| Clin Mol Hepatol > Volume 29(2); 2023 > Article |
|
ABSTRACT
Background/Aims
Methods
Results
Conclusions
ACKNOWLEDGMENTS
FOOTNOTES
Supplementary materials
Supplementary Table 1.
Supplementary Table 2.
Figure 1.
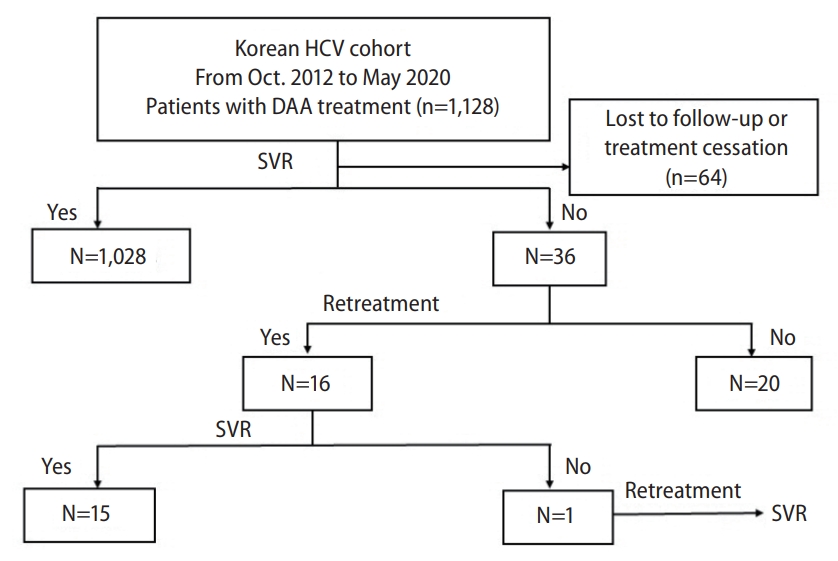
Figure 2.

Table 1.
| Characteristic | Total | Retreatment |
|---|---|---|
| Study population, n | 36 | 16 |
| Age | 63 (56–69) | 62 (55–67) |
| Sex, male/female | 18/18 (50/50) | 10/6 (62.5/37.5) |
| Liver disease status | ||
| Chronic hepatitis | 20 (55.6) | 10 (62.5) |
| Compensated cirrhosis | 5 (13.9) | 3 (6.3) |
| Decompensated cirrhosis | 1 (2.8) | 0 (0) |
| Hepatocellular carcinoma | 10 (27.8) | 4 (25.0) |
| History of HCC* | 3 (8.3) | 2 (12.5) |
| Tumor present† | 7 (19.4) | 2 (12.5) |
| Active tumor‡ | 4 (11.1) | 2 (12.5) |
| HCV Genotype | 20/15/1 (55.6/41.7/2.8) | 8/8/0 (50/50/0) |
| 1b/2/3a | ||
| HCV RNA, log10IU/mL | 6.0 (5.6–6.5) | 6.0 (5.4–6.5) |
| ALT, IU/L | 46 (24–81) | 32 (19–52) |
| Types of failed DAA§ | ||
| Daclatasvir+asunaprevir | 15 (41.7) | 5 (28.4) |
| Sofosbuvir+ribavirin | 13 (36.1) | 8 (47.1) |
| Ledipasvir/sofosbuvir | 6 (16.7) | 4 (23.5) |
| Elbasvir/grazoprevir | 1 (2.8) | 0 (0) |
| Glecaprevir/pibrentasvir | 2 (5.6) | 0 (0) |
| Previous treatment before DAA therapy | ||
| None/IFN/DAA* | 32/4/1 (86.5/10.8/2.7) | 13/3/1 (76.5/17.6/5.9) |
Values are presented as median (interquartile range) or number (%).
DAA, direct-acting antiviral; HCV, hepatitis C virus; ALT, alanine transferase; IFN, interferon; HCC, hepatocellular carcinoma; CT, computed tomography; MRI, magnetic resonance imaging; DCV, daclatasvir; ASN, asunaprevir; LED, ledipasvir; SOF, sofosbubir.
† The presence of a tumor was defined as a lesion on imaging delineated as HCC, including individuals with lesions previously treated with radioembolization or chemoembolization who had evidence of a radiographic tumor response with tumor necrosis;
Table 2.
| Patient No. | Age/sex | Disease status | Failed DAA |
Baseline RAS |
Retreatment |
|||
|---|---|---|---|---|---|---|---|---|
| NS3 | NS5A | NS5B | DAA | SVR | ||||
| 1 | 69/F | CH | DCV+ASN | S122G (0.97)* | Not detected | Not detected | SOF/VEL/VOX 12 w | Yes |
| 2 | 64/F | LC | DCV+ASN | Y56F (0.99) S122 (1) | Y93H (0.51) | S556G (0.48) | LED/SOF+RBV 12 w | No |
| SOF/VEL/VOX 12 w | Yes | |||||||
| 3 | 52/F | CH | DCV+ASN | Not detected | Not detected | Not detected | SOF/VEL/VOX 12 w | Yes |
| 4 | 57/F | CH | DCV+ASN | S122G (0.99) | R30Q (0.99) | C316N (1) | No | |
| 5 | 62/F | HCC | DCV+ASN | Y56F (0.98) | R30Q (1) | S556N (1) | No | |
| 6 | 59/F | CH | DCV+ASN | S122G (1) | Y93N (0.06) Y93C (0.56) | C316N (1) S556G (0.88) | No | |
| 7 | 63/M | LC | DCV+ASN | S122G (0.96) | Y93H (1) | S556G (0.96), C316N (0.98) | No | |
| 8 | 51/M | CH | DCV+ASN | S122G (1) | Not detected | L159F (1), C316N (1), S556G (0.94) | No | |
| 11 | 66/M | CH | LED/SOF | Y56F (0.19) | Y93H (0.34) | C316N (0.87) | GLE/PIB 16 w | Yes |
| 12 | 63/M | HCC | LED/SOF | Not detected | Y93H (1) | Not detected | OMB/PTV/r+DSV+RBV 12 w | Yes |
ASN, asunaprevir; CH, chronic hepatitis; DAA, direct-acting antivirals; HCV, hepatitis C virus; DCV, daclatasvir; DSV, dasabuvir; GLE, glecaprevir; HCC, hepatocellular carcinoma; LC, liver cirrhosis; LED, ledipasvir; OMB, ombitasvir; PIB, pibrentasvir; PTV, paritaprevir; r, ritonavir; RAS, resistance-associated substitution; RBV, ribavirin; SOF, sofosbubir; SVR, sustained virological response; VEL, velpatasvir; VOX, voxilaprevir; w, weeks.
Table 3.
| Patient No. | Age/sex | Disease status | Failed DAA | Time of RAS test |
Posttreatment RAS |
Retreatment |
|||
|---|---|---|---|---|---|---|---|---|---|
| NS3 | NS5A | NS5B | DAA | SVR | |||||
| 1 | 69/F | CH | DCV+ASN | 157 w after ETR | Q80R (0.28)* S122G (1) | L31M (0.99) Y93H (0.99) | Not detected | SOF/VEL/VOX 12 w | Yes |
| 2 | 64/F | LC | DCV+ASN | 14 w after ETR | Y56F (0.99) S122 (1) D168A (0.17) | L31M/V (1) Y93H (1) | S556G (1) | LED/SOF+RBV 12 w | No |
| SOF/VEL/VOX 12 w | Yes | ||||||||
| 9 | 67/F | CH | DCV+ASN | 170 w after ETR | Y56F (0.99) | Y93H (1) | Not detected | SOF/VEL/VOX | Yes |
| 10 | 46/M | CH | DCV+ASN | 25 w after ETR | Y56F (1) S122G (1) | L31M (1) Y93H (0.45) | C316N (1) S556G (0.95) | No | |
| 11 | 66/M | CH | LED/SOF | 166 w after ETR | Not detected | Y93H (1) | Not detected | GLE/PIB 16 w | Yes |
| 13 | 48/M | HCC | SOF+RBV† | 128 w after ETR | Not detected | R30Q (1) Y93H (1) | Not detected | GLE/PIB 8 w | Yes |
ASN, asunaprevir; CH, chronic hepatitis; DAA, direct-acting antivirals; DCV, daclatasvir; ETR, end of treatment; GLE, glecaprevir; HCC, hepatocellular carcinoma; LC, liver cirrhosis; LED, ledipasvir; RAS, resistance-associated substitution; SOF, sofosbubir; SVR, sustained virological response; VEL, velpatasvir; VOX, voxilaprevir; w, weeks
Table 4.
| Patient No. | Age/sex | Disease | Genotype | Failed |
Baseline RAS |
Posttreatment RAS |
Retreatment |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NS3 | NS5A | NS5B | NS3 | NS5A | NS5B | DAA | SVR | |||||
| 1 | 69/F | CH | 1b | DCV+ASN | S122G (0.97)* | Not detected | Not detected | Q80R (0.28) S122G (1) | L31M (0.99) Y93H (0.99) | Not detected | SOF/VEL/VOX 12 w | Yes |
| 2 | 64/F | LC | 1b | DCV+ASN | Y56F (0.99) S122 (1) | Y93H (0.51) | S556G (0.48) | Y56F (0.99) S122 (1) D168A (0.17) | L31M/V (1) Y93H (1) | S556G (1) | LED/SOF+RBV 12 w | No |
| SOF/VEL/VOX 12 w | Yes | |||||||||||
| 11 | 66/M | CH | 1b | LED/SOF | Y56F (0.19) | Y93H (0.34) | C316N (0.87) | Not detected | Y93H (1) | Not detected | GLE/PIB 16 w | Yes |
| 14 | 58/F | CH | 2a/2c | SOF+RBV | Not detected | Not detected | GLE/PIB 8 w | Yes | ||||
| 25 | 48/F | CH | 2a | DCV+ASN† | Not detected | NS5A F28C (1)† | ELB/GRZ+RBV 12 w | Yes | ||||
ASN, asunaprevir; CH, chronic hepatitis; DAA, direct-acting antivirals; DCV, daclatasvir; GLE, glecaprevir; LC, liver cirrhosis; LED, ledipasvir; RAS, resistance-associated substitution; RBV, ribavirin; SOF, sofosbubir; SVR, sustained virological response; VEL, velpatasvir; VOX, voxilaprevir; w, weeks.
Table 5.
| Patient No. | Age/Sex | Disease status | Failed DAA | Baseline RAS | Retreatment | SVR |
|---|---|---|---|---|---|---|
| 14 | 58/F | CH | SOF+RBV | Not detected | GLE/PIB 8 w | Yes |
| 15 | 51/M | CH | SOF+RBV | NS3 Y56F (1)* | GLE/PIB 12 w | Yes |
| 16 | 72/F | CH | SOF+RBV | Not detected | No | |
| 22 | 70/M | HCC | LED/SOF | Not detected | No | |
| 17 | 51/M | HCC | SOF+RBV | Not detected | No | |
| 23 | 48/F | CH | DCV+ASN† | Not detected | ELB/GRZ+RBV 12 w | Yes |
Table 6.
| Patient No. | Age/Sex | Disease status | DAA | Time of RAS test | RAS | Retreatment | SVR |
|---|---|---|---|---|---|---|---|
| 18 | 56/M | CH | SOF+RBV | 76 w after ETR | Not detected | GLE/PIB 12 w | Yes |
| 19 | 76/M | CH | SOF+RBV | 39 w after ETR | Not detected | GLE/PIB 12 w | Yes |
| 20 | 69/M | HCC | SOF+RBV | 30 w after ETR | Not detected | GLE/PIB 12 w | Yes |
| 21 | 69/M | LC | SOF+RBV | 9 w after ETR | Not detected | No | |
| 23 | 48/F | CH | DCV+ASN* | 96 w after ETR | NS5A F28C (1)† | ELB/GRZ+RBV 12 w | Yes |
ASN, asunaprevir; CH, chronic hepatitis; DAA, direct-acting antivirals; DCV, daclatasvir; ELB, elbasvir; ETR, end of treatment; GLE, glecaprevir; GRZ, grazoprevir; HCC, hepatocellular carcinoma; RAS, resistance-associated substitution; RBV, ribavirin; SOF, sofosbuvir; SVR, sustained virological response; w, weeks.
Abbreviations
REFERENCES
- TOOLS
-
METRICS

- ORCID iDs
-
Seungtaek Kim

https://orcid.org/0000-0003-3954-5908Sook Hyang Jeong

https://orcid.org/0000-0002-4916-7990 - Related articles




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement1
Supplement1 Print
Print



