Hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma in the era of chemo-diversity
Article information
Abstract
Recently, treatments for unresectable hepatocellular carcinoma (HCC) have undergone remarkable development. Various systemic chemotherapy drugs have been approved and are recommended by clinical guidelines worldwide. Although systemic treatments are effective and contribute to prolonged patient survival, their effects are unsatisfactory for some specific tumor conditions, such as macrovascular invasion. Hepatic arterial infusion chemotherapy (HAIC) is a traditional treatment for advanced HCC. As yet, there is no worldwide consensus recommending HAIC because no high-quality clinical trials have demonstrated its survival benefit. However, clinical evidence is gradually accumulating that shows its survival benefit, and it is recognized as an effective locoregional treatment for advanced HCC. Several HAIC regimens have been reported, including cisplatin monotherapy, cisplatin plus 5-fluorouracil (low-dose FP), lipiodol-suspended FP, and an oxaliplatin-based regimen. We have entered an era of chemo-diversity in the treatment of advanced HCC. This review aimed to clarify the relevance of HAIC in the era of chemo-diversity. We propose a multidisciplinary therapeutic strategy combining locoregional HAIC treatment with sequential drug therapy, with the aim of becoming cancer-free through conversion therapy.
INTRODUCTION
Recently, therapeutic strategies for intermediate and advanced-stage hepatocellular carcinoma (HCC) have been dramatically changing. Treatment guidelines worldwide recommend systemic therapies as the standard treatment for advanced HCC [1]. Various drugs, such as sorafenib, regorafenib, lenvatinib, ramucirumab, cabozantinib, and atezolizumab plus bevacizumab have been approved [2]. Various treatments are available for intermediate and advanced HCC [3]. Macroscopic vascular invasion (MVI) and extrahepatic spread (EHS) are two factors that define advanced stages of HCC, and these are independent factors of a poor HCC prognosis. Additionally, an indicator of tumor spread in the liver, such as the up-to-seven criteria, and refractory to transcatheter arterial chemoembolization are also important poor prognostic factors [4-6]. Although approved systemic therapies prolong survival even for patients with these HCCs, their therapeutic effects are unsatisfactory because the baseline of such patients’ survival in the intermediate and advanced stages is extremely poor. Therefore, further progress is needed in the treatment of intermediate and advanced HCCs.
Various locoregional treatments have been employed to treat intermediate and advanced HCC, such as radiation therapy, hepatic resection, and transcatheter arterial chemoembolization [7-10]. Hepatic arterial infusion chemotherapy (HAIC) is another such treatment [11]. HAIC is a locoregional treatment using a catheter technique to directly administer anti-cancer drugs into tumors through the hepatic artery. Although HAIC is thought to be a promising therapeutic modality in the treatment of locally advanced HCC, it is not a standard treatment worldwide because of the lack of sufficient evidence [12]. Thus far, no randomized clinical trials have shown survival benefits from HAIC. We have been conducting HAIC treatment for patients with advanced HCC and reported its usefulness [13,14]. We have developed an effective HAIC regimen termed “lipiodol suspended FP/New FP” (FP: cisplatin plus 5-fluorouracil) for the treatment of advanced HCC [15,16]. The regimen consists of cisplatin (CDDP) suspended with lipiodol and 5-fluorouracil (5-FU). Moreover, recent clinical trials regarding HAIC conducted in China revealed survival benefits for patients with advanced HCC. Therefore, the effectiveness of HAIC needs further investigation.
In this review, we updated the current status of HAIC and summarized its therapeutic outcomes. This review clarifies the current status of HAIC in the treatment of advanced HCC in the era of chemo-diversity.
THERAPEUTIC GUIDELINES IN THE TREATMENT OF ADVANCED HCC WORLDWIDE
Various societies of hepatology around the world propose clinical practice guidelines for the treatment of HCC. In this paragraph, we compare the definition of and recommended treatment for advanced HCC from five representative guidelines (Fig. 1): the American Association for the Study of Liver Diseases (AASLD), the European Association for the Study of the Liver (EASL), the Asian Pacific Association for the Study of the Liver (APASL), the Korean Liver Cancer Association, and the Japan Society of Hepatology [17-21]. The definition of advanced HCC is similar among these guidelines. The keywords to diagnose advanced HCC are EHS and MVI, in particular, portal vein tumor thrombus (PVTT). The AASLD and the EASL refer to the Barcelona Clinic Liver Cancer staging system to define advanced HCC [22]. In the Barcelona Clinic Liver Cancer staging system, performance status 1 or 2, preserved liver function in addition to the presence of PVTT or EHS are included in the advanced stage of HCC, which is classified into Barcelona Clinic Liver Cancer stage C. Although MVI and EHS are factors also used to define advanced HCC in the guidelines of the Korean Liver Cancer Association, the APASL and Japan Society of Hepatology, they classify advanced HCC into local progression and EHS, respectively. In every therapeutic guideline, the recommended treatment for advanced HCC is systemic treatment. The AASLD guideline recommends systemic treatment and optionally transcatheter arterial radioembolization for advanced HCC. The EASL guideline only recommends systemic treatment. The recommended treatment for advanced HCC is more complicated in the guidelines of the Korean Liver Cancer Association, the APASL, and Japan Society of Hepatology. Systemic therapies are the first recommendation for the treatment of advanced HCC, especially for patients with EHS. However, several alternative recommendations, such as hepatic resection, transcatheter arterial chemoembolization, external beam radiation, and HAIC are optionally described for the treatment of advanced HCC with MVI.
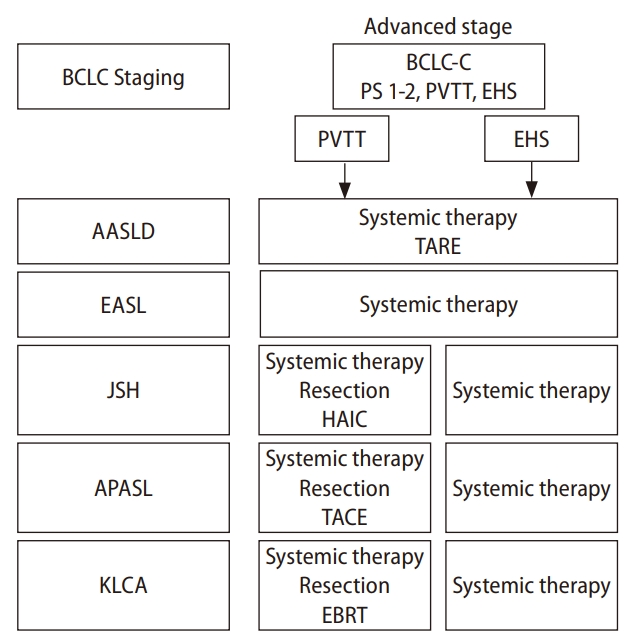
The recommended treatments for advanced HCC in each guideline. HCC, hepatocellular carcinoma; BCLC, Barcelona Clinic Liver Cancer; PVTT, portal vein tumor thrombus; EHS, extrahepatic spread; TARE, Transarterial Radioembolization; AASLD, The American Association for the Study of Liver Diseases; EASL, The European Association for the Study of the Liver; JSH, The Japan Society of Hepatology; APASL, The Asian Pacific Association for the Study of the Liver; KLCA, The Korean Association for the Study of the Liver; HAIC, hepatic arterial infusion chemotherapy; TACE, transcatheter arterial chemoembolization; EBRT, External Beam Radiation Therapy.
THERAPEUTIC OUTCOMES OF SYSTEMIC THERAPIES FOR ADVANCED HCC WITH PVTT
PVTT is one of the most advanced conditions in HCC. Especially, the prognosis of patients with portal invasion into the first branch or main trunk, which is called major PVTT, is extremely poor. Atezo+Beva is the first-line drug for HCC. The sub-analysis of the IMbrave 150 clinical trial revealed that the median survival time (MST) of patients with advanced HCC treated with Atezo+Beva was 14.2 months [23]. The results of Atezo+Beva are promising; however, the data included both MVI and EHS tumor conditions. There is no pure data that shows the efficacy of Atezo+Beva for HCC only with MVI or PVTT. The MST of patients with MVI-HCC treated with sorafenib was 8.1 months but was only 4.9 months in the placebo group [24]. Similar results were also identified in another clinical trial (MST: sorafenib/placebo: 6.5/4.2 months) [25]. The MST of patients with advanced HCC, including MVI treated with lenvatinib, was 6.4 months [26]. Although systemic therapies possess beneficial outcomes even for MVI-HCC, their effects are limited. In particular, the MST of patients with major PVTT is extremely poor even when treated with approved systemic drugs. To aim for prolonged survival of patients with PVTT-HCC, systemic treatment alone might not be sufficient, and more powerful locoregional treatment might be needed to achieve a therapeutic response.
HAIC
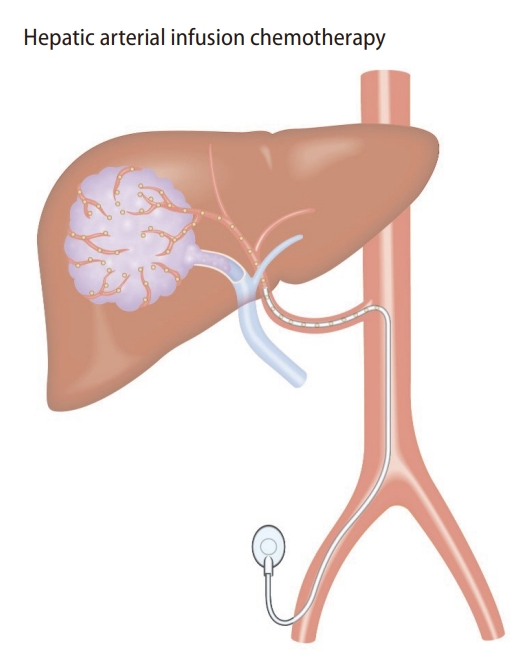
HAIC is a locoregional treatment using a catheter technique. The catheter allows direct and consecutive delivery of anti-cancer drugs to HCC located in the liver (Fig. 2). The benefits of HAIC are increasing local concentrations of anti-cancer drugs in the tumor and reducing systemic adverse events due to anti-cancer drugs. However, to properly perform HAIC, implantation of an indwelling catheter and port system is often needed. Briefly, the catheter is inserted into the femoral, subclavian, or axillary arteries. The catheter is indwelled to appropriately deliver the anti-cancer drugs into the liver. The port system is subcutaneously implanted. Although sophisticated techniques and experiences are needed to perform implantation of the system, this allows repeated intermittent administration of drugs.
STATUS OF HAIC IN THE TREATMENT OF ADVANCED HCC WORLDWIDE
HAIC treatment is not described in the guidelines of the American Association for the Study of Liver Diseases, European Association for the Study of the Liver, or Asian Pacific Association for the Study of the Liver. This is because there is insufficient clinical evidence for HAIC to be able to recommend it in the guidelines. PubMed searches using the terms “hepatic arterial infusion chemotherapy” and “hepatocellular carcinoma” show 411 manuscripts. Most studies regarding HAIC for HCC are retrospective cohort studies with a clinical evidence level of 2a or 2b. Among them, 40 prospective studies and 9 prospective randomized studies evaluated the therapeutic effects of HAIC for HCC. Only 5 studies were large-scale randomized phase 3 trials considered evidence level 1b.
Kudo et al. [27] evaluated the usefulness of low-dose FP, a conventional HAIC regimen, in combination with sorafenib. In this study, low-dose FP did not show a significant additive effect with sorafenib for overall patients with HCC. However, subgroup analysis revealed a significant additive effect in patients with HCC invasion into the portal trunk. Although the primary endpoint was not met in this study, this study suggested that low-dose FP plus sorafenib might be effective in a specific subgroup. In another phase 3 clinical trial by He et al. [29], the Folinic acid, 5-fluorouracil, and Oxaliplatin (FOLFOX) HAIC regimen, which consisted of oxaliplatin, 5-FU, and leucovorin in combination with sorafenib, significantly prolonged the survival of patients with PVTT-HCC when compared to sorafenib monotherapy [28]. This study revealed the usefulness of HAIC, especially the FOLFOX regimen, in combination with sorafenib for HCC with PVTT. Taken together, few studies have demonstrated the effectiveness of HAIC with high clinical evidence. However, recent studies are enhancing the clinical evidence of HAIC by a combination of approved MTAs [29,30]. Recently, the Japanese Society of Implantable Port Assisted Treatment proposed clinical practice guidelines for HAIC with a port system, which will support more widespread application of HAIC [31]. HAIC could be a promising therapeutic modality for locally advanced HCC, such as MVI including PVTT.
HAIC REGIMENS
Various HAIC regimens have been reported in the treatment of advanced HCC. Anticancer drugs that are used in HAIC include doxorubicin, epirubicin, mitomycin, 5-FU, CDDP including a fine-powder CDDP, oxaliplatin, and leucovorin. According to previous reports, monotherapy or combination regimens of 5-FU and platinum-based anticancer drugs including CDDP and oxaliplatin are reported most often and seem to be effective, which might suggest that these drugs should be key drugs in HAIC treatment for advanced HCC. In the following paragraphs, we summarize several representative HAIC regimens for the treatment of HCC. The therapeutic outcomes of each HAIC regimen are listed in Table 1.
CDDP MONOTHERAPY
CDDP is one of the best-known anticancer drugs, which is used for numerous types of cancers. CDDP has the ability to crosslink with purine bases on the DNA, which induces interference with DNA repair and causes DNA damage, followed by inducing apoptosis in cancer cells. Several studies have reported the efficacy of CDDP monotherapy as a HAIC regimen for HCC. In particular, DDP-H is used as a HAIC regimen in Japan [32]. As a HAIC regimen, 65 mg/m2 of CDDP is repeatedly administered every one to two months. Regarding the therapeutic effects of CDDP monotherapy, the objective response rate (ORR) is from 10% to 20%, which seems to be a modest effect. Ikeda et al. [33] reported positive results of CDDP-HAIC monotherapy in combination with sorafenib in a randomized phase 2 trial. In contrast to sorafenib monotherapy, CDDP-HAIC plus sorafenib significantly prolonged the survival of the patients with advanced HCC (P=0.031, hazard ratio 0.60, MST; combination group, 10.6 months/sorafenib monotherapy group, 8.7 months). The sample size of this study was small, but it revealed promising effects of CDDP-HAIC monotherapy in addition to sorafenib. The benefit of this regimen is that it does not require implantation of an indwelling catheter port system.
LOW-DOSE FP
Low-dose FP is the representative HAIC regimen for HCC. This regimen is theoretically effective because CDDP is not only a direct anticancer drug but is also a biochemical modulator to 5-FU, which synergizes antitumor effects. There are several modifications that can be made to the regimen, but in general, one course of low-dose FP consists of 10 mg of CDDP for 30 minutes followed by 250 mg of 5-FU continuously injected for 3 hours administered daily for five days. In principle, this weekly regimen is repeated two or three times in one cycle of low-dose FP. In 2002, Ando et al. [13] reported the therapeutic effect of low-dose FP in HCC with PVTT. The ORR of low-dose FP in this study was 48%. The one-year survival rate was 45% in patients with PVTT-HCC. Large-scale retrospective data regarding low-dose FP was also reported by Nouso et al. [34]. They compared 476 cases treated with low-dose FP and 1,466 cases that received best supportive care using propensity score-matched analysis. Notably, the MST of the patients with HCC who underwent low-dose FP were significantly longer compared to that of the patients who received the best supportive care (P<0.0001, MST; low dose FP 14.0 months/best supportive care 5.2 months). Moreover, the combination therapy of low-dose FP and sorafenib has also drawn attention. Kudo et al. [27] reported a randomized large-scale phase 3 trial of low-dose FP plus sorafenib for advanced HCC. The overall results were negative. However, subgroup analysis revealed that low-dose FP plus sorafenib significantly contributed to prolonged survival in patients with HCC with invasion into the portal trunk (hazard ratio 0.49, MST; low-dose FP plus sorafenib 11.4 months/sorafenib monotherapy 6.5 months).
HIGH-DOSE FP
The promising results regarding high-dose FP regimen are reported in Korea [35-37]. The regimen consists of 60 mg/m2 of cisplatin on day 2 and 500 mg/m2 of 5-FU on days one to three. The dose of high-dose FP is two to three times higher than low-dose FP. The clinical trial performed by the Korean Liver Cancer Study Group showed that high-dose FP showed a better tumor response compared to low-dose FP (ORR: high-dose FP 16.7%, low-dose FP 0%, P=0.024) [38]. The low-dose FP regimen in the study was not the regimen performed in Japan. The effects of high-dose FP for PVTT-HCC are also attractive. Choi et al performed a randomized, prospective, and comparative study to investigate the effects and safety of sorafenib and HAIC for PVTT-HCC [28]. The MST and time to progression were significantly longer in the HAIC group than in the sorafenib group (14.9 vs. 7.2 months, P=0.012 and 4.4 vs. 2.7 months, P=0.010). The objective response rate was 27.6 and 3.4 % in the HAIC and sorafenib, respectively. Although the sample size of the study is relatively small, the study revealed valuable outcomes of HAIC in the randomized and prospective study.
5-FU ARTERIAL INFUSION PLUS INTERFERON THERAPY (FAIT)
Several studies have investigated 5-FU arterial infusion plus interferon therapy, termed FAIT. Interferon not only acts as a biochemical modulator of 5-FU but directly inhibits cell proliferation and angiogenesis. Obi et al. administered FAIT to 116 patients with PVTT-HCC [39]. The ORR of FAIT was 52%, and the complete response rate was 16%. The one-year survival rate was 81% in HCC with PVTT, showing encouraging results for the treatment of advanced HCC. Although FAIT seems to be a promising HAIC regimen, there has been no recent report and no studies with a high level of evidence.
LIPIODOL SUSPENDED FP/NEW FP
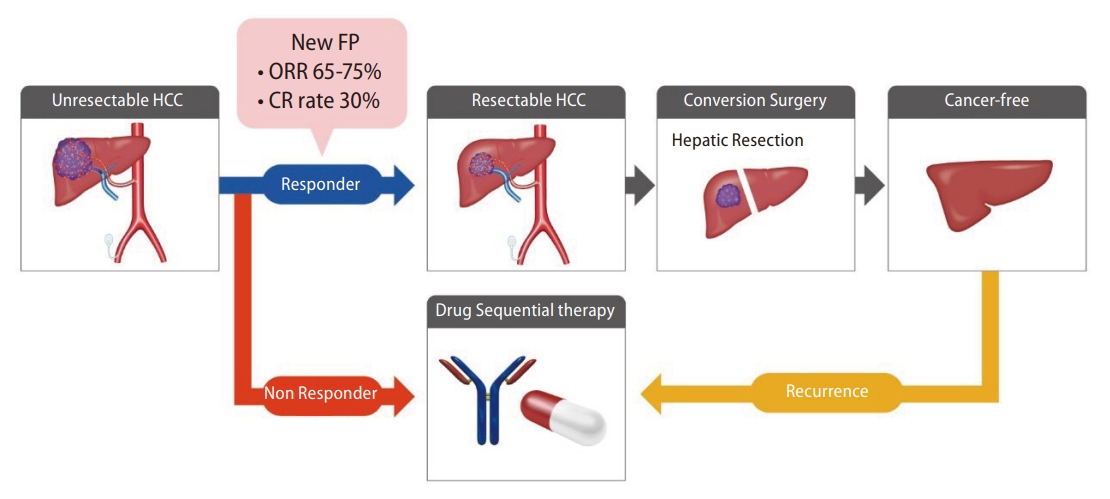
Lipiodol-suspended FP/New FP therapy consists of administration of fine-powder CDDP suspended with lipiodol followed consecutively by 5-FU. A total of 50 mg of fine-powder CDDP was suspended in 5–10 mL of lipiodol. The CDDP-lipiodol suspension was injected using the implanted catheter under angiography, followed by 1,250 mg of 5-FU continuously injected using an infusion balloon pump. Nagamatsu et al. [15] reported the therapeutic effects of New FP in a single-center retrospective study and a multi-center retrospective study. Nagamatsu et al. [40] reported a comparative study of New FP and sorafenib monotherapy as a non-randomized prospective study [40]. The therapeutic outcomes of New FP were attractive. In this study, 64 patients with MVI-HCC, without EHS, and Child-Pugh class A were registered. Among them, 44 patients were treated with New FP, and 20 were treated with sorafenib monotherapy. The median progression-free survival was 5.1 and 9.5 months in the sorafenib and New FP groups, respectively (P=0.001). The MST in the sorafenib and New FP groups was 13.2 months and 30.4 months, respectively (P=0.013). Notably, the complete response rate and ORR of New FP were 23% and 71%, respectively. New FP is thought to have three modes of action (Fig. 3). First, lipiodol itself possesses a mild embolic effect on tumor vessels, which induces tumor necrosis [41]. Second, CDDP has a sustained release effect from the DPP-H-lipiodol suspension [42]. Accumulated CDDP gradually releases from its suspension in a fine-powder CDDP-lipiodol into the tumor. Tanaka et al. [42] reported the sustained release effect from the suspension and emulsion of a fine-powder CDDP and lipiodol, showing that CDDP was released at least for 1 week from the lipiodol suspended with a fine-powder CDDP. Third, continuous administration of 5-FU enhances the time-dependent antitumor effect. The sustained release effects of CDDP enhance the biochemical modulator effect toward 5-FU [43]. Most importantly, this regimen has the aim of achieving a cancer-free state with the addition of conversion therapy. According to Nagamatsu et al. [40], the MST of patients with unresectable PVTT-HCC who achieved cancer-free status was more than 50 months. The high ORR of New FP can lead to down-staging or down-sizing of unresectable HCC, which connects to conversion therapies. The cancer-free rate of patients receiving New FP in this study was 48%. Aiming for conversion surgery is a promising concept for patients with PVTT-HCC, whose prognosis is basically six to twelve months. The therapeutic effects of New FP are promising; however, adverse events remain poorly defined. As lipiodol is injected from the proximal side of the hepatic artery, it is known that cholangitis and cholecystitis can develop [14]. Additionally, the mild embolic effect of lipiodol can cause deterioration of liver function, especially when treating HCC with PVTT. The total amount and distribution of injected lipiodol should therefore be carefully adjusted for each patient. Taken together, New FP could be one of the most promising HAIC regimens. However, prospective trials of New FP therapy with a higher level of evidence are required for this to be accepted as a standard treatment for advanced HCC.
FOLFOX REGIMEN
Recently, a randomized large-scale phase 3 trial showed that the FOLFOX HAIC regimen (HAIF), which consists of oxaliplatin, 5-FU, and leucovorin in combination with sorafenib, significantly prolonged the survival of patients with PVTT-HCC, when compared to sorafenib monotherapy [29]. The MST of patients administered HAIF plus sorafenib and sorafenib monotherapy was 13.37 months and 7.13 months, respectively (P<0.001, hazard ratio 0.35). The ORR of patients administered HAIF plus sorafenib and sorafenib monotherapy was 40.8% and 2.46%, respectively (P<0.01). This HAIF regimen is the most evidenced HAIC regimen, with positive outcomes in the treatment of advanced HCC. The benefit of this regimen is a relatively higher ORR with evidence of prolonged survival. Moreover, the HAIF regimen was conducted without implantation of a catheter and port system, according to the report, which could avoid some procedural difficulties. However, the lack of a catheter and port system means that patients receiving this therapy must lie down during the administration of anticancer drugs. This might be tedious for patients because 5-FU is administered for two days in the HAIF regimen.
HAIC COMPLICATIONS AND ADVERSE EVENTS
Although various effective regimens have been reported for HAIC, complications and adverse events must also be detailed for proper therapeutic management [44]. Catheter-related complications can occur; in particular, complications with implantation of an indwelling catheter and port system are important to watch for. Catheter occlusion, arterial stenosis or occlusion, and migration of the indwelling catheter can also occur. The overall frequency of catheter-related complications is 5–15%. Additionally, adverse events from the cytotoxic agents injected during HAIC also occur. Niizeki et al. [14] reported the adverse events that occurred during HAIC using low-dose FP and lipiodol-suspended FP; the frequencies of severe adverse events were 26.2% and 25.5% in low-dose FP and lipiodol-suspended FP, respectively. Thrombocytopenia was the most frequently observed complication, and deterioration of liver function and drug allergy were also noted. Because of this, sufficient knowledge and experience are required for the proper application of HAIC.
THE ROLE OF HAIC FOR ADVANCED HCC
Until 2008, no systemic chemotherapy had been established for advanced HCC. The first approved systemic therapy, sorafenib, was a breakthrough in the field of systemic chemotherapy for HCC. In 2020, another breakthrough was made with the approval of Atezo+Beva. Currently, many approved MTAs can be used as first-line and later-line drugs in the treatment of HCC [8,45]. In this era of chemo-diversity for the treatment of advanced HCC, sequential drug therapy is a mainstay. The sequential drug therapy dramatically prolongs survival in patients with intermediate and advanced HCC. Shimose et al. [46] reported that intervention with multiple systemic drugs prolonged the period until progression to the advanced stage in intermediate-stage HCC. However, we need to know more about which tumor conditions respond or do not respond to the use of systemic drugs.
Local progression of intra-hepatic lesions, such as MVI, is a critical factor that correlates with the poor prognosis of patients with HCC. However, systemic treatments alone might not control locally advanced HCC. Control of intra-hepatic lesions is also important to prolong the survival of patients with EHS-HCC. Aino et al. [47] evaluated the prognosis of 419 patients with EHS-HCC. Patients with controlled intra-hepatic lesions had longer survival times even than patients with EHS. Moreover, Nakano et al. [48] reported that the progression of intra-hepatic lesions was an independent prognostic factor associated with the poor prognosis of patients with EHS-HCC who received sorafenib treatment. Accordingly, control of advanced intra-hepatic lesions with a combination of other therapeutic modalities, including HAIC, might be a better therapeutic strategy for the treatment of EHS-HCC.
Ueshima et al. [49] compared the therapeutic effects between various HAICs regimens (low dose FP, CDDP monotherapy, and FAIT) and sorafenib in four different cohort groups (cohort 1: with MVI and without EHS, cohort 2: without MVI and EHS, cohort 3: with MVI and EHS, and cohort 4: without MVI and with EHS). In the study, HAIC was significantly effective in cohorts 1 and 3, which means HAIC was effective in the presence of MVI regardless of EHS. The same type of study was conducted with a single regimen of HAIC New FP [6,16]. Even in this study, MVI regardless of EHS was a good target for New FP.
For MVI, in particular, PVTT is a good HAIC target. In general, HAIC seems to be effective for HCC with PVTT. The effects of HAIC are more obvious for HCC with PVTT types III (invasion into the 1st branch of the portal vein) and IV (invasion into the trunk of the portal vein) [50]. Moriguchi et al. [50] reported the effects of HAIC (low dose FP) for HCC with PVTT types III-IV compared with sorafenib treatment (hazard ratio: 0.25). Choi et al. [28] also showed the obvious effects of HAIC when used to treat HCC with PVTT types III-IV (hazard ratio: 0.32). PVTT types III-IV can easily cause liver failure. A high HAIC therapeutic response was more effective in preventing PVTT progression.
A MULTIDISCIPLINARY THERAPEUTIC STRATEGY STARTING FROM HAIC IN THE ERA OF THE CHEMO-DIVERSITY
A multidisciplinary therapeutic strategy is needed in the treatment of advanced HCC [51,52]. The Asian, Korean, and Japanese guideline affirms a multidisciplinary therapeutic strategy combined with systemic treatments and locoregional treatments for advanced HCC, in particular, HCC with PVTT. In the multidisciplinary therapeutic strategy, several patterns are noted. First is a combination treatment of HAIC with systemic therapies. As described before, two large-scale phase 3 studies that combined HAIC with sorafenib were conducted [27,29]. The FOLFOX regimen revealed a significant positive result in combination with sorafenib. Moreover, low-dose FP regimens did not meet the primary endpoint in the whole group but revealed a positive result in the subgroup analysis of the patients with HCC invading the trunk of the portal vein. Additionally, CDDP monotherapy also revealed a positive result in combination with sorafenib in a randomized phase 2 study [33]. Taken together, HAIC is thought to be compatible with systemic therapies. The second pattern noted is the sequential treatment of HAIC with systemic therapies. Recently, Kondo et al. [53] reported a phase 2 trial of sequential HAIC and sorafenib as the initial therapy for HCC, which was named the SCOOP-2 trial. This trial compared a sequential HAIC to sorafenib regimen and sorafenib monotherapy as the initial therapy for advanced HCC. Although the concept of this sequential treatment was interesting, the result of this study was negative. Sequential HAIC using CDDP monotherapy and sorafenib did not improve the survival benefit compared with sorafenib monotherapy. Although the reason for the negative results in this study is not clear, it is thought that the therapeutic power of the CDDP monotherapy regimen in HAIC was insufficient to control advanced HCC. A powerful locoregional treatment such as lipiodol-suspended FP/New FP might be a good candidate for the start of the multidisciplinary treatment. The high ORR can lead to “down-staging” or “down-sizing” for unresectable tumor conditions, with the aim of becoming cancer-free by conversion surgery (Fig. 4). If the initial New FP does not respond, sequential drug therapy should be immediately administered. Sequential drug therapy should also be immediately administered when recurrence develops after the conversion surgery. By choosing multidisciplinary therapy beginning with New FP, we should be able to aim for 5-year survival in patients with MVI-HCC. Multidisciplinary therapy using a powerful locoregional treatment combined with sequential drug therapy should be ideal for treating advanced HCC; however, designing clinical trials for this type of regimen is difficult because the trials are designed as head-to-head studies. Because of this, there is no established clinical evidence for multidisciplinary HAIC therapy with sequential drug therapy. Because of this, a clinical trial examining this combined regimen needs to be designed and performed in the near future, to establish the efficacy of this technique.
CONCLUSION
The clinical evidence of HAIC is gradually increasing; however, clinical evidence supporting its application is still lacking. By increasing the clinical evidence of its efficacy, HAIC should be recognized as a powerful, effective locoregional for advanced HCC. HAIC could be a promising therapeutic modality for the local progression of HCC including PVTT-HCC. HAIC, in particular, lipiodol suspended FP/New FP, has the potential for achieving cancer-free status in combination with conversion therapy in the era of chemo-diversity.
Notes
Authors’ contribution
H.I., T.N., and T.K. conceived the idea of the review. H.I. and S.S. drafted the original manuscript. H.I. and T.K. contributed to visualization of Figures. T.N. and T.K. supervised the content of this review. All authors reviewed the manuscript draft and revised it critically on intellectual content. All authors approved the final version of the manuscript to be published.
Conflicts of Interest
Takumi Kawaguchi received honoraria (lecture fees) from EA Pharma Co., Ltd., Janssen Pharmaceutical K.K., Taisho Pharmaceutical Co., Ltd., and Otsuka Pharmaceutical Co., Ltd. The other authors declare no conflicts of interest.
Acknowledgements
H.I. was supported in part by research grants from The Shinnihon Foundation of Advanced Medical Treatment Research and the Takeda Science Foundation. This work was partly supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant-in-Aid for Scientific Research (C) (Grant Number JP20K08395).
We gratefully acknowledge the work of past and present members our team. A special gratitude we give to Kenji Hirai, Masatoshi Tanaka, Satoshi Itano, and Hiroaki Nagamatsu who significantly contributed to the development of our team.
Abbreviations
5-FU
5-fluorouracil
CDDP
cisplatin
FAIT
5-FU arterial infusion plus interferon therapy
FP
cisplatin plus 5-fluorouracil
EHS
extrahepatic spread
HAIC
hepatic arterial infusion chemotherapy
HCC
hepatocellular carcinoma
MST
median survival time
MTA
molecular targeted agents
PVTT
portal vein tumor thrombus
TACE
transcatheter arterial chemoembolization