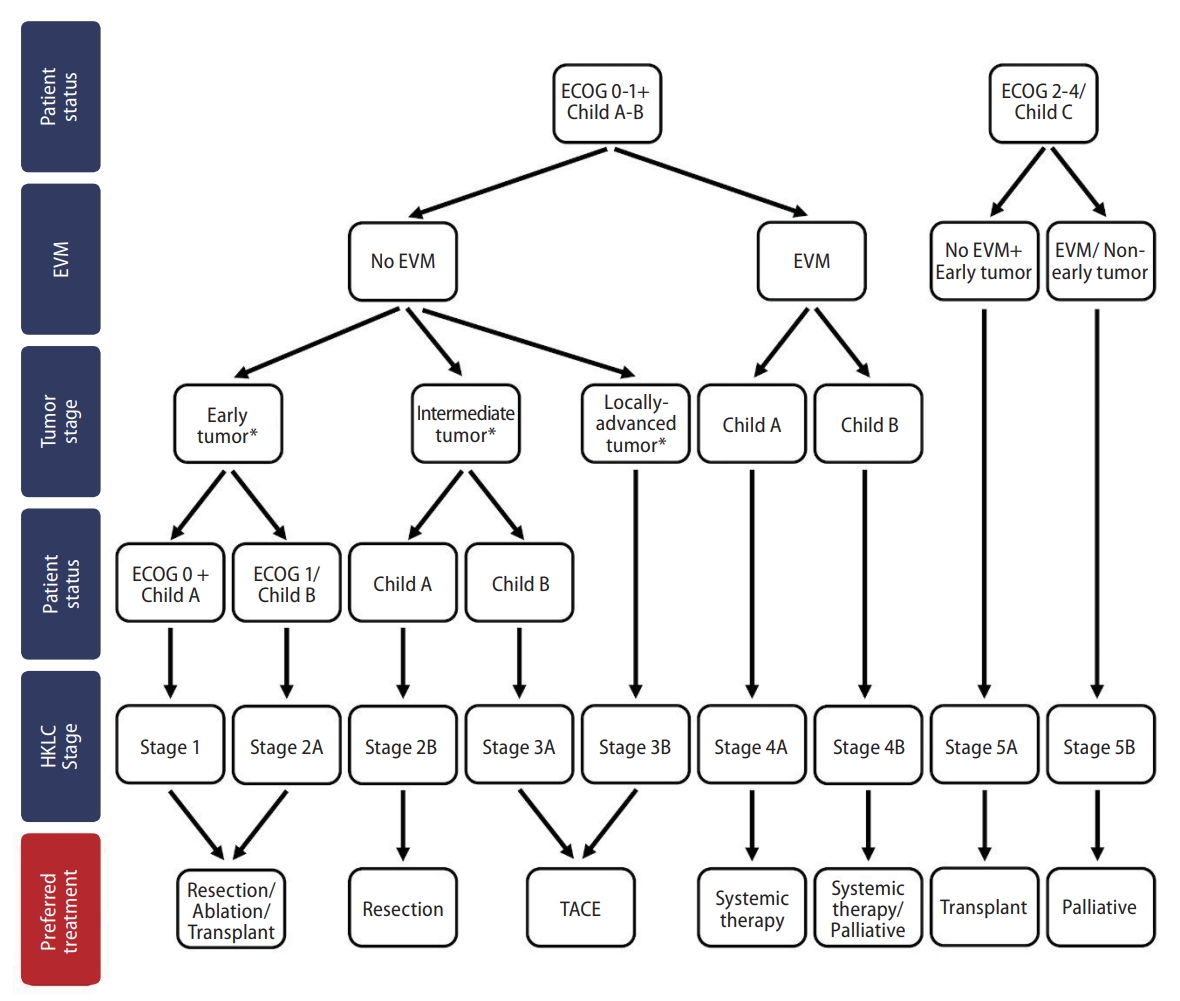
3. Yau T, Tang VY, Yao TJ, Fan ST, Lo CM, Poon RT. Development of Hong Kong Liver Cancer staging system with treatment stratification for patients with hepatocellular carcinoma. Gastroenterology 2014;146:1691-1700.e3.


4. Cancer Expert Working Group on Cancer Prevention and Screening - Center of Health Protection - The Government of the Hong Kong Special Administrative Region. Recommendations on Prevention and Screening for Liver Cancer for Health Professionals 2018. <
https://www.chp.gov.hk/files/pdf/liver_cancer_professional_hp.pdf>. Accessed 1 Dec 2022.
5. Cheung TT, Yu SCH, Chan SL, Poon RTP, Kwok P, Lee AS, et al. The Hong Kong consensus statements on unresectable hepatocellular carcinoma: narrative review and update for 2021. Hepatobiliary Surg Nutr 2022 Feb 10;doi: 10.21037/hbsn-21-405.

7. Yuen MF, Cheng CC, Lauder IJ, Lam SK, Ooi CG, Lai CL. Early detection of hepatocellular carcinoma increases the chance of treatment: Hong Kong experience. Hepatology 2000;31:330-335.


8. Seto WK, Chiu WHK, Cao W, Lui G, Zhou J, Cheng HM, et al. Training, validation and testing of a multiscale three-dimensional deep learning algorithm in accurately diagnosing hepatocellular carcinoma on computed tomography. J Hepatol 2022;77 Suppl 1:S78-S9.

11. Elsayes KM, Kielar AZ, Chernyak V, Morshid A, Furlan A, Masch WR, et al. LI-RADS: a conceptual and historical review from its beginning to its recent integration into AASLD clinical practice guidance. J Hepatocell Carcinoma 2019;6:49-69.


12. Cho C. Gadoxetic acidŌĆōenhanced magnetic resonance imaging and contrast-enhanced ultrasonography in the diagnosis of hepatocellular carcinoma. Hong Kong J Radiol 2017;20:175-191.
13. Cheung TT, Ho CL, Lo CM, Chen S, Chan SC, Chok KS, et al. 11C-acetate and 18F-FDG PET/CT for clinical staging and selection of patients with hepatocellular carcinoma for liver transplantation on the basis of Milan criteria: surgeonŌĆÖs perspective. J Nucl Med 2013;54:192-200.


14. Ho CL, Chen S, Yeung DW, Cheng TK. Dual-tracer PET/CT imaging in evaluation of metastatic hepatocellular carcinoma. J Nucl Med 2007;48:902-909.


16. Cheung TT, Ma KW, She WH, Dai WC, Tsang SHY, Chan ACY, et al. Pure laparoscopic versus open major hepatectomy for hepatocellular carcinoma with liver F4 cirrhosis without routine Pringle maneuver - A propensity analysis in a single center. Surg Oncol 2020;35:315-320.


19. Chiang CL, Chan MKH, Yeung CSY, Ho CHM, Lee FAS, Lee VWY, et al. Combined stereotactic body radiotherapy and transarterial chemoembolization as initial treatment in BCLC stage B-C hepatocellular carcinoma. Strahlenther Onkol 2019;195:254-264.


21. Chan A, Zhang WY, Chok K, Dai J, Ji R, Kwan C, et al. ALPPS versus portal vein embolization for hepatitis-related hepatocellular carcinoma: a changing paradigm in modulation of future liver remnant before major hepatectomy. Ann Surg 2021;273:957-965.

25. Chok KS, Yau TC, Cheung TT, Poon RT, Lo CM. Retrospective study of metachronous lung metastases from primary hepatocellular carcinoma. ANZ J Surg 2016;86:289-293.


27. Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology 2001;33:1394-1403.

28. Ng KK, Lo CM, Chan SC, Chok KS, Cheung TT, Fan ST. Liver transplantation for hepatocellular carcinoma: the Hong Kong experience. J Hepatobiliary Pancreat Sci 2010;17:548-554.


30. Lo CM, Fan ST, Liu CL, Wei WI, Lo RJ, Lai CL, et al. Adult-to-adult living donor liver transplantation using extended right lobe grafts. Ann Surg 1997;226:261-269 discussion 269-270.

31. Mok JHM, Ma KW, Chok KSH. Experience of living donor liver transplantation for hepatocellular carcinoma in the University of Hong Kong Hospital. Hepatoma Res 2022;8:6.

33. Chan SC, Fan ST, Lo CM, Liu CL, Wei WI, Chik BH, et al. A decade of right liver adult-to-adult living donor liver transplantation: the recipient mid-term outcomes. Ann Surg 2008;248:411-419.

40. Wong TC, Chiang CL, Lee AS, Lee VH, Yeung CS, Ho CH, et al. Better survival after stereotactic body radiation therapy following transarterial chemoembolization in nonresectable hepatocellular carcinoma: A propensity score matched analysis. Surg Oncol 2019;28:228-235.


41. She WH, Cheung TT, Yau TC, Chan AC, Chok KS, Chu FS, et al. Survival analysis of transarterial radioembolization with yttrium-90 for hepatocellular carcinoma patients with HBV infection. Hepatobiliary Surg Nutr 2014;3:185-193.


42. Tai YP, Cheung CH, Cheung KM, Cheng HC, Ngan RKC, Kwok PCH. Selective internal radiation therapy for hepatocellular carcinoma: experience from a Hospital in Hong Kong. Hong Kong J Radiol 2017;20:213-219.
43. Kelley RK, Rimassa L, Cheng AL, Kaseb A, Qin S, Zhu AX, et al. Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (COSMIC-312): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 2022;23:995-1008.


45. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al.; IMbrave150 Investigators. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med 2020;382:1894-1905.


46. Yau T, Park JW, Finn RS, Cheng AL, Mathurin P, Edeline J, et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol 2022;23:77-90.


47. Yau T, Kang YK, Kim TY, El-Khoueiry AB, Santoro A, Sangro B, et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: The CheckMate 040 randomized clinical trial. JAMA Oncol 2020;6:e204564. Erratum in: JAMA Oncol 2021;7:140.
48. Tsang J, Wong JSL, Kwok GGW, Li BCW, Leung R, Chiu J, et al. Nivolumab + Ipilimumab for patients with hepatocellular carcinoma previously treated with Sorafenib. Expert Rev Gastroenterol Hepatol 2021;15:589-598.


49. Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 2018;391:1163-1173.


51. Schoeb V. Healthcare service in Hong Kong and its challenges. The role of health professionals within a social model of health. China Perspectives 2016;4:51-58.






 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



