Utility of combining PIVKA-II and AFP in the surveillance and monitoring of hepatocellular carcinoma in the Asia-Pacific region
Article information
Abstract
Even though the combined use of ultrasound (US) and alpha-fetoprotein (AFP) is recommended for the surveillance of hepatocellular carcinoma (HCC), the utilization of AFP has its challenges, including accuracy dependent on its cut-off levels, degree of liver necroinflammation, and etiology of liver disease. Though various studies have demonstrated the utility of protein induced by vitamin K absence II (PIVKA-II) in surveillance, treatment monitoring, and predicting recurrence, it is still not recommended as a routine biomarker test. A panel of 17 experts from Asia-Pacific, gathered to discuss and reach a consensus on the clinical usefulness and value of PIVKA-II for the surveillance and treatment monitoring of HCC, based on six predetermined statements. The experts agreed that PIVKA-II was valuable in the detection of HCC in AFP-negative patients, and could potentially benefit detection of early HCC in combination with AFP. PIVKA-II is clinically useful for monitoring curative and intra-arterial locoregional treatments, outcomes, and recurrence, and could potentially predict microvascular invasion risk and facilitate patient selection for liver transplant. However, combining PIVKA-II with US and AFP for HCC surveillance, including small HCC, still requires more evidence, whilst its role in detecting AFP-negative HCC will potentially increase as more patients are treated for hepatitis-related HCC. PIVKA-II in combination with AFP and US has a clinical role in the Asia-Pacific region for surveillance. However, implementation of PIVKA-II in the region will have some challenges, such as requiring standardization of cut-off values, its cost-effectiveness and improving awareness among healthcare providers.
INTRODUCTION
With an estimated 60.0% increase by 2040 [1], hepatocellular carcinoma (HCC) remains a global disease burden [2,3]. An estimated 85.0% of HCC patients are in low- and middle-resource countries [3], with Asia carrying the largest burden of >20 cases per 100,000 population [2]. Beyond the exposure to risk factors, the incidence and mortality rates of HCC are closely associated with the availability of healthcare resources for detecting early-stage disease, and access to potential curative treatment [3].
The Asian Pacific Association for the Study of the Liver (APASL) guidelines [4] recommend biannual surveillance using a combination of ultrasound (US) and alpha-fetoprotein (AFP) in all high-risk individuals for the early detection of HCC, in order to improve the survival rate of HCC patients.
AFP has had an established role as a biomarker in HCC for decades [5]. It is a standardised test considered more objective than imaging alone, and is easily accessible [4,6]. Its optimal utility in surveillance is in combination with US, and is useful for confirming inconclusive imaging results [5]. However, even in combination with US, AFP has its challenges, including sensitivity and specificity, which are dependent on various factors. These include the cut-off levels used, the degree of necroinflammation of the liver, and the aetiology of the liver disease [4-7]. In addition, up to 80.0% of small HCC (tumour size ≤3 cm) [5,6] and early-stage HCC tumours [4] are not picked up by AFP. Protein induced by vitamin K absence II (PIVKA-II), also known as Des-γ-carboxy (abnormal) prothrombin (DCP), was first described in 1968. In 1984, it was detected in 90.0% of patients with HCC, suggesting that it could have a potential use as an HCC biomarker [8]. Though there are multiple studies on its utility in surveillance, treatment monitoring, and predicting recurrence of HCC, it is not yet recommended as a routine test [4].
The objective of this consensus paper is to discuss the clinical usefulness and value of PIVKA-II in the Asia-Pacific region for the surveillance and treatment monitoring of HCC, its benefits and limitations, and further steps required to improve its utility.
METHODS
A group of 17 experts in hepatology, surgical oncology, medical oncology, and laboratory medicine (Table 1) from countries across Asia-Pacific, was identified to develop this consensus statement that would define the clinical utility of PIVKA-II in the surveillance and treatment monitoring of HCC in the region. The experts convened via an online meeting to share the latest relevant available evidence on PIVKA-II, and to vote on predetermined statements (R1). The votes were to agree or disagree with each statement based on the evidence and the expert’s opinions. Statements that were disagreed on were discussed, and the experts’ points taken into consideration for refinement.
The second (R2) and third (R3) rounds of voting were conducted by emailing each expert the reworded statements presented with the same binary (agree/disagree) options and an open-ended remark column in case of disagreement. After each round, the comments of individual experts were considered and the statements edited further. The final statements, with their agreement, were used to develop the first draft. The agreement for each statement follows a 3-point scale of “inconclusive”, “agree with condition”, and “strongly agree”, based on the proportion of experts agreeing on it (Table 2).
A web review of the draft consensus statement was done to gather feedback from all experts. It included instructions for a final round of voting (R4) on the statements, and a review of the discussion and evidence presented for each statement. All comments were reviewed by the chairpersons, and the manuscript edited. A final round of review was performed by the experts before the manuscript was finalised.
FINDINGS
The final consensus statements, the agreement reached (Table 3), and a summary of the evidence for each, are presented here. For discussions on the implementation of PIVKA-II, the experts’ opinions are presented.
The role of PIVKA-II in HCC surveillance
Statement 1: PIVKA-II in combination with AFP improves the detection of HCC, including small sized tumours (≤3 cm), compared to either biomarker alone
Agreement: Strongly agree
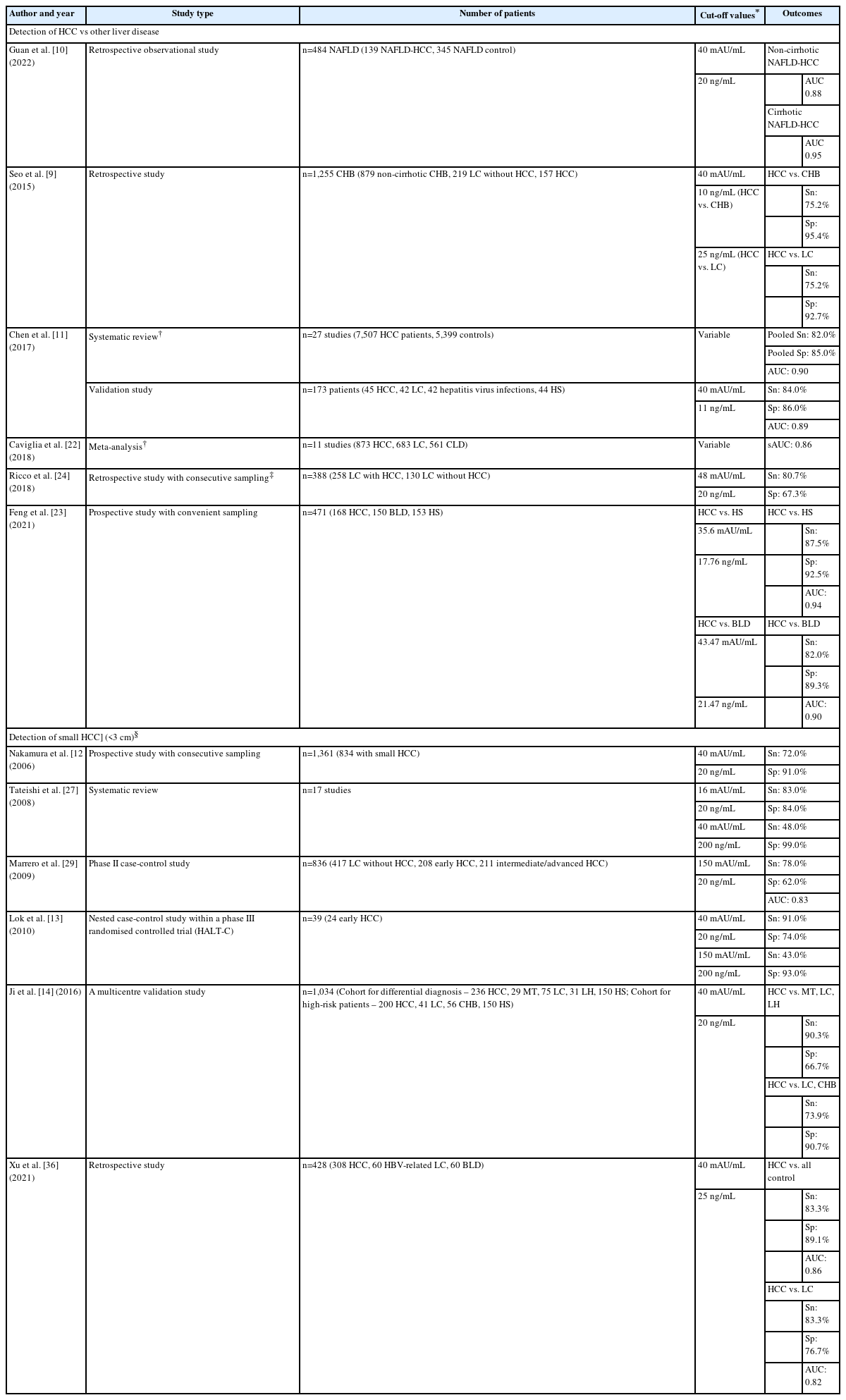
Although PVIKA-II alone has shown adequate accuracy in detecting HCC, combining the test with AFP results in better surveillance (Table 4) across the high-risk groups, as it combines their individual benefits. However, the variable accuracy of both tests, depending on the cut-off values used, must be considered when interpreting the results. The data from studies suggest that the optimal cut-off value for PIVKA-II when used in combination with AFP is 40 mAU/ml but further validation is required [9-14].
Among the studies conducted to determine the accuracy of PIVKA-II alone, AFP alone and combining both biomarkers, only a few meet the optimal level of evidence as described by Early Detection of Research Network (EDRN) [15] and the International Liver Cancer Association [16]. Lok et al. [13] compared the accuracy of AFP and PIVKA-II in the early diagnosis of HCC in a nested-control study within the Hepatitis C Antiviral Long-Term Treatment Against Cirrhosis (HALT-C) Trial [17]. The results demonstrated that combining both biomarkers increased the sensitivity but decreased the specificity of the individual biomarkers to detect early HCC. The sensitivity increased to 91.0% at the time of diagnosis and 73.0%, 12 months prior to diagnosis, and the specificity reduced to 74.0% and 71.0%, at the two time-points respectively [13]. The three other EDRN phase 3 biomarker studies [18-20] to determine accuracy of biomarkers for surveillance of early HCC included an additional biomarker, i.e., lectin-reactive AFP alone or within the GALAD (Gender, Age, AFP-L3, AFP and PIVKA-II), and are beyond the scope of this position paper.
A systematic review of 38 studies with 11,124 cases, revealed that PIVKA-II alone was only moderately accurate in detecting HCC (sensitivity 0.66, 95% confidence interval [CI] 0.65–0.68; specificity 0.88, 95% CI 0.87–0.90; positive likelihood ratio (+LR) 7.13, 95% CI 5.73–8.87; negative likelihood ratio (-LR) 0.33, 95% CI 0.29–0.38) [21].
On the other hand, a pooled analysis demonstrated that combining PIVKA-II and AFP improved sensitivity and specificity compared to either test alone (PIVKA-II+AFP, 82.0% and 85.0% vs. AFP alone, 65.0% and 88.0%, and PIVKA-II alone, 69.0% and 89.0%, respectively) [11]. The AUC also increased by combining both tests (PIVKA-II+AFP, 0.90 vs. AFP, 0.88 and PIVKA-II, 0.75, respectively). These findings were in line with other studies (Table 5) [9,22-24]. Similarly, real-world data demonstrated that PIVKA-II (cut-off value at 40 mAU/mL) is a necessary complement to AFP (cut-off value at 20 ng/mL) and US in surveillance [25].
However, there is a trade-off to be expected with increasing the sensitivity of the tests. Though meta-analyses seeking heterogeneity found that threshold levels do not impact the accuracy of the tests [11,26], higher cut-off values for either marker reduced sensitivity while improving specificity [13]. Additionally, the higher-level evidence studies [13,18] have shown that combination of biomarkers could increase sensitivity, however, could markedly decrease specificity. For surveillance, though specificity of the test has a role, an improved sensitivity is more pertinent so as to rule in cases.
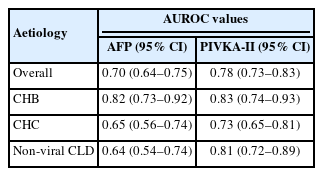
The performance of PIVKA-II and AFP also differ depending on the HCC aetiology [11,13,23,27] The accuracy of AFP and PIVKA-II when analysed in cirrhotic patients with chronic liver disease (n=388) demonstrated that both biomarkers’ performances were significantly influenced by the aetiology and activity of the chronic liver disease (Table 4) [24].
Utility of PIVKA-II and AFP in small HCC
The insidious nature of HCC means that by the time patients are diagnosed, most have very poor outcomes, even for 1-year survival [21,28]. However, it can be cured by surgical resection, orthotopic liver transplantation, or local ablation, if diagnosed early [28]. Small HCC, with nodules of <3 cm, indicates early HCC, and patients with tumours of ≤2 cm have a 5-year survival rate of close to 100% [21]. Hence, the early diagnosis of HCC is essential to improve outcomes for patients.
The recommended method of surveillance (US+AFP) can miss up to 1 in 3 patients with HCC [28], and adding PIVKA-II could improve the detection of early/small HCC. The accuracy of PIVKA-II and AFP levels alone in diagnosing small HCC is still inconclusive, i.e., results showing either one as being more accurate in terms of sensitivity, specificity, and/or AUC [12,26,29,30]. Combining both markers with cut-off levels maximised for sensitivity and specificity indicates an improvement in the detection of small HCC (Table 5). This suggests that combining AFP and PIVKA-II could be useful in picking up HCC where utilising either marker alone might not [12,13,27,29].
An important criterion for tests in HCC surveillance is their ability to differentiate between early HCC and other liver diseases like cirrhosis. At cut-off values of 40 mAU/mL for PIVKA-II and 20 ng/mL for AFP, Ji et al. [14] demonstrated that combining both markers improved sensitivity in differentiating small HCC from disease controls compared to either marker alone, but is dependent on the disease type (Table 5).
The challenge is that the cut-off values for each biomarker used in the combination is still inconclusive. In a systematic review of 17 studies, lower cut-off values of PIVKA-II and AFP appear to have had a better overall accuracy than higher cut-off values [27]. However, the diagnostic odds ratio for the higher cut-off values was 2.4 times better than the lower values(59.8 vs. 25.5, respectively). From the analyses, the authors concluded that the optimal cut-off value was 40 mAU/mL for PIVKA-II and 200 ng/mL for AFP [27]. In another study involving 1,361 HCC patients, of which 61.0% (n=834) had small HCC (<3 cm), PIVKA-II 40 mAU/mL and AFP 20 ng/mL together resulted in a sensitivity of 72.0% and specificity of 91.0% [12].
Statement 2: PIVKA-II is valuable in the detection of HCC in AFP-negative HCC patients
Agreement: Strongly agree
Unlike AFP-positive HCC, AFP-negative HCC (defined as AFP ≤20 ng/mL) are not easily diagnosed, as most present as early or small HCCs [31]. Additionally, the presence of hepatic nodules that resemble HCC tumours on imaging can lead to misdiagnosis. In a large multicentre study, 1,158 patients with HCC were categorised based on AFP levels. The significant proportion of patients had hepatitis B-, hepatitis C- and alcoholic liver disease-related HCC, either alone or in combination. Almost half (46.0%) had normal (<20 ng/mL) AFP levels and only 6.0% (n=66) had AFP levels between 200–400 ng/mL [32]. There is also evidence to suggest a high prevalence of AFP-negative HCC in patients with fatty liver disease, both alcoholic and non-alcoholic [33].
PIVKA-II, on the other hand, has demonstrated the potential utility in improving the detection of early HCC in AFP-negative HCC patients up to 76.0% (Table 6) [14], though most of the studies involved patients with Hepatitis B virus (HBV)-related aetiology [14,23,34-36].
The role of PIVKA-II in prognosis prediction and treatment monitoring
Statement 3: Preoperative PIVKA-II measurement predicts the microvascular invasion (MVI) risk, which may be useful in the assessment of tumour prognosis
Agreement: Strongly agree
Significantly high levels of PIVKA-II based on cut-off values between >40 mAU/mL and >100 mAU/mL appear to predict the occurrence of portal vein tumour thrombosis (PVTT) and MVI, as well as poorer overall survival (OS) and higher risk of recurrence. The challenge in interpreting the data is the wide range of cut-off values used in the studies. Consideration should also be given to the fact that AFP levels and tumour size were also independent predictors for MVI.
A total of 123 newly diagnosed HCC patients (Barcelona Clinic Liver Cancer [BCLC] Stages A–C) were included in a study to determine the correlation of PIVKA-II level to PVTT [37]. PIVKA-II levels were significantly higher in those with PVTT than those without (P=0.003), and had a high area under the receiver operating curve (AUROC) of 0.73, sensitivity of 83.7%, and specificity of 69.2%, at a cut-off of 221.26 mAU/mL [37]. Elevated PIVKA-II levels were also strongly correlated with PVTT (odds ratio [OR] 4.89, P=0.020) [36].
MVI is an independent risk factor for early recurrence in HCC, and impacts prognosis [38]. At a cut-off level of >40 mAU/mL, PIVKA-II was an independent predictor of MVI (hazard ratio [HR] 3.77, 95% CI 1.31–10.88, P=0.014) [39], which in turn is a risk factor for recurrence. Two hundred and seventeen patients with small HCC ≤3 cm, who had three nodules without radiological evidence of vascular invasion, were retrospectively assessed. PIVKA-II of >100 mAU/mL (and AFP of >100 ng/mL) predicted pathological MVI [40]. In another study, HCC with a single ≤3 cm nodule and PIVKA-II level of ≥40 mAU/mL was an independent predictor of MVI (OR 1.79, P=0.0126), as were AFP levels of ≥200 ng/mL (OR 1.82, P=0.0466), and tumours of ≥2 cm (OR 1.84, P=0.0052) [41].
Statement 4: PIVKA-II measurements, before and after curative treatment (resection and radio-frequency ablation [RFA]), are useful for monitoring treatment outcomes and recurrence
Agreement: Strongly agree
Pre-treatment PIVKA-II levels and decrease of PIVKA-II after treatment predicted treatment outcomes for OS and recurrence-free survival (RFS). The changes in PIVKA-II levels appear to have better accuracy than AFP alone; however, combining both tumour markers resulted in better accuracy. PIVKA-II responses have also demonstrated independence in predicting recurrence in very early HCC.
In a meta-analysis that included 15 cohorts with 5,647 patients, pre-RFA elevated PIVKA-II significantly predicted poorer OS and RFS (HR 1.59; 95% CI 1.40–1.82; P<0.001), and (HR 1.76; 95% CI 1.42–2.17; P<0.001) [42], while a significantly large reduction of PIVKA-II (and AFP) levels post RFA, was associated with a reduction of recurrence rate and improving survival time [43]. A PIVKA-II level of ≥100 mAU/mL was an independent risk factor along with AFP ≥15 ng/mL and tumour size ≥2 cm, with relative risks (RR) of 4.19, P=0.003; 3.05, P=0.02, and 3.34, P=0.03, respectively, for recurrence post RFA [44].
Pre-operative elevation of both AFP and PIVKA-II was significantly associated with the development of recurrence, and shorter disease-free survival than the elevation of only one marker (cut-off levels were 20 ng/mL and 40 mAU/mL, respectively) [45]. A systematic review (n=12 studies) that included studies measuring AFP and PIVKA-II responses to various treatment modalities, including liver resection, revealed that a high pre-treatment level of both tests was associated with higher risk of recurrence, including early recurrence (within six months) [46]. Higher pre-treatment AFP and PIVKA-II levels were also associated with unfavourable tumour characteristics, MVI, and multiple tumours. PIVKA-II and AFP levels that did not decline at three months post resection, and had shorter doubling times, also predicted recurrence and significantly poorer OS.
Elevated PIVKA-II levels, before and after resection, independently predicted disease-free survival and OS, as did tumour size and number, and MVI [46]. PIVKA-II levels of >46 mAU/mL were a risk factor for early recurrence (P=0.002), along with the magnitude of tumour necrosis (P=0.012), and the presence of MVI (P=0.029) post curative resection [47]. More patients had elevated PIVKA-II (>40 mAU/mL) than higher AFP levels (>20 ng/mL) at recurrence [48], and PIVKA-II had better specificity and sensitivity than AFP (92.3% vs. 87.2%, and 74.1% vs. 40.7%, respectively). However, a variation in the difference of levels pre- and post-treatment suggests that combining PIVKA-II and AFP may improve the earlier detection of recurrence.
In patients with very early-stage HCC (BCLC Stage 0-A), pre-resection PIVKA-II levels of ≥373.51 mAU/mL demonstrated a strong independent factor in predicting shorter time to progression, post resection [49]. In patients without macroscopic vascular invasion, a pre-resection PIVKA-II level of >445 mAU/mL was an independent risk factor for postoperative tumour recurrence [50]. The tumour-free survival rates at 1 and 2 years for patients with pre-treatment PIVKA-II levels of ≤445 mAU/mL (90.4% and 70.7% respectively) were significantly higher than those with elevated pre-operative levels (73.2% and 50.5%, P=0.048).
Statement 5: PIVKA-II measurements before and after intra-arterial treatment (transarterial chemoembolization [TACE] and Yttrium-90 transarterial radioembolization [TARE]) are clinically useful to indicate response
Agreement: Strongly agree
Pre- and post-treatment PIVKA-II levels have demonstrated an association with treatment outcomes. Lower pre-treatment PIVKA-II levels appear to predict better OS, whilst a good serological response (usually taken as a reduction of ≥20–50% of pre-treatment levels) has correlated with radiological response, better OS and progression-free survival (PFS), and complete (CR) and partial (PR) responses. However, the current data may not be robust enough to support a strong recommendation for pre- and post-intra-arterial treatment PIVKA-II testing.
PIVKA-II and AFP responders to TACE had better time to progression and OS than non-responders (P<0.001) [51]. When the cut-off levels for PIVKA-II and AFP were set at ≥60 mAU/mL and ≥200 ng/mL, respectively, and serological response to ≥50.0% reduction from baseline, serological responders correlated with radiologic responders and had better OS than non-responders (HR: PIVKA-II 3.40 and AFP 4.70; all P<0.001, respectively) [52]. Differences in pre-treatment and 3- and 6-month post-treatment AFP and PIVKA-II levels independently predicted OS, with combined responders doing significantly better than either alone (P=0.011) [46]. Reductions in AFP and PIVKA-II, after TACE treatment were higher in patients with CR and PR vs. stable and progressive disease, but was not associated with better PFS.
PIVKA-II monitoring assists with predicting OS and PFS in TACE [53]. Low pre-treatment PIVKA-II was associated with increased OS (HR 0.65, 95% CI 0.44–0.96), and its response post-TACE of ≥20.0–50.0% reduction was associated with increased OS and PFS (HR 0.39, 95% CI 0.22–0.70 and 0.42, 95% CI 0.23–0.74, respectively). Patients with elevated PIVKA-II levels pre- and post-TACE had poorer survival than those with elevated pre-TACE levels and low post-TACE levels (HR 8.47; P<0.0001) [54]. As the PIVKA-II response was significantly predictive of OS in patients with a high PIVKA-II level at baseline (HR 3.20; P<0.001) [52], it could be a surrogate of immediate and prolonged clinical outcomes post-TACE, especially in patients with high baseline PIVKA-II levels. Monitoring PIVKA-II level trends might be helpful, as it was also strongly associated with objective response rates and disease control rates (P=0.009 and P=0.004, respectively) [54].
A study to determine the predictive values of AFP, PIVKA-II, and modified Response Evaluation Criteria In Solid Tumours (RECIST) response post-TARE, included 63 Child-Pugh Class A patients with AFP >20 ng/mL and PIVKA-II >20 mAU/mL who were treated with TARE [55]. Responses to AFP and PIVKA-II were defined as >50.0% decrease in levels from baseline. Response based on modified RECIST scores was defined as a complete or partial response. PIVKA-II responders had better survival at three and six months, although AFP and modified RECIST responders also demonstrated better survival at three months. The median OS between AFP and PIVKA responders and non-responders at three months were 75.8 months vs. 7.6 months for AFP and 75.8 months vs. 7.1 months for PPIVKA-II, respectively [55].
Statement 6: Pre-liver transplant PIVKA-II levels are associated with the risk of post-operative HCC recurrence, potentially facilitating the patient selection
Agreement: Strongly agree
As HCC recurrence after liver transplant is strongly associated with HCC histological grade, as well as AFP and PIVKA-II levels, measuring pre-liver transplant levels for both these markers can serve as an indication of the expected outcomes in patients who might need the operation. The interest in the association between pre-operative PIVKA-II levels and outcomes post-liver transplant is still fairly recent, and is based on the findings from a systematic review [56]. The number of studies of quality focusing on this association is very small and were mainly done in the Japanese population. Therefore, more studies are required for a stronger recommendation
Pre-operative AFP and PIVKA-II (cut-off values 300 ng/mL and 300 mAU/mL, respectively) were significantly associated with recurrence, post liver transplant, and their combination was a better predictor than either alone [57]. Patients with far advanced HCC and low AFP and PIVKA-II levels (≤300 for both), had significantly better 5-year OS and RFS rates (47.8% and 53.4%, respectively) than those with elevated AFP and PIVKA-II levels (21.0% and 10.8%, respectively). Hence, there might be a role for combining AFP and PIVKA-II levels in patients with advanced HCC, to facilitate selection for transplantation.
Pre-operative PIVKA-II (cut-off values 300–442 mAU/mL) impacted post-liver transplant HCC recurrence. Elevated levels were associated with shorter disease-free survival (HR 5.04, 95% CI 3.32–7.67; P<0.001) indicating that the inclusion of pre-liver transplant PIVKA-II levels could improve the eligibility of HCC patients for liver transplantation [56].
Pre-liver transplant PIVKA-II levels were also inversely correlated with patient survival post liver transplant [58]. When PIVKA-II was ≤100 mAU/mL (n=336), the survival at 1 year was 96.2%, at 3 years was 92.3%, and at 5 years was 91.0%; however, when the levels were >1,000 mAU/mL (n=44), the rates dropped drastically to 71.9%, 37.1%, and 29.7%, respectively. Hence, the Japanese Liver Transplantation Study Group proposed the incorporation of pre-operative AFP and PIVKA-II levels at ≤200 ng/mL and ≤100 mAU/mL, respectively, taken together to facilitate patient selection for liver transplantation.
DISCUSSION
Based on the available evidence and experts’ opinions, PIVKA-II in combination with AFP and US shows potential benefit for surveillance of small and AFP-negative HCC. However, the addition of PIVKA-II for surveillance of small HCC requires stronger evidence, such as a prospective longitudinal study comparing the effectiveness of US and AFP to US, AFP and PIVKA-II. On the other hand, the evidence in utilising PIVKA-II in detecting AFP-negative HCC appears to be stronger. It is important to note the lack of evidence to suggest that the combination of AFP, PIVKA-II and US is superior to utilising AFP and US for the detection of AFP-negative HCC.
A significant limitation for reaching these consensus statements was the paucity of studies with good levels of evidence as recommended by the EDRN and ILCA. Of the four retrieved studies, only one compared PIVKA-II, AFP and their combination, while the rest had included AFP-L3 and/or the GALAD score. However, the phase 3, Level 2a study by Lok et al. [13] demonstrated significant value in combining PIVKA-II and AFP for detection of early HCC.
Viral hepatitis-related HCC is a leading cause of HCC in Asia, particularly chronic hepatitis B virus infection [3]. However, with the availability of a new generation of treatments, chronic hepatitis B viral replication is effectively suppressed and hepatitis C virus infections cured [59]. Antiviral treatment causes viral suppression and reduces the inflammation, which consequently lowers the AFP levels. Hence, cut-off levels for AFP for detection of HCC will have to be lower than the presently accepted thresholds [60,61]. However, more evidence and a consensus are needed to determine the optimal AFP cut-off values for patients treated with antivirals. The addition of PIVKA-II has demonstrated good detection rates in AFP-negative HCC and, therefore, should be incorporated where feasible.
The data also show that PIVKA-II and AFP perform differently depending on the aetiology of the HCC, and also the cut-off values used [11,13,22,23,27]. In patients with chronic hepatitis B-related HCC, PIVKA-II appears to do better than AFP, but on the other hand, the number of studies is comparatively small compared to the evidence for AFP. Therefore, more studies with adequate sample sizes for the different aetiologies for HCC should be performed to further strengthen the position and role of PIVKA-II+AFP in the surveillance of HCC. There is also a lack of studies involving hepatitis C-related HCC patients, as most studies have focussed on chronic hepatitis B-related HCC.
Non-alcoholic fatty liver disease (NAFLD) is a very common disease and also a risk factor for HCC [3]. With the progressive efforts to reduce and potentially eliminate viral hepatitis, and the rampant increase of diabetes mellitus and obesity, NAFLD could become an important cause of HCC [3]. NAFLD has been associated with a 2.6-fold increased risk of HCC, whilst diabetes alone, a 2-3-fold increased risk.3 Furthermore, NAFLD-related HCC frequently occurs without cirrhosis, making patient selection and execution of surveillance programs using AFP and US difficult [62]. A few studies have demonstrated the reliability of PIVKA-II in detecting HCC in patients with NAFLD and non-alcoholic steatohepatitis (NASH) [62,63]. As there were only a few studies performed to determine the utility of PIVKA-II for screening high-risk NAFLD/NASH patients, the experts agreed that a consensus could not be reached at the present time, even though PIVKA-II in combination with age, gender, AFP and AFP-L3 (the GALAD score) might have a role to play in the future [63].
The consensus for the utilisation of PIVKA-II for monitoring the response of curative treatments, particularly post-resection and RFA, was much stronger. The evidence demonstrating the benefits of PIVKA-II in combination with AFP, pre- and post-local curative treatments, was consistent, leading to all of the experts being in agreement (100% agreement) that PIVKA-II can be a recommended biomarker. However, its utility in pre-liver transplant patients requires stronger evidence involving larger sample sizes and the inclusion of populations beyond Japan.
The experts anticipate challenges in implementing PIVKA-II locally. Unlike AFP, PIVKA-II lacks international standardisation, and its values are dependent on the assays used. Furthermore, the cut-off values used in clinical studies vary widely from >20 mAU/mL to >1,000 mAU/mL, making it challenging to implement across different laboratories. To ensure a measure of standardisation and optimisation of PIVKA-II utility, localisation of the reference interval and cut-off values will be required. Conducting localised, small-scale clinical validation studies will help establish the performance and assay-specific cut-off values of PIVKA-II in the local population. Additionally, it will be important for clinicians to understand that baseline PIVKA-II value may be inadequate to detect early HCC and serial monitoring of the biomarker level should be done.
At present, only a few countries like Japan and Taiwan have PIVKA-II reimbursement programs. Hence, the cost and subsequent funding of PIVKA-II will be a major challenge for adopting it in HCC surveillance. To date, cost-effectiveness studies for combining PIVKA-II and AFP for HCC surveillance and monitoring are lacking. Hence, to improve the adoption of PIVKA-II testing as part of HCC surveillance, health economic studies at regional or national levels are required, in order to justify its use. Another area that requires more study is the timing of PIVKA-II elevation and its correlation to HCC development. Longitudinal studies are key to determining this, as the data could influence the schedule for screening, and hence the number of expected tests and its overall cost.
Improving awareness of PIVKA-II among relevant healthcare providers will be essential for its proper use and the interpretation of its results together with AFP levels, tumour clinical characteristics, and factors that might affect its value. Medical education programs, health economic studies, and studies localising PIVKA-II values, will have significant roles before driving its endorsement into regional and local guidelines.
The experts also discussed the utility of using PIVKA-II levels to guide recall for confirmation of HCC and implementation of PIVKA-II in laboratories. Generally, the presence of one or two parameters (elevated AFP levels, elevated PIVKA-II levels, and US findings) could guide recall. Recall of patients might be warranted if elevated AFP and PIVKA-II levels raise suspicion of very small tumours. However, this will depend on the magnitude of the elevation of the biomarkers’ levels. In the absence of US findings, other causes of elevated PIVKA-II and AFP levels should be ruled out, followed by serial monitoring of their levels.
The consensus reached by the experts is strengthened by their collectively vast experience in managing patients with HCC and input from experts who are heads of national laboratories. Articles were also extensively collated for their review, and included meta-analyses and randomised controlled studies, which provide a high level of evidence. On the other hand, the experts agreed that more data, including evidence from cost-effectiveness and longitudinal studies, as well as clinical experience, could advance a stronger recommendation for PIVKA-II utilisation in the region. Currently, other than Japan and South Korea, most countries in the Asia-Pacific region have moderate-to-minimal experience with utilising PIVKA-II extensively in practice.
CONCLUSION
PIVKA-II in combination with AFP and US will be clinically useful in the Asia-Pacific region in surveillance, especially for those with small and AFP-negative HCC, and more so in predicting treatment outcomes in HCC patients. More evidence is required, and stronger consensus at an international level remains to standardise cut-off values and tighten its reference range, in order to support easier applicability of the test. There is also a need for cost-effectiveness studies to justify its use on a broad scale.
Acknowledgements
The writing committee wishes to acknowledge Roche Diagnostics for supporting the manuscript writing via an educational grant.
Notes
Authors’ contribution
Conception and design of the study: J.H. Yu, H.A. Lee, and S.U. Kim; Drafting or revision of the manuscript: J.H. Yu, H.A. Lee, and S.U. Kim; Approval of the final version of the manuscript: J.H. Yu, H.A. Lee, and S.U. Kim.
Conflicts of Interest
This study did not include the use of human or animal subjects.
Below is the list of conflict-of-interest statements: Kim DoYoung, Toan Bao Nguyen, Setiawan Lyana, Huyen Nguyen Nguyen, Mohamed Rosmawati, Hai Thi Thanh Nguyen and Lee Woo-Chang declare that they have no conflict of interest.
Tan Chee-Kiat has received honoraria made to his institution for lectures, presentations, speakers bureaus, manuscript writing and education events from Abbott Laboratories, Astellas and Gilead Sciences. He has also received payment made to his institution for participation on a Data Safety Monitoring Board and Advisory Board for Abbott Laboratories, Bayer, Eissai, Gilead Sciences and Roche Diagnostics.
Hasan Irsan has received honoraria for lectures, presentations, speakers’ bureaus, manuscript writing and education events from Eisai and Roche.
Yu Ming-Lung has received grants from Abbott, BMS, Gilead and Merck. He has also received consulting fees and honoraria for lectures, presentations, speakers’ bureaus, manuscript writing and education events from Abbott, Abbvie, BMS, Gilead, IPSEN, Merck, Roche and Roche Diagnostics.
Namiki Izumi has received honoraria for lectures, presentations, speakers’ bureaus, manuscript writing and education events from Chugai, Eisai and Takeda.
Chow Pierce Kah-Hoe has received grants from AMiLi, MiRXES, Perspectum and Roche. He has received consulting fees for Beigene, Omega Therapeutics and Worrell (LLC), and honoraria for lectures, presentations, speakers’ bureaus, manuscript writing and education events from Abbott, AstraZeneca, Eisai, Roche and Sirtex Medical. He has a patent submitted for “A system and method for classify cancer patients into appropriate cancer treatment groups and compounds for treating the patients. Pub. No.: WO/2019/108135 A1 International; Application No.: PCT/SG2018/050585. He has received payment for participating on Data Safety monitoring Boards and Advisory Boards from AstraZeneca, AUMBioscience, Genentech, Roche, Singapore-Samsung Medical Centre (SG-SMC) Joint Lab and Sirtex Medical. In addition, he declares having stock or stock options in AVATAMED Pte. Ltd.
Chan Stephen Lam has received consulting fees and honoraria for lectures, presentations, speakers’ bureaus, manuscript writing and education events from AstraZeneca, Eisai and MSD. He has participated on a Data Safety Monitoring Board and Advisory Board for AstraZeneca.
Tanwandee Tawesak has received grants from Gilead, Janssen, MSD, Roche and Vir Biotech.
Lee Teng-Yu has received grants from Gilead, MSD and Roche. He has received consulting fees and honoraria for lectures, presentations, speakers’ bureaus, manuscript writing and education events from AbbVie, BMS, Eisai, Gilead and Roche. He has participated on Advisory Boards for BMS, Eisai, Gilead and Roche.
Yang Tian has received honoraria for lectures, presentations, speakers’ bureaus, manuscript writing and education events from Abbott and Roche.
Chan Henry Lik Yuen has received consulting fees from AbbVie, Aligos, Arbutus, Hepion, Gilead, GSK, Janssen, Roche, Vaccitech, Vir Biotechnology and Virion Therapeutics.
He has received honoraria for lectures, presentations, speakers’ bureaus, manuscript writing and education events from Gilead, Roche and Viatris, and received support for attending an overseas conference from Gilead. He has participated on Data Safety Monitoring Boards for Aligos, Roche and Vaccitech.
Abbreviations
US
ultrasound
AFP
alpha-fetoprotein
HCC
hepatocellular carcinoma
PIVKA-II
protein-induced vitamin K absence II
APASL
Asian Pacific Association for the Study of the Liver
MVI
microvascular invasion
RFA
radio-frequency ablation
TACE
transhepatic arterial chemoembolisation
TARE
transhepatic arterial radioembolisation
RECIST
response evaluation criteria in solid tumours
AUC
area under the curve
LR
likelihood ratio
CI
confidence interval
BLD
benign liver disease
CHB
chronic hepatitis B
CLD
chronic liver disease
HS
healthy subjects
LC
liver cirrhosis
LH
liver haemangioma
MT
liver metastasis
Sn
sensitivity
Sp
specificity
PVTT
portal vein tumour thrombosis
BCLC
barcelona clinic liver cancer
AUROC
area under the receiver operating curve
OR
odd ratio
OS
overall survival
RFS
recurrence-free survival
HR
hazard ratio
RR
relative risk
PFS
progression free survival
CR
complete response
PR
partial response
NAFLD
non-alcoholic fatty liver disease