| Clin Mol Hepatol > Volume 29(2); 2023 > Article |
|
See the commentary-article "The imitator of immune-tolerant chronic hepatitis B: A killer in disguise" on page 363.
See the commentary-article "Is liver biopsy essential to identifying the immune tolerant phase of chronic hepatitis B?" on page 367.
ABSTRACT
Background/Aims
Methods
Results
Conclusions
ACKNOWLEDGMENTS
FOOTNOTES
Supplementary materials
Supplementary┬ĀTable┬Ā1.
Supplementary┬ĀFigure┬Ā1.
Supplementary┬ĀFigure┬Ā2.
Supplementary┬ĀFigure┬Ā3.
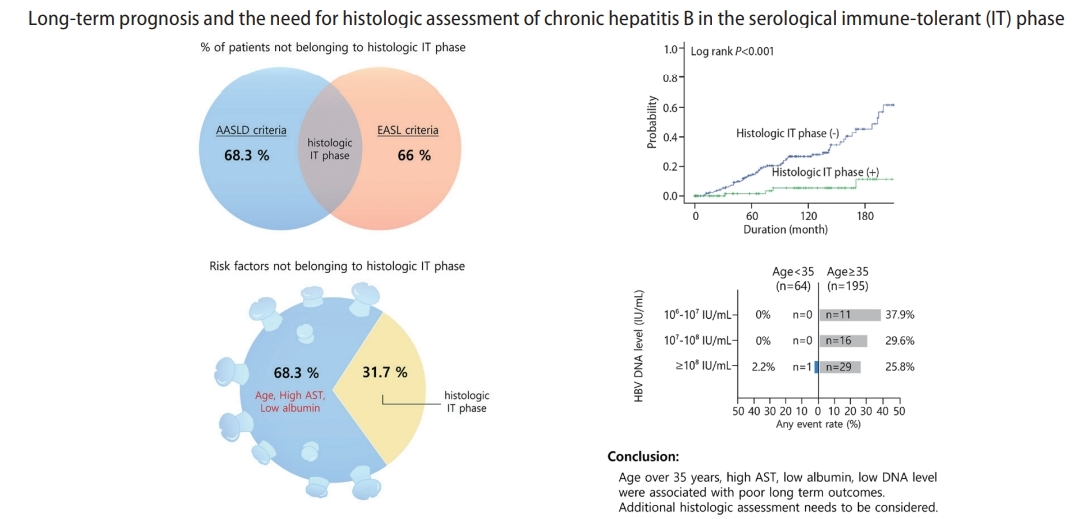
Figure┬Ā1.
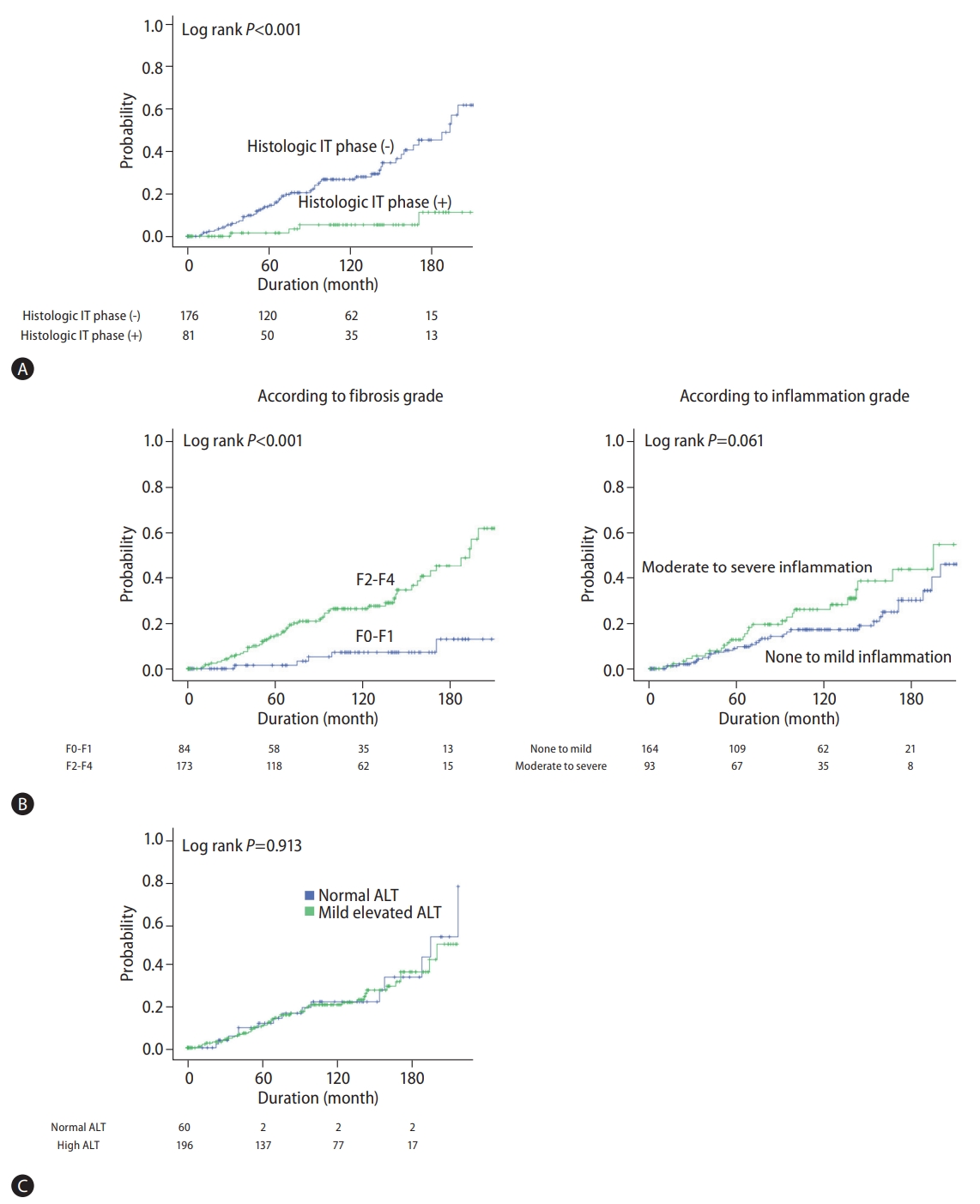
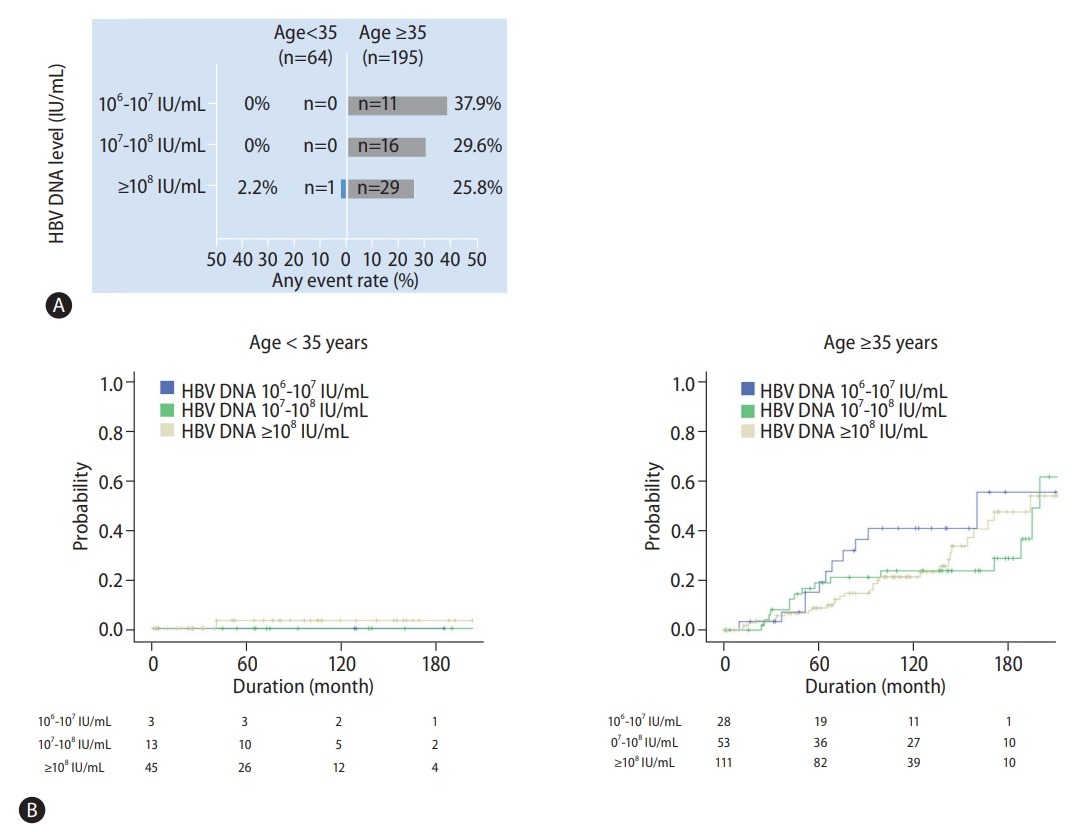
Figure┬Ā2.
Table┬Ā1.
Data are reported as mean┬▒standard deviation or median (interquartile range) for continuous variables and n (%) for categorical variables.
AST, aspartate aminotransferase; ALT, alanine aminotransferase; HBV, hepatitis B virus; IT, immune-tolerant; INR, international normalized ratio; FIB-4, fibrosis-4; APRI, AST to Platelet Ratio Index;
Table┬Ā2.
Table┬Ā3.
Table┬Ā4.
| Outcome: liver-related event* Variable |
Univariate |
Multivariate |
||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Sex | ||||
| ŌĆāMale | 1 (ref) | ŃĆĆ | ||
| ŌĆāFemale | 1.332 (0.775ŌĆō2.289) | 0.300 | ŃĆĆ | ŃĆĆ |
| Age | 1.088 (1.057ŌĆō1.120) | <0.001 | 1.077 (1.045ŌĆō1.110) | <0.001 |
| HBV DNA (IU/mL) | ||||
| ŌĆā106ŌĆō107 | 1.833 (0.917ŌĆō3.662) | 0.086 | ||
| ŌĆā107ŌĆō108 | 0.989 (0.530ŌĆō1.845) | 0.972 | ||
| ŌĆā>108 | 1 (ref) | |||
| Platelet | 0.999 (0.995ŌĆō1.002) | 0.429 | ||
| AST | 0.998 (0.982ŌĆō1.014) | 0.788 | ||
| ALT | 0.985 (0.968ŌĆō1.002) | 0.076 | ||
| Albumin | 0.766 (0.421ŌĆō1.394) | 0.383 | ||
| Total bilirubin | 1.177 (1.005ŌĆō1.379) | 0.044 | ||
| Histologic fibrosis | ŃĆĆ | ŃĆĆ | ŃĆĆ | ŃĆĆ |
| ŌĆāF0-F1 | 1 (ref) | 1 (ref) | ||
| ŌĆāF2-F4 | 5.478 (2.184ŌĆō13.737) | <0.001 | 3.650 (1.375ŌĆō9.694) | 0.009 |
| Histologic inflammation | ||||
| ŌĆāNo to minimal | 1 (ref) | 1 (ref) | ||
| ŌĆāModerate to severe | 1.641 (0.972ŌĆō2.769) | 0.064 | 0.966 (0.556ŌĆō1.679) | 0.904 |
Abbreviations
REFERENCES
- TOOLS
-
METRICS

- ORCID iDs
-
Yeon Seok Seo

https://orcid.org/0000-0003-4171-6331Sang Gyune Kim

https://orcid.org/0000-0001-8694-777X - Related articles
-
Unmet needs of chronic hepatitis C in the era of direct-acting antiviral therapy2020 July;26(3)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement1
Supplement1 Print
Print



