Bariatric surgery for non-alcoholic fatty liver disease: Indications and post-operative management
Article information
Abstract
The prevalence of obesity and metabolic consequences such as nonalcoholic fatty liver diseases (NAFLD) has become a crucial health problem. Lifestyle modifications, especially weight loss, effectively reduces liver injury in NAFLD patients. However, adherence to lifestyle changes is very low in the clinical setting. Bariatric surgery can improve metabolic components and cause long-term weight loss. Therefore, bariatric surgery could serve as an attractive treatment option for NAFLD patients. This review integrates data about the benefits of bariatric surgery on NAFLD but also describes the potential pitfalls.
INTRODUCTION
The global prevalence of obesity has grown dramatically in the last 20 years and has become rapidly a public health issue [1]. The obesity epidemic led to a massive increase in cases of non-alcoholic fatty liver disease (NAFLD). NAFLD represents a spectrum of disease, consisting of non-alcoholic fatty liver (NAFL), nonalcoholic steatohepatitis (NASH), liver fibrosis, cirrhosis and eventually the development of hepatocellular carcinoma (HCC). Recently published data by Harrison et al showed a prevalence of NAFLD, NASH and significant fibrosis in asymptomatic middle-aged Americans of 38%, 14% and 6% respectively, with the highest prevalence in Hispanics (55%) and those with obesity (57%) and diabetes mellitus (70%) [2]. Also in Asia the prevalence is increasing with an estimation of NAFLD prevalence of 20–30% in Korea [3]. Recently published data by Lee et al. [4] showed that even in young Korean men in their early 20s, the NAFLD prevalence consistently increased from 2015 to 2021, respectively from 10.6% to 16.4%. Data from the European Liver Transplant Registry (ELTR) and United Network for Organ Sharing (UNOS) demonstrate that NASH cirrhosis and NAFLD-related HCC are the fastest growing indication for liver transplant in recent years [5,6].
Despite the increasing prevalence of NAFLD and NASH cirrhosis, there are still no Food and Drug Administration (FDA)-approved pharmacotherapies which halt progression in the spectrum of the disease and reduce liver-related complications in patients with NAFLD.
Lifestyle modification with reduced intake of calories combined with increased activity is still the cornerstone of NAFLD treatment. The main driver of NAFLD improvement is the amount of actual weight loss, while the type of diet seems to be less important. Prospective trials comparing various diets are lacking high-quality data. This is nicely summarized in a narrative review by Hydes et al. [7]. The authors concluded that the data only supports reducing saturated fat, refined carbohydrates and red and processed meats in the diet.
It has been shown that a weight reduction of at least 7–10% with conservative lifestyle modification is necessary to resolve NASH and to improve liver fibrosis [8,9]. This was clearly demonstrated in a prospective cohort study with paired liver biopsies in 261 patients. All patients who lost more than 10% of their weight had a 90% complete resolution of their NASH as well as an improvement of fibrosis in 45% [8]. We published data from a prospective study in children and adolescents admitted for severe obesity at a tertiary center (Zeepreventorium, De Haan, Belgium). NAFLD on ultrasound was present in 71.1% of these children. A total of 32.8% of patients had at least fibrosis grade 2, including 10.3% with transient elastography of 9 kPa or greater, compatible with significant fibrosis. All children and adolescents underwent intensive lifestyle therapy encompassing caloric restriction, physical activity, education on a healthy lifestyle, and psychosocial support. After 6 months, the median body weight loss was 16.0%. A significant improvement of steatosis was seen and more importantly, fibrosis improved in 75.0% of the study population (Fig. 1) [10].

Weight loss interventions for the treatment of NAFLD. (A) Lifestyle intervention for pediatric NAFLD. NAFLD was assessed at baseline and after 6 months in 167 patients. Evidence of liver fibrosis was present in 56 patients. After treatment, fibrosis improved in 75% of patients. Figure adapted from the article of Lefere et al. (Clin Gastroenterol Hepatol 2022;20:2317-2326.e4). [10] (B) Bariatric surgery for NAFLD. In a metaanalysis of studies comparing liver biopsy before and after bariatric surgery, complete resolution of fibrosis was observed in 40% of patients. [28] NAFLD, nonalcoholic fatty liver disease; BMI, body mass index; CAP, controlled attenuation parameter.
Although weight loss reduction works, only 5–10% of patients will achieve the target weight loss with structured lifestyle interventions at 1 year and fewer than half of these patients maintain the weight loss 5 years later [11]. Therefore, bariatric surgery could be a therapeutic approach in selected obese patients afflicted with NAFLD.
BARIATRIC SURGERY MECHANISMS
The history of weight loss surgery dates back to 1953 and innovation has continued for years thereafter [12]. A variety of procedures of BS have been developed. Techniques that rely predominantly on malabsorption by deriving digestive juices to the very distal part of the ileum (biliopancreatic diversion [BPD], duodenal switch) lead to large weight loss with severe long-term complications as a consequence, and extreme malabsorptive techniques such as the jejunoileal bypass are therefore abandoned. The most commonly applied techniques currently worldwide are the Roux-en-Y-gastric bypass (RYGB) and sleeve gastrectomy (SG). Very low mortality and morbidity rates are associated with these two procedures performed laparoscopically [13].
The mechanistic effects of BS are complex whereby weight loss due to malabsorption or restriction is not the only mode of action responsible for the potential effects on the liver. Alterations in gut hormone signaling, in bile acid levels and in adipose tissue (AT) inflammation will affect insulin signaling independently of weight loss. Acceleration of gastric emptying in SG and RYGB alters the enterohormonal balance, such as a markedly increased secretion of the gut peptides glucagon-like peptide (GLP-1) and peptide YY [14]. Both RYGB and SG increase the total circulating bile acid pool which play a role as metabolic signaling molecules [15]. BS also causes a reversal of the AT inflammation, and alters the endocrine functions of the AT, such as an increase of adiponectin and a decrease of serum leptin levels. All these physiological changes can contribute to the beneficial effects on the liver (Fig. 2) [16].
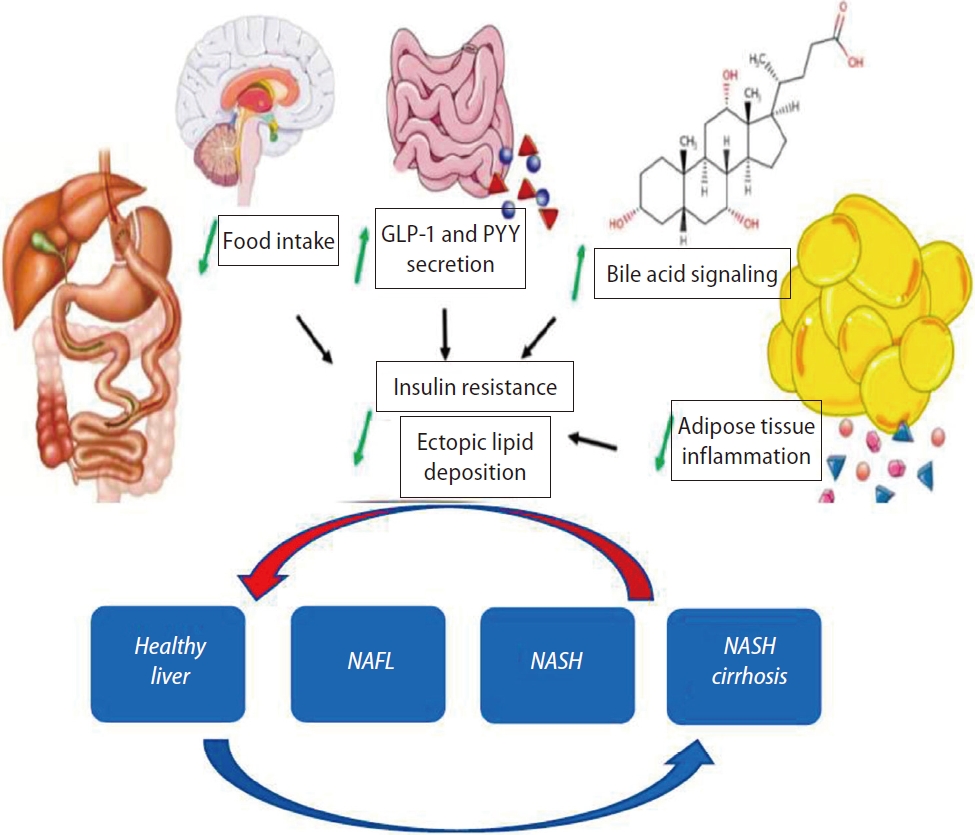
Mechanisms of resolution of non-alcoholic fatty liver disease (NAFLD) after bariatric surgery. Factors including regulation of food intake and food preferences, gut hormone secretion, bile acid signaling and visceral adiposity and adipose tissue inflammation. The potential for reversal of cirrhosis is still debated. GLP-1, glucagon-like peptide; PYY, peptide YY; NAFL, nonalcoholic fatty liver; NASH, nonalcoholic steatohepatitis.
BENEFITS OF BARIATRIC SURGERY IN NAFLD PATIENTS
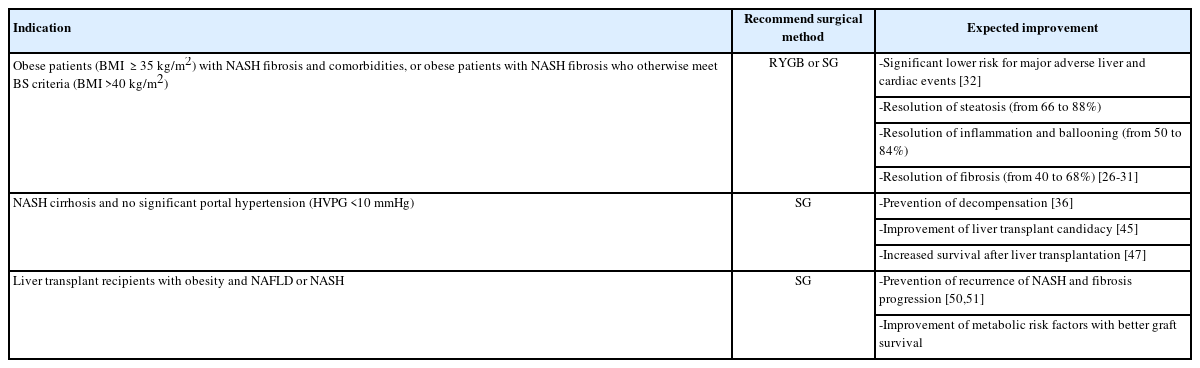
Bariatric surgery induces long-term excess weight loss up to 30% and remission of diabetes mellitus with reducing cardiovascular and cancer-related mortality, the two most frequent causes of death in patients with NASH [17-21]. Patients with obesity who meet the criteria for BS, namely body mass index (BMI) >40 kg/m2 or ≥35 kg/m2 and at least one or more obesity-related co-morbidities, frequently have features of NAFLD or NASH. Studies reported the presence of NAFLD and NASH in morbidly obese adults prior to weight loss surgery in 80.2% to 90% and 14.4%, respectively [22-25]. Despite the high prevalence of NAFLD/NASH in patients undergoing bariatric surgery, this co-morbidity is not consistently determined as an indication. Also screening for fatty liver disease is not routinely done in the preoperative period, nor screening to stage liver fibrosis by liver biopsy or non-invasive markers at the time of surgery.
In the absence of randomized controlled trials, several prospective and retrospective cohort studies and meta-analyses represent that sustained weight loss is associated with a reduction in steatosis, inflammation and fibrosis after BS (Fig. 1) [26-31]. In a recent meta-analysis, twenty-one studies (12 RYGB, 3 adjustable gastric banding [AGB], 2 SG, 1 vertical banded gastroplasty, 3 multiple procedures) enrolling 2,374 patients were included. The pooled proportion of patients who had improvement of steatosis was 88%, steatohepatitis improved in 59% and fibrosis improved or resolved in 30% of patients [30]. Another systematic review and meta-analysis of 32 cohort studies in obese patients, including comparison of 3,093 liver biopsy results before and after BS, confirmed resolution of steatosis in 66%, inflammation in 50%, ballooning degeneration in 76% and fibrosis in 40% of the patients [28]. These beneficial findings suggested in prior systematic reviews and meta-analyses are supported by a prospective long-term follow-up study with consecutive liver biopsies at 1 and 5 years after BS. One year after surgery, NASH resolved in 85% of patients [29]. Similar results were obtained after 5 years BS, indicating the durability of the response. Resolution of NASH without worsening of liver fibrosis was achieved in 84% of patients. Fibrosis regressed gradually and improved in 70% of patients compared to baseline fibrosis after 5 years. Importantly, in patients with advanced fibrosis (stage 3 fibrosis) at baseline, fibrosis improved in 68% and disappeared in 45% of patients at 5 years [31]. Limited weight loss and less improvement of insulin resistance following BS were associated with the persistence of NASH.
A recent retrospective cohort study conducted by Aminian et al. [32] was the first to demonstrate a significant lower risk for major adverse liver and cardiac outcomes in the bariatric surgery group compared with nonsurgical management in patients with biopsy-proven NASH and obesity. Specifically, the cumulative incidence (CI) of major liver outcomes at 10 years was 2.3% in the BS group versus 9.6% in the nonsurgical group. Regarding major adverse cardiac events, CI at 10 years was 8.5% in the bariatric surgery group and 15.7% in the nonsurgical group [32].
Besides preventing liver fibrosis is the development of NAFLD-related malignancies another aspect to consider. Retrospective cohort studies have shown that the adjusted CI of NAFLD-related malignancy (including HCC) is lower in patients who underwent BS vs. not [33]. Lastly the benefits of BS extend beyond the liver to affect diseases of other organ systems, specifically the risks of cardiovascular illnesses, stroke and renal failure [20].
During the last year, endoscopic bariatric therapies have become popular to treat obesity and metabolic conditions. Most of these techniques induce restrictive and metabolic effects. As most studies show that both RYGB and SG improve NAFLD with similar effects [34], endoscopic bariatric techniques could also serve as an option to induce weight loss. Data are currently limited because relatively small sample size in studies, but endoscopic bariatric therapies appear to be effective on NAFLD [35]. These techniques need to be further investigated in the field of fatty liver diseases. BS may be an effective treatment for obese patients (BMI ≥35 kg/m2) with NASH fibrosis or obese patients with NASH fibrosis who otherwise meet BS criteria (BMI >40 kg/m2).
BARIATRIC SURGERY IN CIRRHOSIS AND CONTEXT OF LIVER TRANSPLANTATION
Bariatric surgery in cirrhosis
Obesity is a strong predictor of decompensation in patients with compensated cirrhosis of various etiologies, independent of other predictors such as albumin or portal hypertension [36]. Increased mortality, poor survival after liver transplantation and increased risk of bacterial infections and sepsis related death are correlated with BMI levels >35 kg/m2. Weight loss should therefore be an important therapeutic goal also in patients with compensated cirrhosis.
Data from BS in cirrhotic patients are mostly coming from retrospective analyses of incidental findings at the time of surgery, with a prevalence between 0.5% and 1.5% [37]. In two US nationwide database studies, the in-hospital mortality rate after BS is slightly higher in patients with compensated cirrhosis versus those without cirrhosis (0.9% and 0.6% vs. 0.3% and 0.1%) and markedly increased in patients with decompensated cirrhosis (16.3% and 19.4%) [38,39]. Bariatric surgery is therefore absolutely contra-indicated in patients with decompensated cirrhosis.
The type of surgery is one of the criteria that should be considered in balancing the risks and benefits of BS in patients with compensated cirrhosis. A systematic review of the outcome of 122 patients with compensated cirrhosis undergoing BS showed that mortality related to BS was only observed in BPD and RYGB in 20 and 3.9% respectively. No mortality was observed with SG and AGB [40]. Stable liver function and no progression to liver dysfunction was observed in a small cohort of compensated cirrhotic patients with SG after 10-year follow-up [41]. Currently, a laparoscopic SG is the preferred procedure and seems feasible in compensated cirrhotic patients. Another advantage of SG is the gradual weight loss, absence of malabsorption, and the preservation of endoscopic access to the biliary tree.
A recently published AGA clinical practice guideline for BS in cirrhosis suggests that BS can be considered in selected patients with compensated cirrhosis (Child-Pugh A, model for end-stage liver disease [MELD] score <12) but should only be performed after careful evaluation and management of extrahepatic comorbidities, and after assessing the grade of portal hypertension. It is necessary to exclude those with a history of decompensated cirrhosis or those with significant portal hypertension which could be assessed by an upper endoscopy (presence of varices) or measurement of hepatic venous wedge pressure gradient (>10 mmHg) [42].
Portal hypertension is indeed another criteria to balance the risk of BS in cirrhotic patients. A recent study assessed the prognostic role of hepatic venous pressure gradient (HVPG) in cirrhotic patients undergoing elective extrahepatic surgery. The authors showed that HVPG of more than 16 mmHg is associated with a higher 1-year mortality and a very high risk of death (44%) was seen in the presence of HVPG >20 mmHg [43]. Whether this also applies specifically to BS requires further study, but it is reasonable to follow this guidance and severe portal hypertension (>16 mmHg) must be considered a contraindication for BS. Low-risk for surgery is seen in patients with HVPG less than 10 mmHg [44].
Bariatric surgery before and after liver transplantation (LT)
Treating obesity before liver transplantation can reduce the risk of decompensation on the waiting list and comorbidities, peri-operative and post-operative [45,46]. Takata et al. [45] showed improved LT candidacy in several patients as the BMI dropped. In a systematic review of five studies with the intention of improving LT candidacy, 78% of patients could be listed, and the rate of major and minor complications was 2% and 8%, respectively [47]. Also, in liver transplant candidates, the preferable type of surgery is SG as previously discussed in the section of BS and cirrhosis.
Long-term weight gain and the development of metabolic syndrome are the main concerns post-liver transplant. Recurrent NAFLD/NASH after transplantation is very common, ranging from 10 to 100% and 4 to 28% [48,49]. Probably, the outcomes of NASH cirrhosis liver transplant recipients are not as good as previously thought and this is due to the development of metabolic risk factors. It has been shown that NASH transplant recipients have a 10-year graft survival of 61%, which is significantly lower than other liver diseases [48]. Sleeve gastrectomy is the most performed procedure in this patient group, with the advantage of lack of malabsorption and no interference with immune suppressive drugs. Optimal timing of BS needs to be defined, because delaying too long can cause rapid fibrosis in the graft and reduce patient survival. Reported series described an interval from LT to BS ranging between 27 and 70 months [50,51].
Post-operative management
Monitoring liver function
Due to rapid weight loss during the first few months after surgery, hepatic damage can occur with increasing liver enzymes. Liver function is, however expected to return to normal within a year with a reduction in AST, ALT, and GGT levels already observed 6 months post-surgery [52]. Also, Nickel et al. [53] showed an increase in liver transaminases 1 month after surgery, but normalization within one year was observed. Contributing factors to the short-term elevation of enzymes are the rapid metabolic changes and slow adaptation of liver function after surgery.
Assessment of liver function after BS requires performing routine liver tests including bilirubin, transaminases, GGT, INR, and albumin at months 3, 6, and 12 and afterwards every 1–2 years, if normal findings at 12 months [44].
Strict follow-up of weight loss and supplementation of vitamins and trace elements should be performed even more carefully in patients with known liver disease to avoid further progression in those with pre-cirrhotic stages and to prevent decompensation in those with cirrhosis.
Ideally, the presence and severity of liver disease should be carefully assessed prior to BS. In the general NASH population, a liver stiffness measurement (LSM) cut-off of less than 8 kPa can reliably exclude advanced fibrosis and cirrhosis with a 94 to 100% negative predictive value [54]. Values above 12 to 15 kPa have a high positive predictive value (ranging from 80 to 90%) to detect advanced fibrosis or cirrhosis. Follow-up with noninvasive LSM measurements is not routinely done but data are present where a significant reduction in LSM could be observed in the majority of patients 6 months after surgery. Patients with an intraoperative diagnosis of significant fibrosis or cirrhosis should be referred to liver specialists for further evaluation because of the need for hepatocellular carcinoma screening and close monitoring to prevent episodes of decompensation.
Liver failure after bariatric surgery
BS procedures with a marked malabsorptive component, such as jejunoileal bypass or BPD, were proven to cause life-threatening complications including acute liver failure in up to 10% of patients, and should therefore be abandoned [55]. Liver failure following RYGB and SG is rarely reported. Mahawar et al. [56] reported 10 cases of liver failure after RYGB. Four out of the 10 reports were seen in cirrhotic patients, 2 had extended limb RYGB, 1 distal RYGB and 2 had early or late complications [56]. Extended limb or distal version of RYGB can behave like biliopancreatic diversion with higher potential for malabsorption.
The pathogenesis of liver failure after BS remains poorly understood. Potential contributing factors include rapid weight loss, which increases fatty acid delivery to the liver, and macro- and micronutrient malnutrition. Protein malnutrition plays a pivotal role in liver disease progression. The European practice guidelines on nutrition in chronic liver disease suggests that the optimal daily protein intake should not be lower than the recommended 1.2 to 1.5g/kg [57]. Liver transplantation needs to be considered if reversal of BS is not possible due to severe liver decompensation.
Alcohol use after bariatric surgery
Several studies have suggested that the incidence of alcohol consumption increases over the postoperative period of BS, predominantly in the second postoperative year, with a high prevalence, ranging from 12 to 20% [58,59]. Ibrahim et al. [59] reported an identical risk after RYGB and SG in the second year, although some cohorts described a lower prevalence in restrictive procedures such as SG. Additional studies are needed to clarify the importance of the type of surgery.
BS affects the pharmacokinetics of alcohol with higher peak alcohol concentrations and a greater feeling of drunkenness. Other potential mechanisms involved in post-bariatric alcohol use disorder (AUD) are still debated. Alterations in secretion of gut hormones like incretins and ghrelin, bile acid alterations, vagal nerve signaling, and changes in gut microbiota might impact the central nervous system processes and increase the sensitivity for alternative rewards such as alcohol [16].
We recently published single-center data showing that 6% of 188 patients transplanted for alcoholic liver disease between 2008 and 2018 had a history of BS. These patients were significantly younger and presented with more severe decompensated liver disease [60]. Similarly, a recent study reported that a history of BS is increasingly common in patients presenting with acute alcoholic hepatitis. Although BS patients were younger at presentation, survival was similar [61]. In a retrospective observational analysis of obese adults based on insurance claims, women had undergone BS had twofold increased risk of alcoholic cirrhosis and alcohol misuse compared to women without prior surgery [62].
BS patients should therefore be educated about the possible risks of alcohol use which can lead rapidly to the development of alcoholic cirrhosis, and surgeons should be reluctant to perform BS in patients with a history of AUD.
CONCLUSION
The major impact of NASH on the risk of cirrhosis and hepatocellular carcinoma highlights the urgent need for effective therapies to reverse the disease. Weight loss is the cornerstone in the treatment of NAFLD but difficult to reach and to keep long-term the target goals with only conservative lifestyle changes.
Obese patients with NASH fibrosis could benefit from BS. There is evidence that BS is safe, improves steatosis, inflammation and fibrosis score and reduces the risk for mortality from cardiovascular disease and NAFLD-associated HCC. Patients with cirrhosis need to be carefully selected by a multidisciplinary team of specialists to assess of the risk and the choice of type of surgery (Table 1).
Severe malnutrition related to excessive rapid weight loss after BS and de novo alcohol misuse are the most important contributors for the deterioration of liver function after BS. Prevention and early recognition of alcohol misuse pre- and post-surgery is a major unmet need.
Notes
Authors’ contribution
Conceptualization and writing: AG, SL.
Conflicts of Interest
The authors have no conflicts to disclose.
Acknowledgements
We cordially thank all members of our lab and collaborating scientists for all helpful discussions. AG is a senior clinical researcher of the Research Foundation Flanders (1805718N). SL is supported by a grant from the Research Foundation Flanders (12R0321N).
Abbreviations
NAFLD
non-alcoholic fatty liver disease
NASH
non-alcoholic steatohepatitis
HCC
hepatocellular carcinoma
ELTR
European Liver Transplant Registry
UNOS
United Network for Organ Sharing
BS
bariatric surgery
BPD
biliopancreatic diversion
RYGB
Roux-en-Y-gastric bypass
SG
Sleeve gastrectomy
AT
adipose tissue
EWL
excess weight loss
BMI
body mass index
AGB
adjustable gastric banding
CI
cumulative incidence
HVPG
hepatic venous pressure gradient
LT
liver transplantation
AUD
alcohol use disorder