 AASLD guidance [13] has no recommendation regarding screening in children who are overweight and obese, due to a paucity of evidence.
GENETIC SUSCEPTIBILITY
Knowledge of the genetic component of NAFLD has grown exponentially, in part owing to genome-wide association studies and the advent of high-throughput omics technologies. Currently, at least five variants in different genes have been robustly associated with NAFLD [64], such as patatin-like phospholipase domain-containing protein 3 (PNPLA3), transmembrane 6 superfamily member 2 (TM6SF2), membrane bound O-acyltransferase domain-containing 7 (MBOAT7), glucokinase regulator (GCKR), and Hydroxysteroid 17-Beta Dehydrogenase 13 (HSD17B13). Carriers of the PNPLA3 I148M [65-67] and the TM6SF2 E167K variants [68,69] have a higher liver fat content and increased risk of NASH. Nevertheless, the incorporation of NAFLD genetic markers into routine clinical testing for the dynamic assessment of disease status and response to therapy has been protracted. While PNPLA3 I148M is the best-characterized genetic variant associated with NAFLD, its contribution to NAFLD heritability remains modest [70,71]. Accordingly, the EASL-EASD-EASO Clinical Practice Guidelines 2016 [12] do not recommend the testing of these genetic variants in routine clinical practice, although genotyping may be considered in selected patients and clinical studies.
FIRST-DEGREE FAMILY RELATIVES
The risk of undiagnosed liver disease in first-degree relatives of NAFLD patients has been of concern, particularly in those who have more advanced fibrosis. By using magnetic resonance elastography to quantify hepatic fibrosis in siblings, parents, and offspring of patients with NAFLD-cirrhosis [72], first-degree relatives of patients with NAFLD-cirrhosis have a 12 times higher risk of advanced fibrosis than healthy controls, even after adjustment for age, sex, ethnicity, BMI, and diabetes status, signifying that screening for advanced fibrosis in first-degree relatives of patients with NAFLD-cirrhosis can be beneficial. With that being said, both the 2016 EASL-EASD-EASO [12] and 2018 AASLD guidelines [13] stated that, until further evidence emerges, systematic screening of family members for NAFLD is not advisable currently.
SCREENING IN THE PRIMARY CARE SETTING
Primary care would be taking up the main bulk of identifying patients with diabetes, dyslipidemia, hypertension, and components of metabolic syndrome; and are the optimal providers to identify patients with NAFLD, make appropriate referrals to specialists, and arrange appropriate surveillance. Once patients develop advanced fibrosis, the risk of liver-related mortality is exponentially increased [73]. Therefore, the challenge for primary care providers is the early identification of high-risk patients for specialist referral.
A prospective cohort study was designed to assess 1,118 patients with incidental abnormal liver function tests in the primary care setting and found the incidence rate of NAFLD to be 26.4% [74]. However, the number of primary care patients with abnormal liver enzymes may underestimate the true underlying prevalence, given the poor association between liver enzyme derangement and the presence of NAFLD. In terms of identifying patients with advanced fibrosis using the Enhanced Liver Fibrosis (ELF) test, with the low population prevalence of advanced fibrosis in the primary care setting, the positive predictive value of non-invasive testing was similarly low [75]. The use of non-invasive blood tests (a two-step algorithm combining FIB-4 score and ELF) for liver fibrosis improves the detection of advanced fibrosis and cirrhosis while reducing unnecessary referrals in patients with NAFLD [76]. With that being said, in order to implement primary care as a first-stage triage screening, primary care physicians need to be aware of the asymptomatic presentation of most NAFLD patients and understand the differences between NAFLD and NASH [77].
MODALITIES OF SCREENING
Liver biopsy is essential for the diagnosis of NASH, and is the only procedure that reliably differentiates NAFL from NASH [78]. A histologically-based scoring system, NAFLD activity score (NAS) [79,80], was developed and validated to fulfill the diagnostic criteria for NASH and include the full spectrum of NAFLD. Recent accurate quantitative assessments of liver fibrosis based on liver biopsy, such as second harmonic generation/two-photon excitation fluorescence (SHG/TPEF) microscopy imaging [81], can improve the efficacy endpoint for fibrosis in NASH clinical trials and give a more precise method for NASH staging. According to the 2018 AASLD guideline [13], liver biopsy should be considered in patients with NAFLD who are at increased risk of steatohepatitis and advanced fibrosis. However, the risks of percutaneous liver biopsy, including bleeding, organ perforation, sepsis, and death, are also critical [82].
With the vast majority of NAFLD patients being stable and asymptomatic, performing liver biopsies on all patients is unfeasible and unethical for disease screening, diagnosis, or progression assessment. Non-invasive diagnostic methods using plasma samples, ultrasonography, liver elastography (including both transient and magnetic resonance) have been developed with good diagnostic performance for liver steatosis and fibrosis [83,84]. These methods have been widely used for early steatosis detection, disease severity assessment, identification of patients needing a liver biopsy for confirmatory diagnosis (e.g., after discrepant results) and for the assessment of fibrosis progression. While avoiding the risks associated with a liver biopsy, these non-invasive tools, with the possible exception of transient elastography, are also hampered by several limitations, including suboptimal sensitivity to evaluate the complete spectrum of NAFLD histological lesions and the lack of validity to be used for routine diagnosis (Table 3).
Several scoring systems have been established for further elucidation of the presence of NAFLD [85-93]. The FIB-4 index (calculated by four clinical variables: age, aspartate aminotransferase [AST], ALT, and platelet count) [94] and NFS (age, BMI, impaired fasting glucose and/or diabetes, AST, ALT, platelet count, and albumin) [95-97] have been recommended by the EASL-EASD-EASO guidelines [12] as part of the diagnostic algorithm for ruling out advanced fibrosis. Importantly, the NFS has been shown to predict liver decompensation and mortality in patients with NAFLD [95].
Conventional ultrasonography is the most common method for the qualitative assessment of hepatic steatosis due to its accessibility and low cost [98]. However, the ability to detect steatosis in patients with NASH is limited by the presence of advanced fibrosis [99]. Ultrasonography is useful at detecting moderate-to-severe steatosis with high diagnostic accuracy, with an area under the receiver operating characteristic curve (AUROC) of 0.93 [100], but is unable to discriminate between steatosis, fibrosis, inflammation, or NASH [101]. Furthermore, ultrasonography is also limited by both inter- and intra-observer reliability [102].
Vibration-controlled transient elastography (VCTE) is the most validated and commonly used elastography method worldwide [103]. VCTE measures the tissue elasticity, which is directly related to liver stiffness, and in turn, is related to the degree of fibrosis [104]. Besides liver stiffness assessment, controlled attenuation parameter (CAP) is obtained by VCTE to quantify the liver fat [105]. A CAP value ≥248 dB/m is the commonly used cut-off to define hepatic steatosis [106,107]. Mild (equivalent to number of affected hepatocytes: 5–33%), moderate (34–66%), and severe (>66%) steatosis are defined as CAP 248–267 dB/m, CAP 268–279 dB/m, and CAP ≥280 dB/m, respectively [106]. According to recently published cut-offs in a large multicenter study [108] and a meta-analysis [109], low risk of advanced fibrosis was defined as liver stiffness measurements <8.0 kPa, intermediate risk (8.0–12.0 kPa), and high risk >12.0 kPa.
MRI provides high specificity and sensitivity in detecting liver steatosis, especially MRI-PDFF. MRI-PDFF enables fat mapping of the entire liver, which is more accurate than CAP in detecting all grades of steatosis in NAFLD patients (AUROC 0.99) [110]. MRI-PDFF is usually used as a research tool and is not easily accessible in clinical practice due to the logistical complexities, lengthy scan time, and lack of required expertise at the majority of medical imaging centers [111]. Additionally, H-magnetic resonance spectroscopy (H-MRS) is a well-established and validated method of non-invasive liver fat quantification by directly measuring chemical composition of tissue [88]. H-MRS is highly accurate for even minimal amounts of steatosis [112], but its widespread application is also hampered by its cost and availability.
MRE enables non-invasive assessment of hepatic fibrosis, and is currently considered the most accurate non-invasive modality. MRE uses a modified phase-contrast method to image the propagation of the shear wave in the liver parenchyma for quantitatively assessing tissue stiffness [113,114]. A meta-analysis found that MRE detected fibrosis in NAFLD with a high level of accuracy (AUROC 0.86–0.91) for all stages [115]. This technique is more accurate than VCTE in detecting F2 fibrosis (AUROC 0.86–0.89 vs. AUROC 0.84) and F4 fibrosis (AUROC 0.88–0.97 vs. AUROC 0.95) [110,116]. However, its wider application is limited by cost, expertise, and availability. Currently, MRI-related techniques are unlikely to be applied as a first-line screening method in clinical practice.
Shear wave elastography (SWE) was developed based on the technological foundation of conventional ultrasonography. A potential advantage of SWE is the ability to perform measurements over a wider region of interest, thereby reducing sampling error [117]. Point shear wave elastography (pSWE) has similar advantages to VCTE in that the performance is better for severe fibrosis and cirrhosis than for the lower stages of fibrosis [88,117]. Unfortunately, pSWE does not allow for the assessment or quantification of steatosis. Values obtained with pSWE have a narrow range (0.5–4.4 m/s), which limits the definitions of cut-off values for discriminating different fibrosis stages, reducing its impact on management decisions [118]. There are no well-established cutoffs for pSWE in NAFLD patients.
In addition to the currently used screening modalities mentioned above, there are also various serum, metabolomic, stool, and device-based approaches (Table 4) that have potential for screening. Measuring the mean fluorescence intensity of perilipin-2 (PLIN2) or ras-related protein 14 (RAB14) in peripheral blood monocytes has been demonstrated to be an accurate liquid biopsy for NASH [119]; however, since it is detected by flow cytometry, its practicality for screening remains uncertain. Other promising markers, including serum thrombospondin-2 (TSP2) [120] and lipocalin-2 (LCN2) [121], lack validation and well-established cut-off values. Multi-spectral electrical impedance tomography (EIT) [122] is a self-administrative medical device for liver steatosis, but it is still in very early phases of development. Other methods with potential include metabolomic-based markers for fibrosis, ballooning and NASH [123-125], fecal-based bacterial signatures [126], and the 13C-methacetin breath test [127-129].
SURVEILLANCE AND FOLLOW-UP ARRANGEMENT
Most of the screening algorithms proposed to use these non-invasive assessments in a sequential algorithm [130,131]. A stepwise ultrasonography-FIB-4/NFS-VCTE strategy to screen for NAFLD is shown in Figure 1. First, ultrasonography is the preferred first-line diagnostic procedure for imaging of NAFLD. Fatty liver index (FLI), SteatoTest, and NAFLD liver fat score are acceptable alternatives for the diagnosis of steatosis if imaging tools are not available or feasible [12]. For fibrosis assessment, a non-invasive test with a single cut-off is performed in primary care or endocrinology units to exclude patients with a low risk of advanced fibrosis. FIB-4 or NFS are inexpensive, easy-to-perform tests for the exclusion of advanced fibrosis using a single cut-off (NFS <-1.455 and FIB-4 <1.30), and can be used as a first screening option for intermediate-to-high–risk patients. Both these tests may be influenced by age and should use a different cut-off for patients aged >65 years (NFS <0.12 and FIB-4 <2.0).
Once FIB-4 yields intermediate or high results, second-line VCTE can be used to improve the identification of advanced fibrosis, which has been shown to reduce the need for liver biopsy [131,132]. Patients can then undergo VCTE when advanced fibrosis cannot be excluded [133]. The cut-off for advanced fibrosis with VCTE is 8.0 kPa (M probe) or 6.2 kPa (XL probe) for the exclusion of advanced fibrosis. The XL probe is highly recommended in obese patients. Patients above the recommended thresholds should be referred to a hepatologist for subsequent management.
The optimal surveillance strategy for patients with NAFLD is undetermined. The variable risk of progression of both the hepatic disease and the underlying metabolic conditions, as well as the cost and workload for healthcare providers, need to be considered. According to the EASL-EASD-EASO algorithm [12], monitoring should include routine biochemistry, assessment of comorbidities, and non-invasive monitoring of fibrosis. NAFLD patients without worsening of metabolic risk factors, should be monitored at 2- to 3-year intervals. Patients with NASH and/or fibrosis should be monitored annually, and those with NASH cirrhosis at 6-month intervals. If indicated on a case-by-case basis, liver biopsy could be repeated after 5 years.
COST-EFFECTIVENESS OF SCREENING
The question of whether NAFLD screening should be undertaken is deeply influenced by cost-effectiveness. High direct and indirect costs could be a barrier to screening. The AASLD guidelines do not recommend population screening for NAFLD [13]. Screening for liver fibrosis by VCTE at primary care centers is a highly cost-effective intervention and leads to earlier identification of patients in European and Asian populations, better than by standard of care alongside or using serum biomarkers [134]. Whether a two-step screening program using serum biomarkers followed by VCTE is more cost-effective and cost-saving in population screening should be tested in future studies. Moreover, the use of non-invasive liver fibrosis tests (FIB-4, ELF, or VCTE) in primary care increases early detection of advanced liver fibrosis, reduces unnecessary referral of patients with mild disease, and is cost-efficient [135]. Adopting a two-tier approach improves resource utilization [135].
For high-risk populations, one study found screening for NASH in T2DM (age >50 years) by ultrasonography to lack cost-effectiveness; however, that may in part be related to the study’s design, with the outcome measures of HCC and liver transplantation not being considered [136]. More recent data have supported the cost-effectiveness of screening. A comprehensive cost-utility analysis indicated that screening for NAFLD in patients with T2DM in the United States using an algorithm-based approach, starting with ultrasound and liver biochemistry and followed by VCTE for fibrosis to detect those most likely to have advanced fibrosis, was more cost-effective than the status quo of no screening [137]. Moreover, screening at a younger age will increase cost-effectiveness. However, comparisons of the cost-effectiveness of screening for NAFLD in general populations versus high-risk populations are still required.
FIB-4 followed by either VCTE, MRE, or liver biopsy can be cost-effective strategies for identifying cirrhosis in populations in whom the prevalence of cirrhosis varies between 0.27% and 4% [138]. Based on the U.S. health system, the combination of FIB-4 and VCTE, was the most cost-effective and the least costly, followed by the combination of FIB-4 and MRE. FIB-4 and VCTE remained the most cost-effective strategy if the aim were to avoid liver biopsy. Again, these findings require validation in other healthcare jurisdictions.
CONCLUSIONS
To this end, identifying high-risk populations based on the risk factors and metabolic characteristics for non-invasive screening is crucial. Screening all populations is generally not advisable and is not cost-effective [136]. Despite variations in international guidelines regarding how and who to screen, patients with T2DM, metabolic syndrome or persistently elevated liver enzymes may benefit the most from screening (Fig. 1). Screening for NAFLD in these high-risk patients, starting with ultrasound and liver biochemistry, and followed by non-invasive testing for fibrosis to detect advanced liver fibrosis, is more cost-effective than not screening this population [137]. The increasing availability of novel non-invasive tools, including transient elastography and MRI-based methods, will accurately quantify the severity of NAFLD and may help in screening and monitoring disease outcomes. The stepwise FIB-4/NFS-VCTE algorithm has been developed to rule out patients with a low risk of advanced fibrosis.
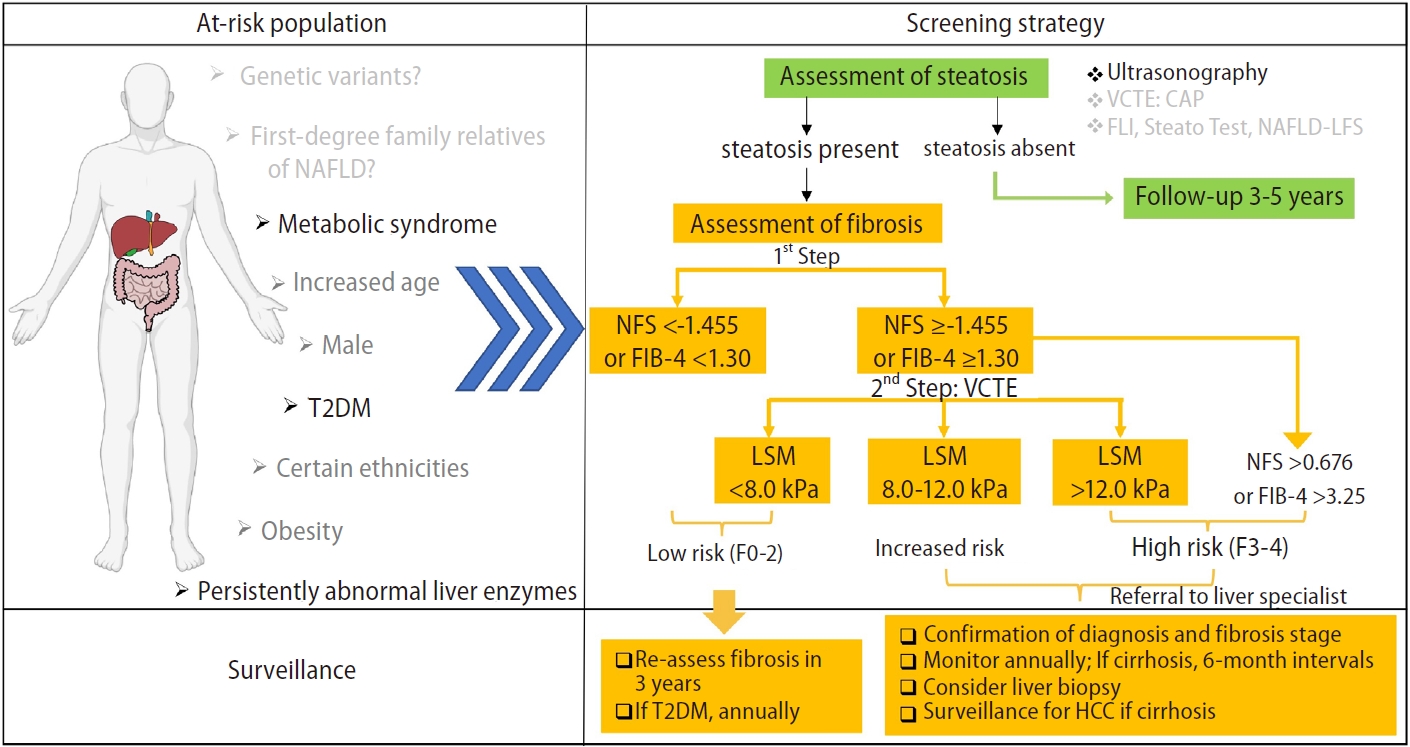
Diagnostic flow-chart to assess and monitor disease severity in the presence of suspected NAFLD. NFS threshold: -1.455 in patients aged <65 years, 0.12 in patients aged ≥65 years. FIB-4 threshold: 1.30 in patients aged <65 years, 2.0 in patients aged ≥65 years. CAP, controlled attenuation parameter; FIB-4, fibrosis-4 index; FLI, fatty liver index; HCC, hepatocellular carcinoma; NAFLD, nonalcoholic fatty liver disease; NFS, NAFLD fibrosis score; VCTE, vibration controlled transient elastography; T2DM, type 2 diabetes mellitus; LSM, liver stiffness measurement.
Regardless of screening strategies, patient participation will always be a key determinant of success. This is a social and behavioral challenge, as screening is a personal choice that is ideally based on informed decision-making. Increased patient participation [139] and physician awareness of the importance of screening will be crucial in reducing the morbidity and mortality related to NAFLD.
Notes
Authors’ contribution
S Zhang: Conceptualization, Literature Review, Writing and Original Draft Preparation; LY Mak: Review, Critical Revision; MF Yuen: Review, Critical revision, final approval of published version; WK Seto: Review, Critical revision, final approval of published version.
Conflicts of Interest
MF Yuen is an advisory board member and/or received research funding from AbbVie, Arbutus Biopharma, Assembly Biosciences, Bristol Myer Squibb, Dicerna Pharmaceuticals, GlaxoSmithKline, Gilead Sciences, Janssen, Merck Sharp and Dohme, Clear B Therapeutics, Springbank Pharmaceuticals; and received research funding from Arrowhead Pharmaceuticals, Fujirebio Incorporation and Sysmex Corporation. WK Seto received speaker’s fees from AstraZeneca and Mylan, is an advisory board member of CSL Behring, is an advisory board member and received speaker’s fees from AbbVie, and is an advisory board member, received speaker’s fees and researching funding from Gilead Sciences. No other authors have any conflict of interest to disclose.
Abbreviations
NAFLD
non-alcoholic fatty liver disease
NASH
nonalcoholic steatohepatitis
HCC
hepatocellular carcinoma
AASLD
American Association for the Study of Liver Diseases
BMI
body mass index
EASL
European Association for the Study of Liver
EASD
European Association for the Study of Diabetes
EASO
European Association for the Study of Obesity
APASL
Asian Pacific Association for the Study of the Liver
KASL
Korean Association for the Study of the Liver
T2DM
type 2 diabetes mellitus
T1DM
type 1 diabetes mellitus
1H-MRS
proton-magnetic resonance spectroscopy
MRI-PDFF
magnetic resonance imaging-estimated proton density fat fraction
MRE
magnetic resonance elastography
NFS
NAFLD fibrosis score
MAFLD
metabolic dysfunction-associated fatty liver disease
NASPGHAN
North American Society of Pediatric Gastroenterology
AST
aspartate aminotransferase
ALT
alanine aminotransferase
ELF
enhanced liver fibrosis
NAS
NAFLD activity score
SHG/TPEF
second harmonic generation/two-photon excitation fluorescence
VCTE
vibration-controlled transient elastography
CAP
controlled attenuation parameter
SWE
shear wave elastography
pSWE
point shear wave elastography
PLIN2
perilipin-2
RAB14
ras-related protein 14
TSP2
thrombospondin-2
LCN2
lipocalin-2
EIT
electrical impedance tomography
FLI
fatty liver index