Hepatitis B core-related antigen: A novel and promising surrogate biomarker to guide anti-hepatitis B virus therapy
Article information
Abstract
The current requirement for biomarkers to detect hepatitis B virus (HBV) infection is polarized. One is a fully-automated and highly sensitive measurement system; the other is a simple system for point-of-care testing (POCT) in resource-limited areas. Hepatitis B core-related antigen (HBcrAg) reflects intrahepatic covalently closed circular DNA and serum HBV DNA. Even in patients with undetectable serum HBV DNA or HBsAg loss, HBcrAg may remain detectable. Decreased HBcrAg levels are associated with reduction of the occurrence of hepatocellular carcinoma (HCC) in chronic hepatitis B. Recently, a fully-automated, novel high-sensitivity HBcrAg assay (iTACT-HBcrAg, cut-off value: 2.1 logIU/mL) has been developed. This attractive assay has been released in Japan very recently. iTACT-HBcrAg can be useful for monitoring HBV reactivation and prediction of HCC occurrence, as an alternative to HBV DNA. Moreover, monitoring HBcrAg may be suitable for determining the therapeutic effectiveness of approved drugs and novel drugs under development. Presently, international guidelines recommend anti-HBV prophylaxis for pregnant women with high viral loads to prevent mother-to-child transmission of HBV. However, >95% of HBV-infected individuals live in countries where HBV DNA quantification is not available. Worldwide elimination of HBV needs the scaling-up of examination and medication services in resource-limited areas. Based on this situation, a rapid and easy HBcrAg assay as a POCT is valuable. This review provides the latest information regarding the clinical use of a new surrogate marker, HBcrAg, in HBV management, based on iTACT-HBcrAg or POCT, and introduces novel agents targeting HBV RNA/protein.
INTRODUCTION
Unfortunately, because hepatitis B virus (HBV) relaxed circular DNA is continually converted to covalently closed circular DNA (cccDNA) in the nuclei of hepatocytes [1,2], a life‐long commitment to chronic hepatitis B (CHB) treatment is required. The current therapeutic goal is to achieve a functional cure, which is characterized by sustained undetectable HBV DNA with the seroclearance of hepatitis B surface antigen (HBsAg) [3,4]. Highly sensitive HBsAg assays have been developed to confirm a functional cure of HBV infection [5-8].
Determining the amount and transcriptional activity of cccDNA is also essential for evaluating disease progression and the clinical outcomes of CHB patients [9]. Hepatitis B core-related antigen (HBcrAg) is a surrogate marker of intrahepatic HBV replication. It has shown a good correlation with conventional HBV biomarkers, including HBV DNA and HBsAg [9-11]. HBcrAg is a sensitive biomarker of the continued transcription of cccDNA in hepatitis B e antigen (HBeAg)-negative patients, despite marked HBV DNA suppression by treatment with nucleos(t)ide analogues (NAs) [12]. In addition, monitoring HBcrAg may help determine therapeutic effectiveness, as many new prospective therapeutic anti-HBV agents are premised on concomitant use of NAs [9].
Currently, the majority of new HBV infections occur in highly endemic areas such as China, Southeast Asia, and sub-Saharan Africa [13]. The World Health Organization (WHO) has just published guidance, including options for the validation of elimination of hepatitis B and C as a public health problem [14]. Anti-HBV prophylaxis is recommended for pregnant women with high viral loads to prevent mother-to-child transmission of HBV (DNA ≥200,000 IU/mL) [15-17]. Four options for countryspecific aims are set out in the guidance, including the elimination of mother-to-child transmission of HBV, which is discussed in “The first global guidance from the WHO for country validation of hepatitis B and C elimination” [14]. Despite the fundamental role of HBV DNA in the management of CHB, >95% of HBV-infected people live in countries where HBV DNA quantification is not readily available [18]. Worldwide HBV elimination needs the scaling-up of examination and medication services in low-income and middle-income countries [18]. Based on these circumstances, a rapid and simple HBcrAg assay is effective for point of care testing (POCT) in regions with limited resources.
As mentioned above, the current demand for HBV biomarkers seems to be polarized: an automated and highly sensitive measurement system vs. a rapid and easy system which can be used for POCT in areas with limited resources. This review describes the clinical use of a new surrogate marker, HBcrAg, in the treatment of CHB or HBV reactivation based on a high-sensitivity HBcrAg assay (iTACT-HBcrAg) and a novel strategy of HBV prevention based on POCT. We also introduce novel anti-HBV agents targeting HBV RNA/protein.
HBcrAg: A SURROGATE MARKER OF INTRAHEPATIC HBV REPLICATION
Japan was the first country to recommend testing for HBcrAg in clinical guidelines for CHB management, followed by the greater Asian region and then Europe [16,19,20]. In this section, we introduce the unique features of the HBcrAg assay and focus on its use in the management of CHB, which includes the ability to predict hepatocellular carcinoma (HCC) (both occurrence and recurrence) and HBV reactivation.
Components of HBcrAg and the development of its assays
In 2002, HBcrAg was first reported as a target in the development of a sensitive enzyme immunoassay specific for hepatitis B core antigen (HBcAg) and HBeAg [21]. Further progress was achieved with the development of an assay for the detection of HBcrAg. HBcrAg comprises three products encoded by the precore/core gene on the HBV genome. HBeAg is a circulating peptide, derived from the precore protein by proteolysis and secreted from the hepatocytes [22]. HBcAg is a component of the virion and forms the inner nucleocapsid that surrounds the viral DNA. Empty enveloped particles, which contain the 22-kDa precore protein (p22cr/PreC) and phosphorylated HBcAg (pHBcAg), are DNA- and HBcAg-deficient virion-like particles [23,24]. All three proteins are derived from an identical 149 amino acid sequence [23,25]. Now, HBcAg, empty particles, and HBeAg can all be measured as HBcrAg by serological testing [26,27]. The replication cycle of HBV and components of HBcrAg are shown as Figure 1.
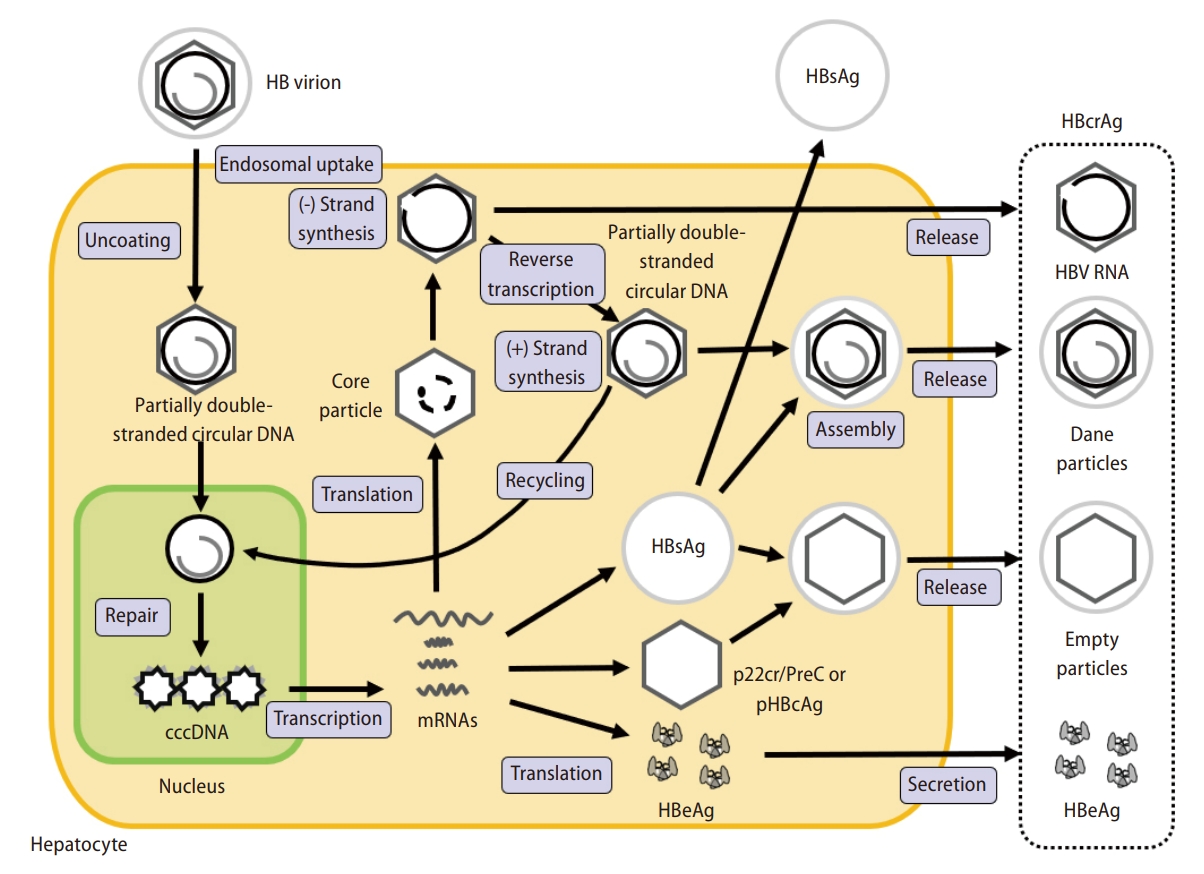
Schema of the life cycle of HBV and HBcrAg. The cccDNA is present as minichromosomes. There are 5 to 50 per hepatocyte. The minichromosomes are recycled as cccDNA to maintain the amount of cccDNA [13]. HBcrAg is produced from cccDNA. HBcrAg is coded from the precore/core region and is a denatured mixture consisting of HBV RNA, HBeAg, HBcAg, and empty particles [21,26,30]. This figure is based on Inoue et al. [9] HBeAg: information on the replication status of the virus. Empty particle. HBcAg: undetectable protein in blood specimens because it is always part of a complex. These different types of protein antigens are translated from mRNAs that are transcription products of cccDNA, which itself is generated by the HBV replication process in hepatocytes [23]. Serologic testing can measure HBeAg, empty particles, and HBcAg altogether as HBcrAg [26,27]. HBV, hepatitis B virus; cccDNA, covalently closed circular DNA; HBcrAg, hepatitis B core-related antigen; HBeAg, hepatitis B envelope antigen; HBcAg, hepatitis B core antigen; pgRNA, pregenomic RNA.
The clinical performance of this assay was evaluated in CHB patients and the HBcrAg concentration correlated positively with the HBV DNA concentration (P<0.001) over a 100,000-fold range [26].
The role of HBcrAg in recent clinical assessments
HBcrAg has been used to support the monitoring of CHB and the prediction of clinical outcomes. In this section, we describe the role of HBcrAg in recent clinical applications.
Changes of the amount of serum HBcrAg during NA therapy
In CHB, HBcrAg production persists, even during NA therapy. Among patients positive or negative for HBeAg and treated with NA, 98% had undetectable serum HBV DNA, although intrahepatic cccDNA still could be detected in 51% [1]. Similar findings have been described in several reports [28-31].
Additionally, serum HBcrAg levels at baseline and changes during NA therapy may also serve as suitable indicators in CHB patients [26,32-35]. The details are described in published reviews [9-11,36].
HBcrAg for assessing the possibility of NA cessation
Assessments of serum HBcrAg levels seem to be effective for identifying patients who do not relapse after stopping NA therapy. Elevated levels of HBcrAg (median, 4.9 logIU/mL) predicted relapse in patients treated with lamivudine (LAM) and who had undetectable HBV DNA for at least 6 months prior to stopping therapy [37]. In a similar study, the virological relapse rate within one year of cessation of NA treatment was evaluated in 68 HBeAg-negative patients with CHB. Virological relapse occurred in 37 (54.4%) of them and subsequent analyses revealed that age (>40 years) and an end-of-treatment HBcrAg level above 3.7 logIU/mL were factors associated with virological relapse [38]. Conversely, an HBcrAg level below 3.4 logIU/mL at the time of cessation of LAM therapy was the only independent factor predictive of not relapsing after the cessation of NA therapy. No patients with HBcrAg levels <3.0 logIU/mL at the cessation of therapy developed alanine aminotransferase (ALT) flares [39].
With reference to the first published papers [37,39], the Japan Society of Hepatology Guidelines for the Management of HBV Infection [19] set out the criteria for the cessation of NA therapy. The three laboratory criteria for the cessation of NA therapy are: (A) at least two years administration of NAs; (B) undetectable serum HBV DNA (using real-time PCR); (C) negative serum HBeAg at time of NA treatment cessation; and (D) when the above criteria are met, it is possible to predict the risk of relapse from HBsAg and HBcrAg levels at the time of cessation of therapy; low serum HBsAg (<80 IU/mL) and HBcrAg (<3.0 logIU/mL) could indicate a group at low-risk of relapse [19].
Regarding entecavir (ETV) and tenofovir disoproxil fumarate (TDF), the HBcrAg level at NA cessation is an independent predictor of relapse, in addition to HBsAg, age, ALT levels, and TDF use [40]. Therefore, the measurement of serum HBcrAg may provide a better assessment of patients for whom NA cessation is planned.
The CREATE study group examined the predictors of the loss of detectable HBsAg in a worldwide cohort of 1,216 HBeAg-negative patients with undetectable HBV DNA who discontinued long-term NA therapy [41]. The probability of HBsAg loss after NA cessation varied according to patient ethnicity, HBV genotype and end-of-treatment viral antigen levels. Patients with low HBsAg (<100 IU/mL) and/or undetectable HBcrAg levels (<2 logIU/mL), particularly those of non-Asian ethnicity or infected with HBV genotype C, appear to be the best candidates for NA cessation [41]. This report shows that HBcrAg is suitable in predicting HBsAg loss after NA cessation.
HBcrAg for predicting HCC occurrence and recurrence
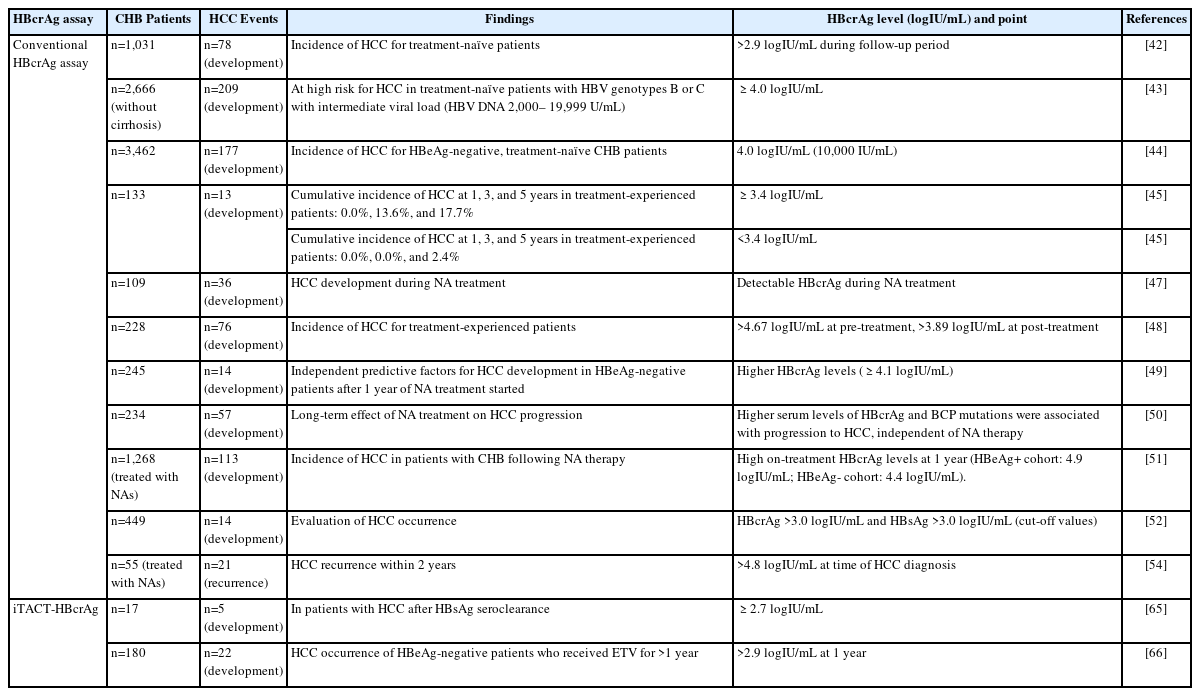
In this subsection, we introduce the relationship between serum HBcrAg levels and HCC occurrence or recurrence in patients receiving NA treatment or not (Table 1).
In treatment-naïve CHB patients, HBcrAg was superior to HBV DNA as a predictor of HCC [42]. During a median follow-up period of 10.7 years, an HBcrAg level >2.9 logIU/mL was independently associated with HCC development in CHB patients without NA treatment [42]. In another report, among treatmentnaïve patients with CHB and an intermediate viral load (serum HBV DNA 2,000–19,999 IU/mL), a serum HBcrAg level of 4.0 logIU/mL was an independent risk factor for HCC [43]. Similarly, a serum HBcrAg level of 10,000 IU/mL (4.0 logIU/mL) stratifies HCC risk in HBeAg-negative patients in the indeterminate phase [44].
For treatment-experienced patients, NA reduced, but did not eradicate, the risk of HCC occurrence [45,46]. In treatment-experienced patients, at the time of disappearance of HBV DNA, the cumulative incidence of HCC at 1, 3, and 5 years was 0.0%, 13.6%, and 17.7%, respectively, in patients with serum HBcrAg levels ≥3.4 logIU/mL but was 0.0%, 0.0%, and 2.4%, respectively, in patients with serum HBcrAg levels <3.4 logIU/mL (P=0.005) [45]. In another report, detectable serum HBcrAg (≥3.0 logIU/mL) in CHB patients who received NA treatment for at least two years was an independent risk factor for HCC (hazard ratio [HR]=3.53) [47]. Additionally, a post-treatment HBcrAg >3.89 logIU/mL predicted progression to HCC (odds ratio [OR]=3.53). Especially, regarding non-cirrhotic patients, HBcrAg >3.90 logIU/mL predicted HCC (OR=5.95) [48]. Moreover, in HBeAg-negative patients after one year from the start of NA treatment, high HBcrAg levels (≥4.1 logIU/mL) were independent predictive factors for HCC development [49]. Regarding the long-term effects of NA treatment on HCC progression, high HBcrAg levels and basal core promoter mutations were associated with HCC progression, independent of NA therapy [50]. Hosaka et al. [51] showed the associated between the reduction in HBcrAg and the risk reduction of HCC incidence during NA treatment. They concluded that patients with persistently high on-treatment HBcrAg levels (cut-off values: 4.9 log U/mL for HBeAg-positive cohort and 4.4 logIU/mL for HBeAg-negative cohort) were more likely to develop HCC, despite sustained viral suppression via long-term NA treatment.
Incidentally, the concomitant presence of HBsAg and HBcrAg can be an efficient biomarker for assessing HCC in patients with CHB. When the cut-off values of serum HBsAg and HBcrAg were set at 3.0 lU/mL and 3.0 logIU/mL, respectively, patients with a history of HCC were frequently found among those with low serum HBsAg and high serum HBcrAg levels [52].
Regarding HCC recurrence-free survival rates, these were significantly lower in HCC patients with high intrahepatic cccDNA and serum HBcrAg levels than those with low cccDNA/HBcrAg levels (P=0.035, P=0.003, respectively) [53]. Recently, the predictive value of the serum level of HBcrAg for HCC recurrence after curative surgical treatment was validated. In a survey of 55 CHB patients, a serum HBcrAg level >4.8 logIU/mL at the time of presurgical HCC detection was related to HCC recurrence within two years of surgery (HR=8.96) [54].
HBV reactivation
There have been few reports on the effectiveness of conventional HBcrAg measurements for predicting HBV reactivation. Among HBsAg-negative and anti-HBc-positive patients undergoing high-risk immunosuppressive therapy, including rituximab for allogeneic hematopoietic stem cell transplantation, those who were HBcrAg-positive had a significantly higher 2-year HBV reactivation rate than those who were HBcrAg-negative (71.8% vs. 31%, P=0.002) [55].
A comparison between HBcrAg and HBV RNA assays
In this section, the features of serum HBV RNA detection, an HBV biomarker with similar applications to HBcrAg, are presented briefly. Circulating HBV RNA might be used as a new serum biomarker for HBV infection, treatment and prognosis [56,57]. Huang et al. [58] examined the correlation between serum HBV RNA and intrahepatic cccDNA levels and found that serum HBV RNA reflected the cccDNA level in HBeAg-positive CHB patients [59]. That is, serum HBV RNA levels differ significantly between HBeAg-positive and HBeAg-negative patients.
Meanwhile, serum HBcrAg correlates with intrahepatic cccDNA levels better than HBV RNA and HBsAg, regardless of HBeAg status. Chen et al. [60] also assessed the correlation of serum HBcrAg with HBV RNA and HBsAg, and investigated whether serum HBcrAg was more useful as an indicator of intrahepatic cccDNA in HBeAg-positive and HBeAg-negative CHB patients. Serum HBcrAg was found to be better correlated with intrahepatic cccDNA than serum HBV RNA and HBsAg, regardless of HBeAg status [60].
A HIGH-SENSITIVITY HBcrAg ASSAY PUT INTO PRACTICAL USE IN JAPAN
Characteristics of the new high-sensitivity HBcrAg assay
We recently developed and described a fully automated high-sensitivity chemiluminescent enzyme immunoassay (CLEIA) for detecting HBcrAg [61]. This novel, high-sensitivity HBcrAg assay (iTACT-HBcrAg; Fujirebio, Inc., Tokyo, Japan) is less expensive than serum HBV DNA assays and can measure serum HBcrAg levels within 30 minutes. The procedure involved in the iTACT-HBcrAg assay is summarized in Figure 2. The sensitivity (2.1 logIU/mL) of the iTACT-HBcrAg assay is approximately 10-fold higher than that of a conventional HBcrAg assay (2.8 logIU/mL). This attractive assay has been launched in Japan recently and has made its way into clinical practice. In this section, we discuss which components of HBcrAg can be detected to contribute to its high sensitivity and the reported clinical use of this assay.

Summary of the process involved in the iTACT-HBcrAg assay [61]. HBV particles and HBeAg/anti-HBe immunocomplexes are modified by pretreatment, and anti-HBe and anti-HBc antibodies are inactivated. RNA particles are produced in patients undergoing nucleos(t)ide analogue treatment. Because the iTACT-HBcrAg assay contains reducing agents in the pretreatment solution, the molecular configuration of HBcrAg can be altered. This action enhances immunoreactivity by increasing the number of detectable molecules and allows the presentation of a novel epitope that was blocked. This figure is based on Inoue et al. [61] HBcrAg, hepatitis B core-related antigen; HBV, hepatitis B virus; HBeAg, hepatitis B e antigen; anti-HBe, hepatitis B e antibody; anti-HBc, hepatitis B core antibody; HBcAg, hepatitis B core antigen; iTACT-HBcrAg, immunoassay for total antigen including complexes via pretreatment (high-sensitivity) HBcrAg assay; AMPPD, 3-(2'-spiroadamantane) 4-methoxy -4-(3"-phosphoryloxy) phenyl-1,2-dioxetane; CLEIA, chemiluminescent enzyme immunoassay.
What does iTACT-HBcrAg detect in the sera of patients with HBV reactivation?
The first report of the iTACT-HBcrAg assay included the early detection of HBcrAg using OptiPrep® density gradient centrifugation analysis [61].
The analysis of HBV-related antigens in the serum of a patient prior to HBV reactivation suggested that HBcrAg detected 133 days before HBV DNA became detectable might be in empty particles. At that time, both serum HBeAg and HBsAg were undetectable by a conventional assay and ultra-high-sensitivity HBsAg immune complex transfer-CLEIA (ICT-CLEIA; Sysmex Corporation, Kobe, Japan) [7], respectively. HBcrAg in the fractions that corresponded to the empty particles in a serum sample obtained 49 days after HBV reactivation were in enveloped empty particles. At that point, HBV DNA was again undetectable, HBeAg was positive (2.6 cut-off index), and HBsAg was positive (0.0135 IU/mL by ICT-CLEIA).
These data suggest that the particles detected 133 days before HBV reactivation are similar to the enveloped empty particles detected 49 days after HBV reactivation, and not consistent with unenveloped particles with a greater density [61,62]. To test this hypothesis, an even more sensitive HBsAg assay than ICT-CLEIA [7] is needed, in addition to iTACT-HBcrAg.
HBV reactivation
iTACT-HBcrAg has an advantage for monitoring patients in the early phase of HBV reactivation. The first report regarding iTACT-HBcrAg confirmed that HBcrAg was detectable by iTACT-HBcrAg before and at the time of HBV DNA positivity in 9 and 2 of 13 patients diagnosed as HBV reactivation [61]. Subsequently, several papers have been published that support this initial report and show more comprehensive clinical features of iTACT-HBcrAg.
Recently, the clinical usefulness of iTACT-HBcrAg was determined in patients with resolved HBV infection after NA treatment for HBV reactivation [63]. Twenty-seven patients with HBV reactivation and systemic chemotherapy for hematological malignancies were analyzed retrospectively. Of 25 patients with detectable iTACT-HBcrAg at the initiation of NA treatment, 17 (68%) achieved iTACT-HBcrAg loss. Recurrence of HBV reactivation after NA cessation was not observed in seven of eight patients who achieved iTACT-HBcrAg loss or were seropositive for anti-HBs during follow-up, except for one without anti-HBs after allogeneic transplantation. These results indicated that iTACT-HBcrAg could be a potential surrogate marker for safe NA cessation in patients with resolved HBV infection after HBV reactivation, in addition to diagnosis of HBV reactivation in the early phase [63].
In another report, the efficacies of measuring iTACT-HBcrAg to detect HBV reactivation and to determine the initiation of NA treatment were assessed [64]. Of 44 patients with HBV reactivation enrolled in the study, 27 had quantifiable serum HBV DNA (≥1.3 logIU/mL), serum HBV DNA being unquantifiable (<1.3 logIU/mL) in the remaining 17 patients. Of the 11 patients with undetectable HBcrAg by iTACT-HBcrAg at HBV reactivation and/or thereafter, 10 had unquantifiable HBV DNA and none developed HBV reactivation-related hepatitis. Taken together, iTACT-HBcrAg is suitable for monitoring HBV reactivation to determine the initiation of NA treatment [64].
Prediction of HCC development
iTACT-HBcrAg might also be useful for predicting HCC development (Table 1).
Recently, the occurrence of HBcrAg ≥2.7 logIU/mL in patients with HCC after HBsAg seroclearance was reported to be significantly higher than the level of HBcrAg in patients who did not develop HCC (100% [5/5] vs. 33% [4/12], P=0.029). In that report, a low but detectable level of HBcrAg measured by iTACT-HBcrAg potentially predicted HCC development, even if HBsAg seroclearance was achieved according to a conventional assay [65].
In a more recent report, a retrospective cohort study of 180 HBeAg-negative patients who received ETV for more than one year was conducted [66]. During follow-up, 22 patients developed HCC. The baseline HBsAg levels were not associated with HCC development. However, high HBcrAg levels at baseline and at year 1 were significantly associated with HCC development (P<0.001). The adjusted HR for HCC incidence was significantly lower in patients with HBcrAg ≤2.9 logIU/mL at year 1 than in those in the high HBcrAg group. These results indicated that the iTACT-HBcrAg assay predicted the occurrence of HCC during anti-HBV treatment better than the conventional assay [66].
Detection of occult HBV infection in CHB patients with HBsAg loss
Wong et al. evaluated the usefulness of iTACT-HBsAg and iTACT-HBcrAg assays in 96 CHB patients with HBsAg seroclearance, documented by standard assays [67]. At 10 years after HBsAg seroclearance, 20.4% and 64.5% of the patients still had detectable HBsAg and HBcrAg, respectively, using the iTACT-assays. Cumulatively, 66 (71%) patients had detectable HBsAg and/or HBcrAg.
These results showed that the iTACT assays detected a low level of HBsAg and/or HBcrAg in >70% of patients, even 10 years after HBsAg seroclearance. In other words, CHB patients with a functional cure may still harbor a low level of HBV protein expression.
Similarly, there is a report that testing for HBcrAg can be valuable in HBV-resolved patients who are negative for HBsAg [68]. In an HBV endemic region, more than 80% of non-hepatitis B/non-hepatitis C-HCC patients were anti-HBc seropositive, and occult HBV infection, a difficult-to-diagnose liver disease, was considered an important risk factor for non-hepatitis B/non-hepatitis C-HCC development. Hsieh et al. [68] reported the efficacy of using HBcrAg to identify a subset of non-hepatitis B/non-hepatitis C individuals with high sufficient risk of HCC in that area.
Contribution of HBcrAg in the development of new drugs
HBcrAg is associated with cccDNA, and plays a pivotal role in the evaluation of cccDNA during the development of new agents against CHB. On the other hand, most anti-HBV agents currently under development are likely to be used in combination with NA, rendering the HBV DNA assay unsuitable for drug efficacy evaluation. HBcrAg, which is unaffected by NA, may be useful to monitor cccDNA activity in hepatocytes during NA treatment. This will be discussed in detail in the later chapter.
CLINICAL APPLICATION OF THE HBcrAg ASSAY IN REGIONS WITH LIMITED RESOURCES
HBV DNA quantification assays and CLEIA for HBcrAg have limited availability and are expensive in resource-limited areas. In this section, we describe recent reports of the clinical application of a simple and unique HBcrAg assay in resource-limited regions such as Africa.
The first global guidance from the WHO for country validation of hepatitis B and C elimination
New WHO Guidance for country validation of viral hepatitis B and C elimination was released during a joint the European Association for the Study of the Liver (EASL)—the Centers for Disease Control and Prevention (CDC)—the European Centre for Disease Prevention and Control (ECDC) and WHO symposium at the EASL International Liver Congress 2021 [14]. This new guidance can contribute to reducing both new infections with hepatitis B and C viruses and deaths from liver cirrhosis and cancer, reaching high coverage (>90%) with program interventions.
Countries are encouraged to pursue the elimination of viral hepatitis B and C together; however, they may choose to apply separately for one of four certification options described below: Option A: elimination of mother-to-child transmission of HBV (as part of triple elimination of human immunodeficiency virus [HIV], syphilis and HBV, or HIV/HBV). Option B: hepatitis C virus (HCV) as a public health problem; Option C: HBV as a public health problem (including elimination of mother-to-child HBV transmission); and Option D: elimination of HBV and HCV together as a public health problem [14].
In this guidance, elimination of mother-to-child transmission is a major focus. To prevent mother-to-child transmission, NA treatment of HBV is recommended for pregnant women with high viral loads (≥200,000 IU/mL) [15-17]. However, >95% of HBV-infected people live in countries where HBV DNA quantification is not easily available [18]. Based on these circumstances, a rapid and easy HBcrAg assay is required in regions with limited resources.
A simple treatment algorithm based on HBcrAg assay
A quantitative serum HBcrAg assay is 5- to 10-fold less expensive than a quantitative serum HBV DNA assay. Identification of Gambian patients with CHB who had indications for anti-HBV treatment was assessed using a new experimental algorithm that did not include a serum HBV DNA assay [69]. A simple treatment algorithm based on an HBcrAg assay alone, without an HBV DNA assay, yielded a large area under the receiver operating characteristic curve (0.91, 95% confidence interval [CI]=0.88–0.95), with a sensitivity of 96.6% and specificity of 85.8% [69]. These results support the concept that the HBcrAg assay might be suitable to replace a serum HBV DNA assay for identifying patients with CHB who need treatment.
Quantification of HBcrAg using dried blood spots
In a recent report, a standardized method to quantify HBcrAg in dried blood spots to identify HBV-infected people with high levels of viremia was developed and assessed [70]. The limit of detection of HBcrAg in dried blood spots in relation to HBV DNA levels was 19,115 IU/mL (4.281 logIU/mL) across the major HBV genotypes (A, B, C, D, and E). A strong linear correlation was confirmed between HBcrAg levels in dried blood spots and HBV DNA levels (r=0.94, P<0.0001) in samples with high viral loads (range: 3.7–7.0 logIU/mL). The tool which uses HBcrAg in dried blood spots shows promise for identifying HBV-infected patients with high levels of viremia who require anti-HBV therapy [70].
A rapid test for HBcrAg based on immunochromatography
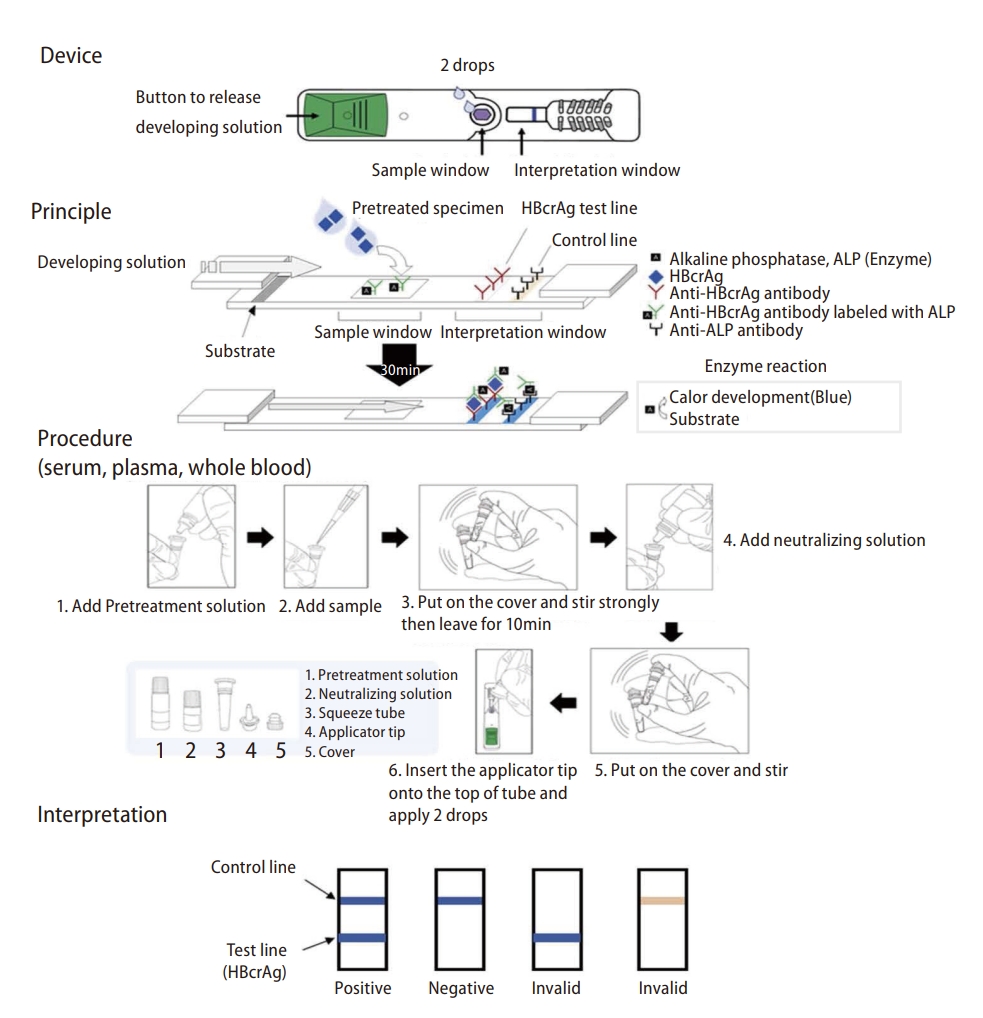
The measurement of HBcrAg requires CLEIA, which remains unavailable in decentralized locations in regions with limited resources. Therefore, a rapid diagnostic test, based on immunochromatography and enabling the detection of HBcrAg (HBcrAg-RDT), was developed and subjected to analytical/clinical validation [71].
HBcrAg-RDT can detect high HBcrAg levels and high viremia in serum, plasma, or whole blood. The sensitivity and specificity of HBcrAg-RDT to diagnose HBV DNA levels were 72.7% and 91.7% for ≥2,000 IU/mL, 86.7% and 88.7% for ≥20,000 IU/mL, and 91.4% and 86.3% for ≥200,000 IU/mL. The sensitivity of HBcrAg-RDT was comparable to HBcrAg-CLEIA and its performance did not vary across HBV genotypes. Its low production cost (USD <5), simple specimen preparation, no requirement for equipment/cold chains, operating temperature (39°C), and rapid turnaround time (45 minutes) all favor its use in regions with limited resources [71]. The HBcrAg-RDT methodology is shown in Figure 3.
DEVELOPMENT OF ANTI-HBV THERAPY AND HBcrAg
NAs, currently approved for the treatment of CHB, are excellent agents with potent activity against HBV DNA. However, the efficacy of NAs against HBsAg is poor, and the probability of achieving HBsAg clearance, a “functional cure”, is low with current treatment methods alone. Therefore, novel HBV therapies aimed at HBsAg clearance are being developed vigorously.
Capsid assembly modulator (CAM)
CAM, a novel agent in development, inhibits the reverse transcription of pgRNA into HBV DNA by blocking capsid formation, the site of reverse transcription. In a phase I study, JNJ-6379 reduced serum HBV DNA levels to -2.86 logIU/mL after 4 weeks of treatment, with no change in HBsAg levels [72]. Although CAM is expected to be used in combination with NA, it is difficult to evaluate the efficacy of CAM by HBV DNA during combination therapy with NA. On the other hand, HBcrAg, which is barely influenced by NA, is a useful test for determining the efficacy of CAM combined with NA. However, because the efficacy of CAM monotherapy is limited with little reduction of HBsAg and resistant mutations have emerged, the focus of development has shifted to HBV RNA inhibitors [72].
Development of HBV RNA inhibitors, agents targeting HBV RNA
HBV RNA inhibitors, a class of direct-acting antiviral agents, have shown good results in suppressing HBsAg and several drugs have entered clinical trials. In addition, a new class of HBV RNA inhibitor is also being developed and is attracting much attention. This chapter outlines the possible mechanisms of action of the HBV RNA inhibitors and the newer therapeutic agents under development and clinical trials.
HBV RNAs, the 3.5 kb messenger RNA (mRNA) that also functions as pregenomic RNA (pgRNA), 2.4 kb and 2.1 kb mRNAs encoding HBsAg, and 0.7 kb mRNA encoding the HBx protein, are transcribed from the HBV genome, present as cccDNA in the nuclei of hepatocytes (Fig. 4). HBV RNA inhibitors include (1) small interfering RNA (siRNA) and (2) antisense oligonucleotide (ASO), which are oligonucleotide therapeutics, and clinical trials are undergoing. In addition, a new drug class, (3) RNA-destabilizer, RNA-binding protein inhibitors, is under development.

Roles of RNA inhibitors. HBV-RNA transcribed from cccDNA is used for HBV replication as mRNA and pregenomic RNA. RNA inhibitors suppress these HBV RNAs. (1), (2) siRNAs and ASOs bind to complementary sequences of HBV RNAs and promote RNA degradation. (3) RNA destabilizers promote RNA degradation by inhibiting RNA-binding proteins and destabilizing HBV-RNAs. These reagents degrade HBV RNAs and inhibit their translation into HBV proteins followed by decreasing of HBsAg and HBcrAg. NAs suppress only reverse transcription. HBV, hepatitis B virus; cccDNA, covalently closed circular DNA; mRNA, messenger RNA; siRNA, small interfering RNA; ASO, antisense oligonucleotide; HBsAg, hepatitis B surface antigen; HBcrAg, hepatitis B core-related antigen; NA, nucleos(t)ide analogue.
siRNA targeting HBV RNA
siRNA comprise small double-stranded RNA molecules, consisting of 21–23 nucleotides, that act directly on the target RNA, together with the AGO complex, and cause RNA interference (RNAi) to degrade the mRNA and inhibit expression of the target gene. Currently, several siRNA-based HBV therapeutics are under development. Studies using human liver chimeric uPA/SCID mice (PXB mice) showed that siRNA targeting HBV-RNA reduced serum HBsAg levels by ten-fold or more [73].
JNJ-3989 is a drug complexed with N-acetylgalactosamine (GalNAc), a liver-directed drug delivery system (DDS). GalNAc binds to asialoglycoproteins expressed on the surfaces of hepatocytes, and its use as a DDS enables efficient liver uptake of target molecules. In a phase I/II clinical trial in combination with NA, monthly subcutaneous administration of JNJ-3989 resulted in a decrease HBsAg of at least 1 logIU/mL in all patients and reduced HBsAg to below 100 IU/mL in 88% of patients. Furthermore, the average HBsAg reduction of more than 1 log IU/mL was maintained up to 6 months after the end of the study [74]. JNJ-3989 and other siRNAs currently in phase 2 trials, VIR-2218, RG-6346, and AB-729, showed potent HBsAg-lowering activity, with 85–92% of subjects achieving HBsAg reductions of ≥1 logIU/mL and more than 50% of subjects achieving less than 100 IU/mL. In addition, a phase IIb study (Reef-1 Study) of a three-drug combination treatment comprising JNJ-3989, NA, and CAM (JNJ-6379) is currently underway. It is also important to analyze whether combination therapy reduces cccDNA in future trials, and HBcrAg may contribute, as its effect on cccDNA can be evaluated even during NA administration.
Antisense oligonucleotides (ASO)
ASOs are short single-stranded DNA or RNA molecules, less than 20 nucleotides long, that are complementary to the target sequence. ASO binds to the complementary RNA and forms a DNA/RNA hybrid, followed by rapid degradation via RNase H1.
ASOs designed for anti-HBV therapy bind to HBV RNA, as 8–10 base DNA strands, with modification to resist degradation by nucleases, and cleavage by RNase H1 prevents translation of the viral protein, which cannot be targeted by NAs.
In a phase II study of GSK3228836 (Beprovirsen, an ASO), of which a primary outcome was a loss of HBsAg and HBV DNA for 24 weeks after the end of treatment, HBsAg was significantly reduced by subcutaneous administration twice a week at the start and once a week thereafter [75]. A higher rate of patients with low baseline HBsAg (≤3 log10IU/mL) achieved the primary outcome (Arm 1: 16% vs. 6%) compared to those with high levels (>3 log10IU/mL). A phase II study is also ongoing for GSK 4388067A.
Adverse events in ASO treatment are mostly mild/moderate, including injection site redness at the time of administration, and the safety profile is considered good [76].
Drugs targeting HBV RNA-binding proteins
A new class of drugs is inhibitors of the RNA-binding proteins that are important for RNA regulation. For HBV RNA, inhibitors of non-classical poly(A) polymerases 5 and 7 (PAPD5/7) are under development as anti-HBV therapeutic agents and have been shown to reduce HBsAg in HBV-infected mouse models [77].
RG-7834 is a novel oral HBV therapeutic agent, belonging to the dihydroquinolidinone family of compounds (DHQ), and acts as an RNA destabilizer by inhibiting PAPD5/7 to degrade HBV RNA [78]. RG-7834 has been shown to selectively inhibit HBV transcription in HBV-infected PXB mice. Although the development of RG-7834 has been suspended because of undisclosed adverse effects, it has been modified to avoid effects on other organs by combination with a liver-specific delivery system [79]. AB-452 has also been shown to act as an RNA destabilizer targeting PAPD5/7, and to have superior HBsAg-lowering properties in vitro [80].
The PAPD5/7 inhibitor GSK3965193 is currently in phase I and II trials as a single agent and in combination with Bepirovirsen, an ASO. In preclinical studies of GSK3965193 and Bepirovirsen in an HBV-infected mouse model using AAVHBV, dose studies were conducted with GSK3965193 and Bepirovirsen as single agents and combination therapy with the two agents [81]. The results showed that GSK3965193 administered per oral as a single agent, resulted in a maximum 1 log reduction of HBsAg levels. Bepirovirsen was also administered subcutaneously with a maximum 2 log reduction of HBsAg levels. Furthermore, a 3-log reduction of HBsAg was observed when both drugs were used in combination, exceeding the effect of either as monotherapy.
DISCUSSION
Here, we have discussed the future prospects for HBcrAg diagnostics. HBcrAg is a useful novel biomarker for the management of CHB, including HBV reactivation and predicting HCC occurrence. HBcrAg is appropriate for evaluating the amount of intrahepatic cccDNA in CHB patients. As noted in the introduction, when we look at HBV infection on a global scale, there now appear to be two separate demands for HBV marker assays. One is an automated and highly sensitive assay system and the other is a simple assay system that can be used as a POCT in resource-limited areas.
Just recently, iTACT-HBcrAg was launched in Japan and appeared in clinical practice. The advantages of this assay include that it does not require specific skills, it is less expensive than serum HBV DNA assays, and it has a more rapid turnaround time than the conventional HBcrAg assay, with results available within 30 minutes from the automated system, rather than the approximately 7 hours required for serum HBV DNA assays [61]. Additionally, iTACT-HBcrAg should be valuable for creating and assessing the anti-HBV treatments that are currently under development and that target intrahepatic cccDNA or function as fibrolytic agents.
Recently, Wu et al. mentioned the pros and cons of HBcrAg in their review [82]. At this time, we would like to present data that does not give HBcrAg much credit and ponder points for the future use of HBcrAg. The promising surrogate marker should reflect the kinetics of cccDNA [83]. The serum HBcrAg level strongly reflects the amount of cccDNA prior to NA treatment. Wang et al. [84] have reported that the decrease of HBcrAg correlated with the decline of cccDNA level after 96 weeks’ NAs therapy in HBeAg-positive patients (r=0.282, P=0.043) [84].
In their study investigating the ability of HBcrAg to predict HBV relapse in HBeAg-negative patients after cessation of ETV therapy, Huang et al. [85] reported that a baseline HBcrAg of 4 logIU/mL was the optimal cut-off value for predicting HBV relapse. Meanwhile, HBcrAg at the end of treatment was not a significant predictor of virological or clinical relapse after the cessation of ETV [85]. In another report, Seto et al. [86] described the effectiveness of measuring HBV RNA for determining the suitability of treatment cessation. While end-of-treatment serum HBV RNA and off-treatment serial HBV RNA were both independently associated with HBV DNA >2,000 IU/mL, serum HBcrAg measurement is not essential for deciding on ETV cessation in patients with CHB, especially those with low HBsAg levels [86]. Based on these reports, we have to conclude that the serum HBcrAg level can be valuable for treatment-naïve CHB patients; nevertheless, its value might be limited for patients on-treatment. We need to continue to accumulate and examine clinical data regarding HBV biomarkers, including HBcrAg, HBsAg and HBV RNA, to clarify further the situations in which HBcrAg can be used appropriately.
Moreover, the clinical utility of HBcrAg, compared to conventional markers of HBV replication and disease activity, is unclear. Ghany et al. [87] categorized untreated participants according to the phase of CHB, based on HBsAg and HBeAg status and HBV DNA and ALT levels. Associations of a higher HBcrAg level with higher ALT, APRI, and Fibrosis-4 levels were consistent in the HBeAg-negative, but not HBeAg-positive, phases. Despite clear relationships between the HBcrAg level and CHB phases, these markers have limited additional value in differentiating CHB phases because of their strong association with HBV DNA [87].
Meanwhile, the development of POCT for more convenient and accurate HBV markers was a long-held desire to efficiently identify individuals infected with HBV. An immunochromatography assay for HBcrAg and the system which uses dried blood spots has shown promise for identifying HBV-infected patients with high levels of viremia who require antiHBV therapy and its use in regions with limited resources is reasonable. In locations like Africa, where the testing site and home are far apart, we must consider the possibility that once a person leaves the testing site or clinic, he/she may never return. In this respect, the HBcrAg-RDT reported recently [71] makes sense and is an excellent technique consistent with local requirements.
HBV RNA inhibitors potently suppress surface protein synthesis and have excellent HBsAg-lowering properties. They may aim to achieve a functional cure by suppressing the production of HBsAg and contributing to the recovery of exhausted host immune responses. In addition, HBV RNA inhibitors target almost all RNAs transcribed from cccDNA and are expected to be superior HBcrAg inhibitors, among all drugs currently in development. On the other hand, the oligonucleotide therapeutics, siRNA and ASO, are inevitably associated with the problems of possible resistance mutations and off-target effects on the host. In addition, the new agents need further studies not only to prove efficacy but also to define safety better. In uncontrolled clinical trials of Bepirovirsen, adverse events occurred frequently; in particular, elevated ALT levels, which were more common in patients without NA than with NA [88]. It may be better to use NA therapy in combination with these new agents.
RNA destabilizers, a new class of anti-HBV agents, target RNA-binding proteins derived from the host and are therefore unlikely to induce resistance mutations. In addition, they are orally administered drugs, which may contribute to good adherence. Notably, AB-452, an RNA destabilizer, made the pgRNA bands almost completely invisible when used in combination with GLS-4, a CAM [80]. The HBV RNA destabilizer, when used in combination with CAM, may work more effectively by inhibiting capsid formation and keeping pgRNAs bare state. In other words, combining an HBV RNA destabilizer with CAM may have a more potent effect and bring about cccDNA and HBcrAg reduction.
Although siRNA, ASO, and RNA destabilizers are promising drugs with excellent therapeutic effects, they are considered to have limitations in monotherapy as HBsAg may rise again after discontinuation of medication. Therefore, in order to achieve a functional cure, it will be important to develop combination therapy with currently approved NAs, and agents under development that inhibit HBV DNA synthesis, such as core assembly modulators, and combined with immune modulators such as PD1 inhibitors and TLR agonists. HBcrAg could be very useful and make a significant contribution to the development of new therapies.
CONCLUSION
In this review, we have described the clinical use of a novel surrogate marker HBcrAg in the management of CHB and new anti-HBV therapies targeting HBV RNA. With further validation with global studies, HBcrAg is expected to become a next-generation biomarker for many characteristics of clinical practice in CHB.
Notes
Authors’ contribution
Conceptualization, T.I., T.W. and Y.T.; Writing the Original Draft, T.I. and T.W.; Writing, Review, & Editing, Y.T.
Conflicts of Interest
Lecture Fees: Gilead Sciences, Inc and GlaxoSmithKline PLC. (Yasuhito Tanaka).
Research fundings: Fujirebio, Inc. and Sysmex Corporation (Takako Inoue and Yasuhito Tanaka),Gilead Sciences, Inc., Janssen Pharmaceutical K.K., GlaxoSmithKline PLC. (Yasuhito Tanaka).
Acknowledgements
Figure 1 in this article will be published in Comprehensive Guide to Hepatitis Advances, 1st edition, Editors: Wai-Kay Seto, Mohammed Eslam, Noninvasive assessments of disease severity: biomarkers, ISBN: 9780323983686, Copyright Elsevier (2023).
Abbreviations
HBV
hepatitis B virus
POCT
point-of-care testing
HBcrAg
hepatitis B core-related antigen
HCC
hepatocellular carcinoma
CHB
chronic hepatitis B
cccDNA
covalently closed circular DNA
HBsAg
hepatitis B surface antigen
HBeAg
hepatitis B e antigen
NAs
nucleos(t)ide analogues
HBcAg
hepatitis B core antigen
pHBcAg
phosphorylated HBcAg
LAM
lamivudine
ALT
alanine aminotransferase
ETV
entecavir
TDF
tenofovir disoproxil fumarate
CLEIA
chemiluminescent enzyme immunoassay
COI
cut-off index
EASL
European Association for the Study of the Liver
CDC
Centers for Disease Control and Prevention
ECDC
European Centre for Disease Prevention and Control
HIV
human immunodeficiency virus
HCV
hepatitis C virus
CAM
capsid assembly modulator
ASO
anti-sense oligonucleotide
DDS
drug delivery system
iTACT-HBcrAg
high-sensitivity HBcrAg assay
PD1
programmed cell death 1
TLR
toll-like receptor
BCP
basal core promoter