| Clin Mol Hepatol > Volume 29(2); 2023 > Article |
|
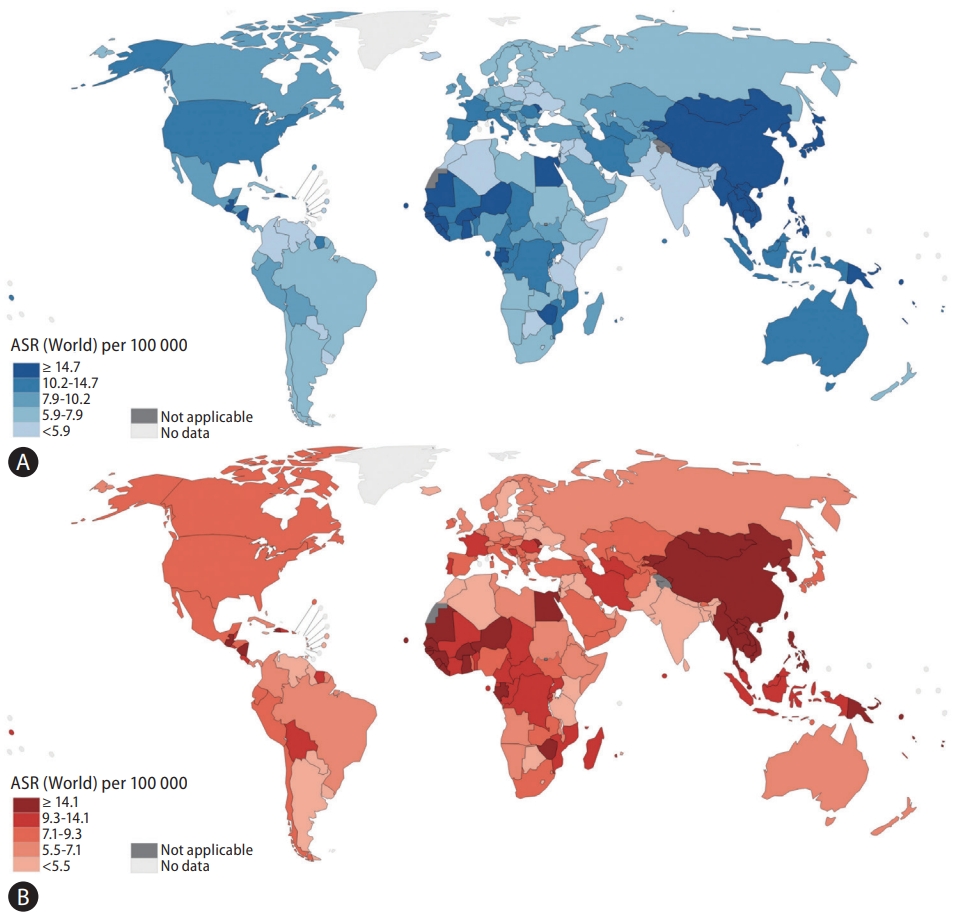
The face of primary liver cancer has evolved over the past century and is one of the most commonly diagnosed cancers in the world. Despite advancements in screening and treatment, primary liver cancer remains deadly, with global mortality rates closely matching incidence. According to data from Global Cancer Statistics 2020, liver cancer is the sixth most common type of cancer, yet it has the third highest mortality rate [1]. There is a male predominance in the burden of primary liver cancer with a ratio of 2-3:1, such that primary liver cancer is the second leading cause of cancer-related mortality among men [1]. Primary liver cancer may refer to any malignancies within the liver, including angiosarcoma or intrahepatic cholangiocarcinoma, though it usually refers to hepatocellular carcinoma (HCC), which makes up 75–85% of all cases [1]. HCC typically develops in patients with liver cirrhosis, and the most important etiologies of underlying liver disease associated with the development of HCC are chronic hepatitis B virus (HBV) infection, chronic hepatitis C virus (HCV) infection, alcohol-related liver disease (ALD), and nonalcoholic fatty liver disease (NAFLD) [2]. Incidence and mortality of primary liver cancer vary widely across the globe mainly due to variations in risk factor profiles and etiology from region to region (Fig. 1) [1,2]. Many nations have expanded programs for screening, treatment, and prevention of primary liver cancer and its predominant risk factors, which have already begun affecting trends in cancer incidence [3]. For example, China reported a 65% reduction in HCC-related mortality between 1982 and 2009 after enacting agricultural policy reforms in the 1980s, which improved storage methods of rice and maize, subsequently reducing the burden of aflatoxin exposure in the country [4].
In a recent issue of the Clinical and Molecular Hepatology, Choi and colleagues [5] reported global trends in age-standardized rates of incidence, mortality, and disease-adjusted life years (DALYs) for primary liver cancer between 1990 and 2019 in order to better characterize the effect of targeted interventions at reducing the burden of primary liver cancer and its main risk factors. This study used data from the Global Burden of Disease (GBD) database from 1990 to 2019, which includes data from 204 countries and territories extracted from censuses, household surveys, civil registration and vital statistics, disease registries, health service use, and other sources [6]. In this study, age-standardized rates of prevalence (10.2 [95% uncertainty interval, UI]: 9.2–11.3] to 9.1 [95% UI: 8.3– 10.0]) and incidence (9 [95% UI: 8.1–10] to 6.5 [95% UI: 5.9– 7.2]) showed a decline between 1990 and 2019. Similarly, global age-standardized DALYs and mortality rates decreased between 1990 and 2019 by 41.5% (95% UI: 31.5–49.8) and 33.4% (95% UI: 23.2–41.9), respectively. Most of this decline happened between 1996 and 2012, after which the agestandardized mortality rates remained stable without further decrease. Despite the decline in age-standardized data, crude numbers of DALYs and deaths from liver cancer increased over time. This effect is likely caused by a net improvement in disease outcomes alongside an increasing population of older adults. This study showed that HBV remains the leading cause of liver cancer mortality and incidence, followed by HCV, ALD, and NAFLD. In regions with a relatively high burden of HCC related to HBV, such as East Asia and Asia Pacific, age-standardized mortality and incidence rates were highest, though they experienced the most dramatic decrease over the study period. Conversely, agestandardized DALYs rates increased most dramatically in Central Asia (150.2%), followed by North America (107%) and Australasia (94.8%), where rates of ALD and NAFLD are on the rise.
It is important to note that, though Western Sub-Saharan Africa and Southeast Asia had higher age-standardized DALYs rates in 2019 than East Asia (76.7 and 176.4 vs. 69.1, respectively), neither region experienced as drastic of a percent decrease between 1990 and 2019 as was seen in East Asia (-17.2% and -12.2% vs. -65.5%, respectively). This difference is likely related to variance in implementing national vaccination programs against HBV and accessing treatment with nucleos(t)ide analogue antivirals [2]. After China adopted a universal immunization program against HBV in 1992, there was a decline in the seroprevalence of hepatitis B surface antigen in children under 5 years old, which fell to <1.0% by 2006 [7]. Taiwan, whose universal HBV vaccination program started 8 years earlier, experienced a similar effect [4]. In contrast, only seven countries in Sub-Saharan Africa had formalized a plan to combat viral hepatitis by 2017, and only 10 of 47 countries in Africa included a birth dose in their routine recommended immunization program [8].
Additionally, the lack of nationalized HCC surveillance programs and lower access to healthcare resources result in higher rates of advanced-stage liver cancer or severe liver dysfunction at presentation in Sub-Saharan Africa [9]. A multinational retrospective observational cohort study from 2016 showed that 72% of patients in Sub-Saharan Africa had Barcelona- Clinic Liver Cancer stage D HCC at the time of diagnosis, and only 3% would go on to receive any HCC-directed treatment [10]. Even in high-income countries like the United States, racial and ethnic minorities experience disparities regarding HCC outcomes, as non-Hispanic Blacks are less likely to be diagnosed with early-stage disease [11]. In addition, non- Hispanic Blacks experience 12% higher HCC-related mortality than non-Hispanic Whites, based on the data from the Surveillance, Epidemiology, and End Results program between 1995–2006 [12].
In this study, the regions with the highest age-standardized DALYs rate for HCV-associated liver cancer in 2019 were highincome Asia Pacific, North Africa, and Middle East [5]. This reflects the HCV epidemics in Japan and Egypt that resulted from the use of shared needles as part of nationwide antischistosomal campaigns that started in the 1950s [4,13]. In response to this epidemic, Egypt established a national program for screening and treating viral hepatitis and aimed to eliminate HCV by 2030 [14]. Other nations have made significant progress in controlling HCV as well. For example, age-standardized HCC-related mortality decreased in the United States by an annual rate of -3.5% (95% confidence interval, -5.9 to -1.1) between 2014 and 2018, reflecting the impact of direct-acting antiviral agents for the treatment of HCV [15].
While national programs for screening, vaccination, and treatment of viral hepatitis played a prominent role in reducing age-standardized DALYs and mortality rates [16], the rates of ALD and NAFLD-related liver cancer are increasing at an alarming rate. Data from GBD do not even capture the anticipated rise in ALD and NAFLD due to increased alcohol consumption and rates of obesity observed during and after the coronavirus disease 2019 (COVID-19) pandemic [17]. What is particularly alarming about this trend is how challenging it is to address these conditions compared to viral hepatitis. Unlike viral hepatitis, which can be cured or suppressed with oral antiviral agents, treatment of ALD and NAFLD requires multimodal and multidisciplinary approaches to treatment beyond medication.
This study skillfully utilized one of the only major multinational cancer databases to demonstrate trends in primary liver cancer across the globe, including the patient-centered composite metric of DALYs. Most limitations of the study pertain directly to the source data. While both GBD and Globocan are established and well-designed, they take different approaches to data collection. Globocan prioritizes data from national registries. This strategy results in high-quality inputs when data is available, but many low-income countries do not have national cancer registries [18]. Globocan works around this by estimating a county’s cancer burden by extrapolating data from neighboring countries, which may have drastically different geopolitical influences affecting the epidemiology of certain liver cancer risk factors. When national registries are not available for a population, GBD uses alternative sources of information, such as surveys or verbal autopsies. Because of these heterogeneous approaches, noticeable discrepancies have been observed between the two databases. For example, age-standardized incidence rates of lip and oral cancers dramatically differed between Globocan 2020 and GBD 2019 for Papua New Guinea, Vietnam, China, Pakistan, and Indonesia, likely because these countries did not have quality data regarding lip and oral cancers in their national registries [19]. Additionally, the data in question is now more than 3 years old. Though the most recent iteration of Globocan includes data from 2020, both major data sets have yet to capture any effects of the recent COVID-19 global pandemic. Lastly, these databases describe cancer in terms of site of occurrence. Since the different types of primary liver cancer have different risk factors, it would be helpful to be able to examine trends specifically for HCC, separate from other primary liver cancers.
In summary, Choi and colleagues [5] utilize cancer epidemiological data from the large, multinational GBD 2019 database to demonstrate that age-standardized rates of DALYs, mortality, incidence, and prevalence declined from 1990 to 2019. While HBV remains the world’s predominant risk factor for liver cancer, the global burden of viral hepatitis is decreasing, while ALD- and NAFLD-related liver cancer is on the rise. The necessary next steps should be identifying how the COVID-19 pandemic and the continued advancement of treatment strategies for chronic liver disease have altered these trends.
ACKNOWLEDGMENTS
The work had no funding sources, which could have influenced the study design and conduct, analysis, interpretation of the data, review, and approval of the manuscript.
FOOTNOTES
Authors’ contributions
Dr. Peter Konyn was involved in the study concept and design, acquisition of data, interpretation of data, and drafting of the manuscript. Dr. Aijaz Ahmed and Dr. Donghee Kim were involved in the study concept and design, interpretation of data, drafting of the manuscript, critical revision of the manuscript, and study supervision.
Figure 1.
Estimated global age-standardized incidence rates and mortality rates for primary liver cancer in adults (over 20 years of age), 2020. (A) Global age-standardized incidence rates for primary liver cancer in adults. (B) Global age-standardized mortality rates for primary liver cancer in adults. ASR, age-standardized rate. Data source: World Health Organization GLOBOCAN 2020 <http://gco.iarc.fr/today> Accessed 15 Feb, 2023 [18].
REFERENCES
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209-249.



2. Konyn P, Ahmed A, Kim D. Current epidemiology in hepatocellular carcinoma. Expert Rev Gastroenterol Hepatol 2021;15:1295-1307.


3. Korean Liver Cancer Association (KLCA) and National Cancer Center (NCC) Korea. 2022 KLCA-NCC Korea practice guidelines for the management of hepatocellular carcinoma. Clin Mol Hepatol 2022;28:583-705.




4. Goh GB, Chang PE, Tan CK. Changing epidemiology of hepatocellular carcinoma in Asia. Best Pract Res Clin Gastroenterol 2015;29:919-928.


5. Choi S, Kim BK, Yon DK, Lee SW, Lee HG, Chang HH, et al. Global burden of primary liver cancer and its association with underlying aetiologies, sociodemographic status, and sex differences, 1990-2019: a DALYs-based analysis of the Global Burden of Disease 2019 study. Clin Mol Hepatol 2023;29:433-452.



6. GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020;396:1204-1222 Erratum in: Lancet 2020;396:1562.


7. Liang X, Bi S, Yang W, Wang L, Cui G, Cui F, et al. Epidemiological serosurvey of hepatitis B in China--declining HBV prevalence due to hepatitis B vaccination. Vaccine 2009;27:6550-6557.


8. Okeke E, Davwar PM, Roberts L, Sartorius K, Spearman W, Malu A, et al. Epidemiology of liver cancer in Africa: Current and future trends. Semin Liver Dis 2020;40:111-123.


9. Kedar Mukthinuthalapati VVP, Sewram V, Ndlovu N, Kimani S, Abdelaziz AO, Chiao EY, et al. Hepatocellular carcinoma in Sub-Saharan Africa. JCO Glob Oncol 2021;7:756-766.

10. Yang JD, Mohamed EA, Aziz AO, Shousha HI, Hashem MB, Nabeel MM, et al.; Africa Network for Gastrointestinal and Liver Diseases. Characteristics, management, and outcomes of patients with hepatocellular carcinoma in Africa: a multicountry observational study from the Africa Liver Cancer Consortium. Lancet Gastroenterol Hepatol 2017;2:103-111 Erratum in: Lancet Gastroenterol Hepatol 2022;7:704.


11. Rich NE, Hester C, Odewole M, Murphy CC, Parikh ND, Marrero JA, et al. Racial and ethnic differences in presentation and outcomes of hepatocellular carcinoma. Clin Gastroenterol Hepatol 2019;17:551-559.e1.



12. Mathur AK, Osborne NH, Lynch RJ, Ghaferi AA, Dimick JB, Sonnenday CJ. Racial/ethnic disparities in access to care and survival for patients with early-stage hepatocellular carcinoma. Arch Surg 2010;145:1158-1163.


13. Gomaa A, Allam N, Elsharkawy A, El Kassas M, Waked I. Hepatitis C infection in Egypt: prevalence, impact and management strategies. Hepat Med 2017;9:17-25 Erratum in: Hepat Med 2017;9:35.




14. Hassanin A, Kamel S, Waked I, Fort M. Egypt’s ambitious strategy to eliminate hepatitis C virus: A case study. Glob Health Sci Pract 2021;9:187-200.



15. Kim D, Konyn P, Cholankeril G, Wong RJ, Younossi ZM, Ahmed A; Hepatocellular Carcinoma Research Committee for Chronic Liver Disease Foundation. Decline in annual mortality of hepatitis c virus-related hepatocellular carcinoma in the United States, from 2009 to 2018. Gastroenterology 2020;159:1558-1560.e2.


16. Goh MJ, Sinn DH, Kim JM, Lee MW, Hyun DH, Yu JI, et al. Clinical practice guideline and real-life practice in hepatocellular carcinoma: Korea perspective. Clin Mol Hepatol 2023;29:197-205.



17. Kim D, Alshuwaykh O, Dennis BB, Cholankeril G, Ahmed A. Trends in etiology-based mortality from chronic liver disease before and during COVID-19 pandemic in the United States. Clin Gastroenterol Hepatol 2022;20:2307-2316.e3.



18. Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, et al. Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer, 2020.
-
METRICS

-
- 0 Crossref
- 0 Scopus
- 2,576 View
- 57 Download
- ORCID iDs
-
Donghee Kim

https://orcid.org/0000-0003-1919-6800 - Related articles
-
The latest global burden of liver cancer: A past and present threat2023 April;29(2)
Recent trends in the treatment of chronic hepatitis C2012 March;18(1)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



