Acute-on-chronic liver failure: Terminology, mechanisms and management
Article information
Abstract
Acute-on-chronic liver failure is an acute deterioration of liver function manifesting as jaundice and coagulopathy with the development of ascites, with a high probability of extrahepatic organ involvement and high 28-day mortality. The pathogenesis involves extensive hepatic necrosis, which is associated with severe systemic inflammation and subsequently causes the cytokine storm, leading to portal hypertension, organ dysfunction, and organ failure. These patients have increased gut permeability, releasing lipopolysaccharide (LPS) and damage-associated molecular patterns (DAMPS) in the blood, leading to hyper-immune activation and the secretion of cytokines, followed by immune paralysis, causing the development of infections and organ failure in a proportion of patients. Early detection and the institution of treatment, especially in the "Golden Window" period of 7 days, gives an opportunity for reversal of the syndrome. Scores like the Asian Pacific Association for the Study of the Liver (APASL) ACLF research consortium (AARC) score, a model for end stage liver disease (MELD), and the CLIF Consortium acute-on-chronic liver failure (CLIF-C ACLF) score can help in the prediction of mortality. Treatment strategy includes treatment of acute insult. Patients should be considered for early transplant with MELD score >28, AARC score >10, high-grade hepatic encephalopathy, and in the absence of >2 organ failure or overt sepsis to improve survival of up to 80% at five years. Patients, with no option of transplant, can be treated with emerging therapies like faecal microbial transplant, plasma exchange, etc., which need further evaluation.
INTRODUCTION
Acute-on-chronic liver failure (ACLF) is an acute deterioration in the function of the liver, with significant systemic inflammation and high short-term mortality [1].People with liver failure have a very high mortality and morbidity rate. The list of causes of liver failure is constantly growing, and treating these patients is certainly very challenging. Liver failure can manifest as acute liver failure or as ACLF. When an acute insult causes liver failure in a patient with underlying chronic liver disease, it is referred to as ACLF. While various societies define the ACLF condition differently, the symptoms usually involve acute deterioration of liver function manifesting as liver failure and other non-hepatic organ failures with high short-term mortality [1].
CURRENT DEFINITIONS AND CONCEPT
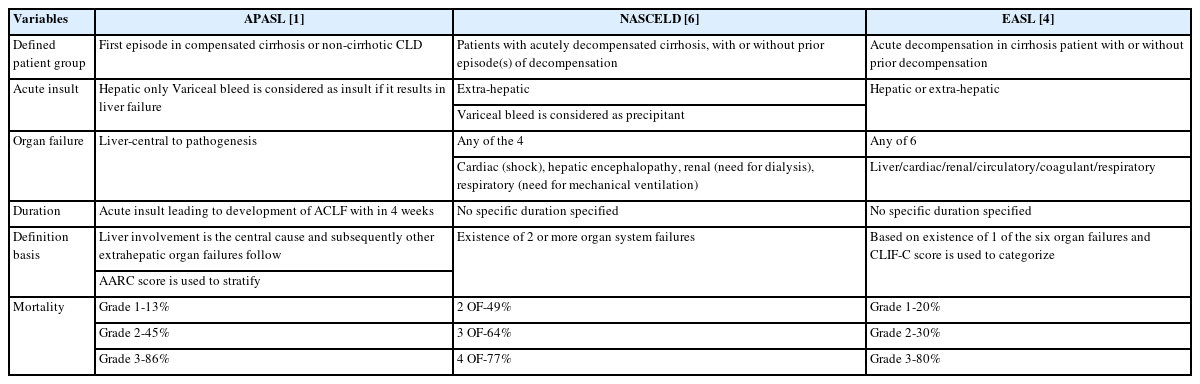
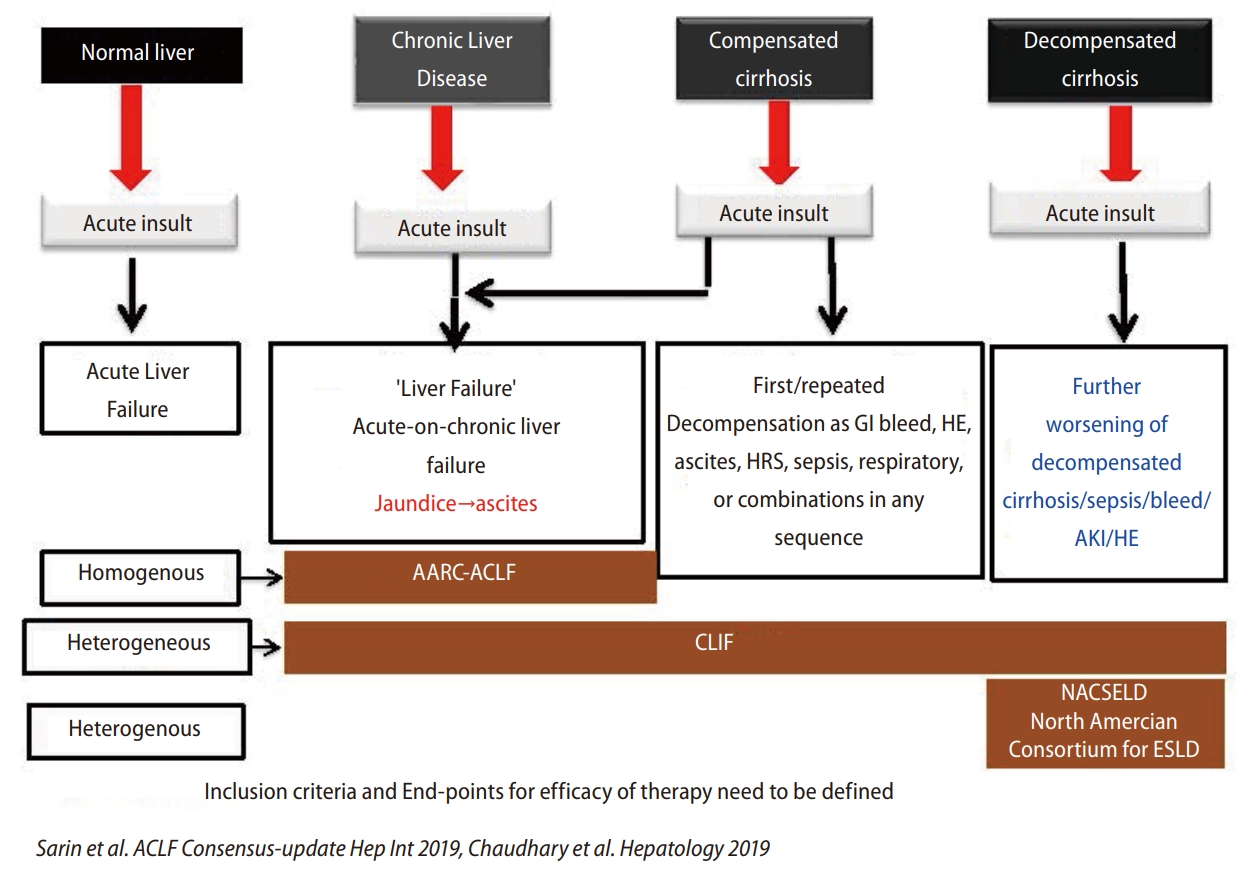
Asian Pacific Association for Study of Liver (APASL) has defined this condition as a syndrome with an acute hepatic insult manifesting as jaundice (serum bilirubin ≥5 mg/dL (≥85 micromole/L) and coagulopathy (INR ≥1.5 or prothrombin activity <40%) complicated within 4 weeks by clinical ascites and/or hepatic encephalopathy (HE) in a patient with previously diagnosed or undiagnosed chronic liver disease, i.e., with or without cirrhosis, with high 28-day mortality. While European association of study of liver (EASL) defines it as acute decompensation in a cirrhotic patient with or without prior decompensation [1,2]. The difference between APASL, EASL, and the North American Consortium for the Study of End-Stage Liver Disease (NACSELD-ACLF) criteria is shown in Table 1. According to the APASL definition, ACLF does develop in patients with grade 3 or 4 fibrosis and not necessarily in cirrhosis. This has been elucidated in Figure 1, which shows the spectrum of the disease and various definitions from different societies [1,2].
Various definitions of liver failure. Illustrating the differences in the definition by various societies and its basis and time frame. APASL, Asian Pacific Association for the Study for the Liver; EASL, European Association for the Study of the Liver; NASCELD-ACLF, North American Consortium for the Study of End-Stage Liver Disease's definition of acute-on-chronic liver failure; GI, gastro-intestinal bleed; HE, hepatic encephalopathy; HRS, Hepatorenal syndrome; AK, acute kidney injury).
Baveno VII guidelines suggested the concept of compensated advanced chronic liver disease (cACLD) in 2021 to describe the continuum of severe fibrosis and cirrhosis in patients with chronic liver disease based on liver stiffness measurement (LSM). This helps in stratifying the risk of clinically significant portal hypertension (CSPH) and decompensation at the point of care.3 Studies have shown that if ACLF patients survive the first 4 weeks of illness, they have a high chance of resolution of ascites, jaundice, and hepatic encephalopathy [1,2].
EPIDEMIOLOGY AND PATHOGENESIS
ACLF is a severe medical ailment that affects a large population across the globe. Its prevalence ranges from 20–35% among the population at risk, for example, those suffering from viral hepatitis (hepatitis B virus [HBV] or hepatitis C virus [HCV] infection), excessive alcohol intake, or suffering from NASH, etc [1-4]. With the rising epidemics of obesity and NASH, the proportion of ACLF is bound to rise with increased mortality. As demonstrated in a follow-up study of 80,383 cirrhosis patients for 3.5 years, 574 (APASL definition), 4,276 (EASL definition), and 783 (both definitions) patients developed ACLF [1-6]. Mortality was also high among grade III ACLF patients, with a mortality rate of up to 74% vs. 1.9 percent among those without ACLF presentation [7-10].
Acute precipitating events and chronic insults
Acute precipitating events
The precipitating events can be classified as hepatic or non-hepatic, among the most common causes are a reactivation of the chronic HBV infection, acute hepatitis A and E virus (HAV/HEV) infection, alcohol-associated hepatitis (AH), and an acute bacterial infection. Among the Asian population, precipitating events can be identified in 95% of cases [1]. While in the western population, alcoholism and bacterial infection were important triggering events [2], which can be identified among 60% of cases at presentation [11,12]. HAV is responsible for 10–30% of acute hepatitis and about 5–15% of liver failure cases in India [13,14]. HEV infection is endemic in Asia and Africa, with a median incidence of 21% (range 4–72%) [15]. In India, HEV accounts for 10–40% of acute hepatitis and 15–45% of liver failure. In the west, the frequency is low, with a French study quoting an incidence of 3.2% [16-18]. Since any chemical or drug is mostly metabolised by the liver using an effective cytochrome system. The possible drug-induced liver injury usually presents as acute liver failure. Studies involving ACLF patients are few. However, most studies like Devarbhavi et al. [19] showed that they have high mortality, up to 17%. In a multinational Asian study of 660 patients, drug-induced liver injury (DILI) contributed as an acute insult in 9.1% of patients, and in 53.3% of these patients, the acute insult was attributed to anti-tubercular drugs followed by complementary alternative medications [20].
Chronic underlying liver conditions
Viral causes: In some regions, chronic HBV and HCV are important causes of underlying chronic liver disease or cirrhosis [21-23]. It is estimated that 325 million people worldwide are living with chronic HBV or HCV infection [24]. HBV infection can present as an acute infection in a patient with chronic liver disease (CLD) due to another aetiology or HBV reactivation of chronic HBV infection, which can be spontaneous or due to chemotherapy or immunosuppression therapy, etc. ACLF-HBV-related is associated with mortality ranging from 30% to 70% [25]. Hepatitis B surface antigen (HBsAg) positivity in the general population ranges from 1.1% to 12.2%, with an average prevalence of 3–4%. Chronic HBV infection is seen in approximately 40 million people, accounting for 20% of cases of cirrhosis and 40% of hepatocellular carcinoma (HCC) cases [24-26]. In published data from coastal India, among the 123 ACLF patients, HBV-related disease presentation was seen in 11.3% [14].
Anti-HCV antibody prevalence in the general population is estimated to be between 0.09 and 15%. As per the available data, about 1.75 million people were estimated to be newly infected with HCV in 2015, increasing the total number of people living with hepatitis C to 71 million.
Alcohol: It is one of the important precipitant causes. A recent history of binge drinking plays a key role in precipitating ACLF with alcoholic hepatitis. Patients with significant alcohol intake, present with alcoholic hepatitis with jaundice and no other obvious cause for hepatitis [1,26].
Non-alcoholic fatty liver disease: With the epidemic of obesity increasing all over the world, non alcoholic fatty liver disease (NAFLD) is fast becoming one of the leading causes of underlying chronic liver disease [1,27].
The pathophysiology involves the accumulation of reactive oxygen species, which plays an important role in leading to apoptosis. Hepatic injury leads to the release of various cytokines with inhibition of survival genes (Met) and induction of proapoptotic signalling molecules TNF and perforin and apoptosis stimulation fragment ligand known as FAS ligand (FasL) which accelerates apoptosis which is demonstrated in Figure 2 [27].
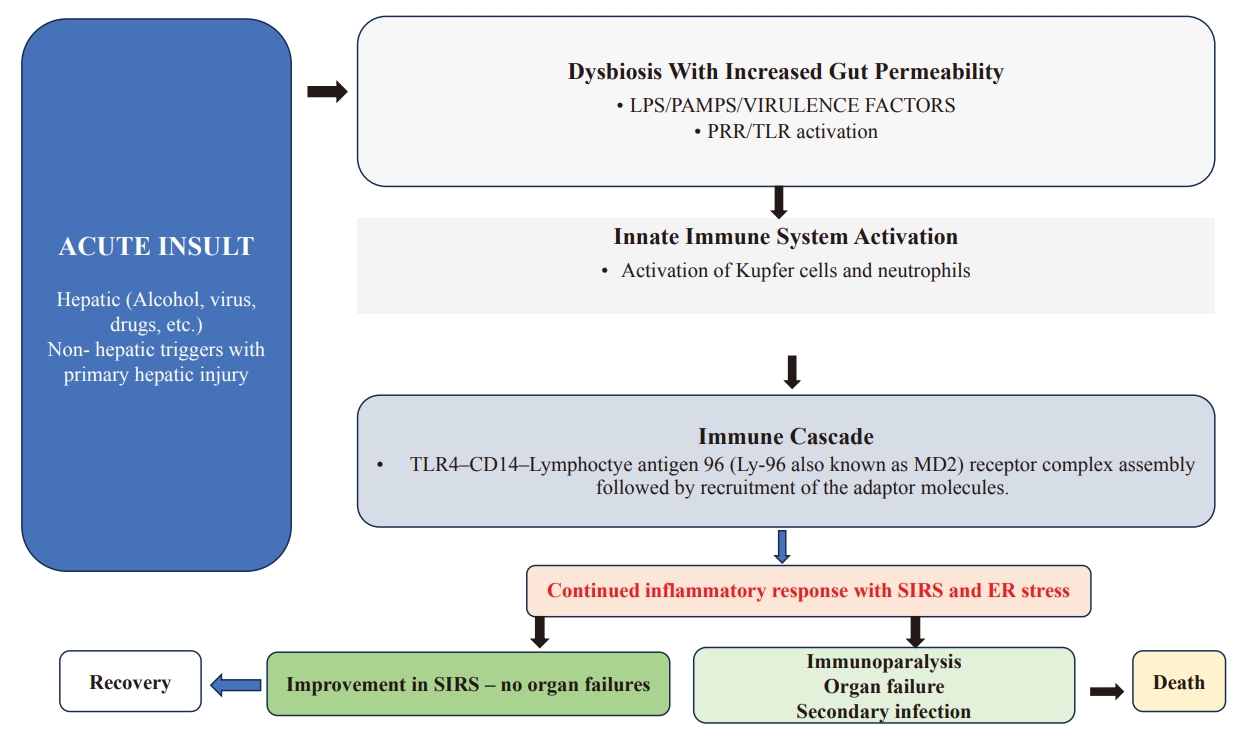
Insights into of Pathophysiology of Acute on Chronic Liver Failure. Figure illustrates the importance of Gut-liver axis and immune activation, causing SIRS which leads to various organ failures. Above figure shows Dysbiosis with release of products leads to release of DAMPS/HMGB1/ATP/IL-1a/IL-33/S100 protein superfamily and also release of mitochondrial DNA, N-formyl peptides causes PRR/TLR activation. Subsequently leads to activation of innate immune system with abnormal phagocytosis and cell damage along with mitochondrial dysfunction leading to NLRP3 inflammasome. Later immune activation and molecular cascade causes TLR4–CD14–Lymphoctye antigen 96 (Ly-96 also known as MD2) receptor complex assembly followed by recruitment of the adaptor molecules, myeloid-differentiation factor 88 (MYD88) and TIR domain-containing adaptor molecule 1 (TRIF) which can result in recovery or death based on improvement in the inflammatory response. LPS, lipopolysaccharides; DAMP, damage associated molecular pattern’s; PAMP’S, pathogen associated molecular patterns; PRR/TLR, pattern recognition receptors/TOLL like receptors; i-NOS, Nitric oxide synthase; ER- endoplasmic reticulum; NLRP3, NLR family pyrin domain containing 3; RAAS, Renin-angiotensin-aldosterone system; HMGB1, High mobility group box 1 protein.
Liver failure is a driver of inflammation, organ failure, and sepsis
APASL’s definition encompasses a more similar group of patients, where liver failure drives extrahepatic organ failure and mortality. It can help in detecting these patients in the early part of the illness [3]. It also identifies the separate group of patients who develop decompensation in patients with known CLD or cirrhosis or prior decompensation, which develops in less than 3 months and is known as acute decompensation (AD) [1,5]. This is in comparison to the western ACLF definition, which includes organ failure in the definition and sepsis as one of the prominent precipitating causes in the criteria. In a nutshell, the western definition includes acute decompensation of cirrhosis with the presence of organ failures (hepatic and extrahepatic) and hence has very high short-term mortality [5,6].
Infection, especially a bacterial infection, is present in about 1/3 of the patients at presentation and, when associated with organ failure (OF), has an increased risk of mortality. A decompensated cirrhosis patient with infection has an 8% mortality rate, while an infected ACLF patient with 1 OF has a 27% mortality rate, and the rate increases to 77% with 4 OF [28]. Similarly, patients with infection and sepsis had a high chance of cerebral failure (31% vs. 10%), circulatory failure (34% vs. 18%), and respiratory failure (20% vs. 10%) when compared to patients without the infection. Infected patients had higher mortality, i.e., with infection vs. without infection, of 51% vs. 35% [29,30]. Since infection itself can result in liver failure, the inclusion of both extrahepatic organ failure and sepsis in the definition of ACLF would, therefore, delay diagnosis in all cases and also permit the inclusion of a heterogeneous group of patients.
Golden window period and critical functioning hepatic reserve
The initial 1-week period from the symptom onset and presentation is very important and usually, the necrosis of the liver produces a systemic inflammatory response (SIRS), which has several important consequences. The prime drivers of the patient outcome at different time points are a combination of the critical functional hepatic reserve and the nature and severity of the acute insult. Once SIRS develops, it augments further systemic inflammation, leading to extrahepatic organ failure. So, this period up to the development of immune paralysis and subsequent development of sepsis is considered the “golden window of opportunity.” This “golden window” period can be modulated using immune-modulation therapy, etc., for the prevention of SIRS progression and the development of organ failure, thus changing the course of the disease (Fig. 3) [1,30,31].
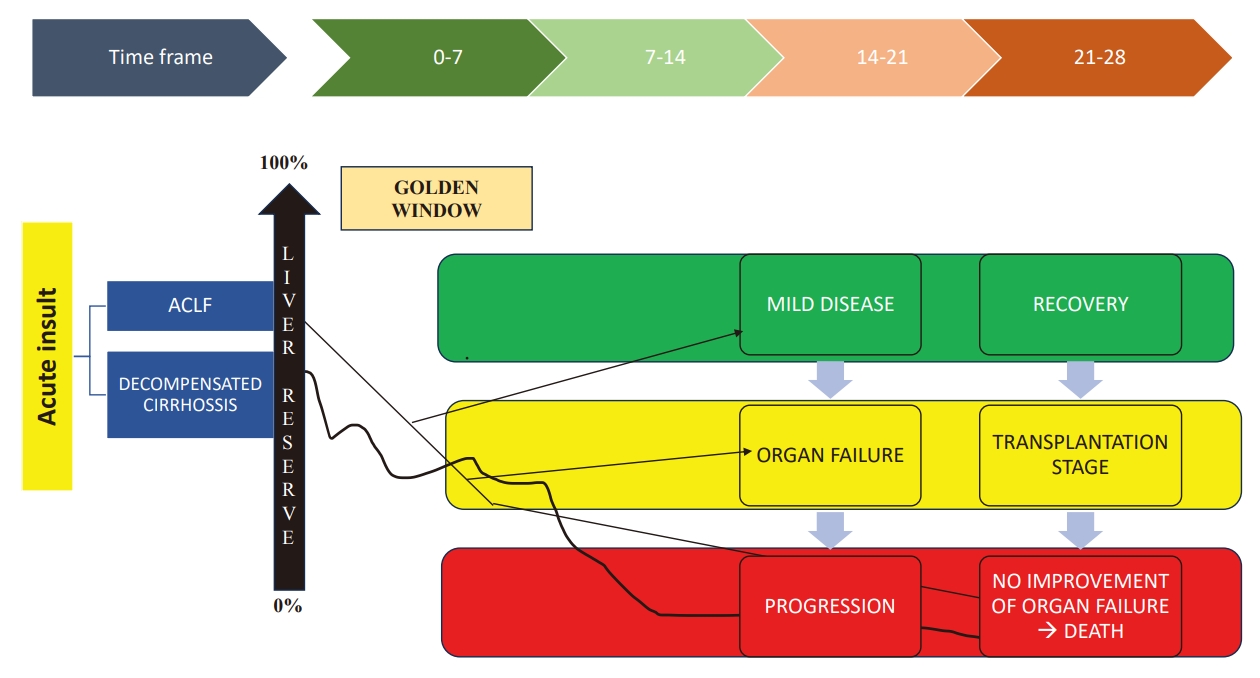
Concept of Golden window. This period may be considered as the initial 1-week period of the disease presentation. An acute hepatic insult leading to hepatic decompensation is the main driver resulting in the subsequent extra-hepatic organ failure. This is essentially due to the failure of recovery or regeneration. The period from acute insult and the development of immune paralysis and subsequent the development of sepsis is considered as the golden window of opportunity. Prevention of SIRS or its progression to sepsis and using immune modulation in the golden window period provides therapeutic opportunity and may benefit the patient [1,31].
CLINICAL FEATURES AND NATURAL HISTORY
As mentioned in the definition, liver failure, i.e., the development of jaundice and coagulopathy, is an important event. Later, these patients will develop ascites and encephalopathy in the form of altered consciousness, which can range from trivial unawareness to a significant coma [1,3]. Other clinical features that can be present at the time of presentation are acute variceal bleeding or presentation with an infection like pneumonia, spontaneous bacterial peritonitis (SBP), urinary tract infection, etc. Reactivation of HBV infection and acute HAV/HEV infections are usually associated with symptoms of fatigue, fever, and other prodromal symptoms. A history of intake of complementary and alternative medicines (CAMs) or treatment of tuberculosis (TB) infection with anti-tubercular therapy could also be present [1,2,32]. In the APASL ACLF research consortium (AARC) database, among 1,028 patients, 15% had obesity, 14% had type 2 diabetes mellitus (T2DM), 7% had hypertension (HTN), and 15% had dyslipidaemia [1,33]. ACLF patients generally have higher MELD scores and high AARC scores with high mortality (Table 3). In the Canonic study, a prior history of decompensation was present in up to 23% of the patients [1]. Usually, the presence of decompensation favours the diagnosis of AD.
Role of the liver biopsy
Patients with ACLF are often sick with severe coagulopathy, so invasive procedures might be risky. However, liver biopsy with a trans-jugular route is considered safe, and percutaneous biopsy could be unsafe due to coagulopathy and bleeding risk. In some patients where trans-jugular cannot be performed, one can also try for laparoscopic liver biopsy [34].
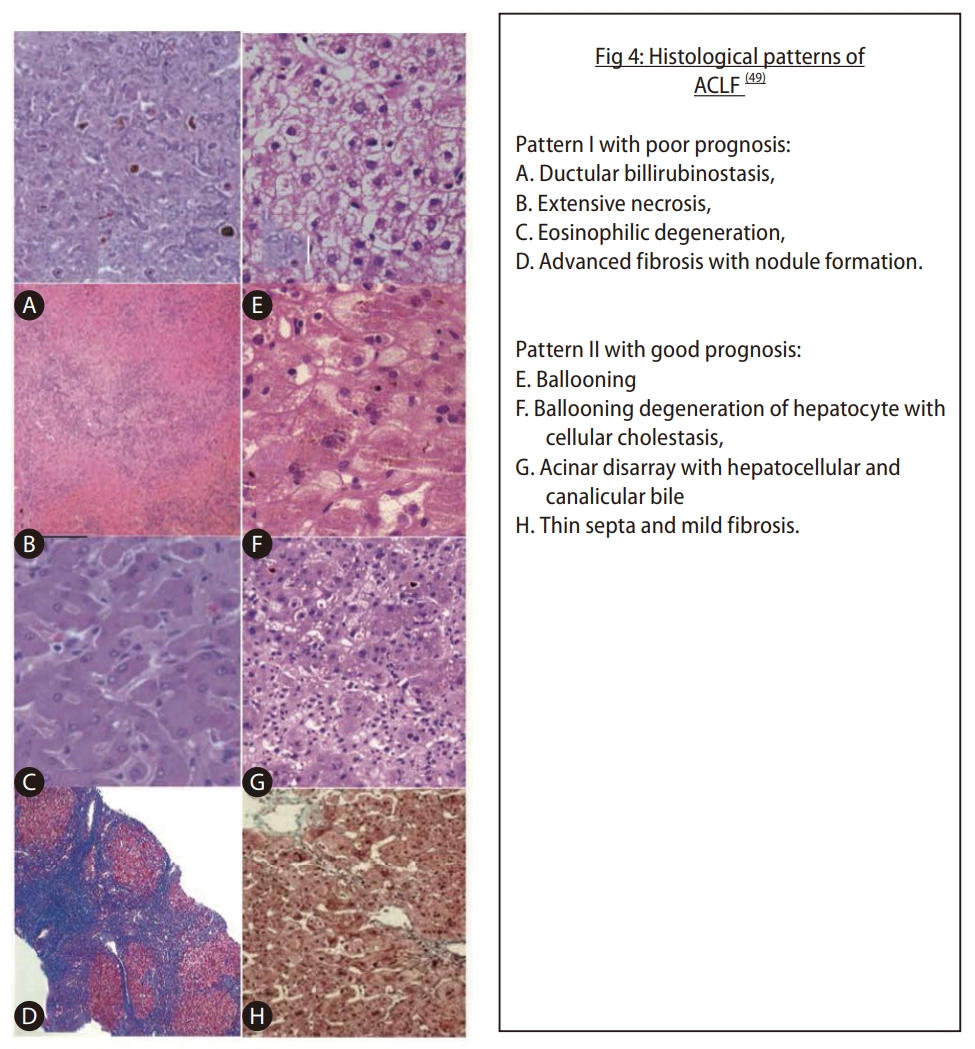
ACLF generally has pathologic findings of fibrous bands (spurs or bridges) and ductular proliferation. While the features of bile duct proliferation and cholestasis are common in acute injuries [34]. Diagnostic stains for fibrosis or necrosis can be performed (using Shikata’s orcein stain). Rastogi et al. [34] have proposed two patterns that predict patient outcomes when comparing those who survived to those who died (box 1; Fig. 4). Pattern I is associated with high mortality, and pattern II is associated with better survival, irrespective of the etiology [34].
Sub-massive hepatic necrosis is characterised by extensive and confluent necrosis, cholestasis, and ductular bilirubinostasis, and it is predictive of poor outcomes in patients with HBV-related ACLF. The extent of necrosis, liver damage, and fibrosis are important; the finding of bilirubinostasis and eosinophilic degeneration of hepatocytes usually has an unfavourable outcome, while ballooning can denote the potential for regeneration [34,35]. Other methods, like transient elastography, may be helpful to see the extent of fibrosis, etc. but needs more data on reliability (Fig. 4) [35].
ORGAN FAILURE AND OUTCOME ASSESSMENT IN ACLF PATIENTS
Renal failure
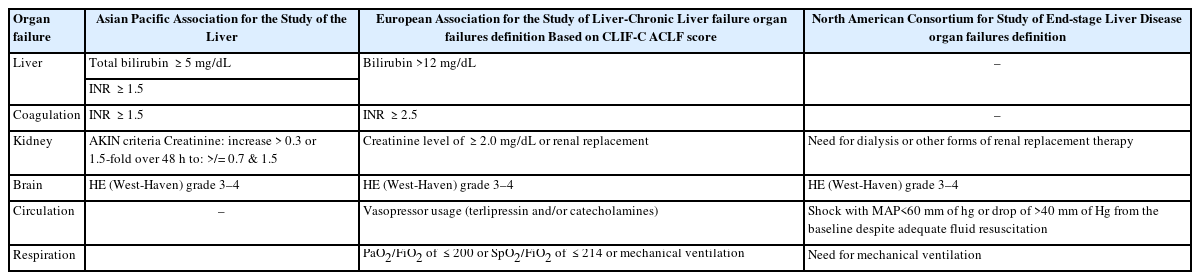
According to EASL, renal involvement is an important organ failure, and it is incorporated in the case definition at the time of presentation [2]. While in the APASL definition, the co-hort showed that acute kidney injury (AKI) was present in 30% and that it further developed in another 23–34% during the course of the disease. Splanchnic vasodilation with hormonal abnormalities forms the basis of the pathogenesis; SIRS and sepsis also play a very definitive role [35,36]. These patients usually show a partial or full response with stoppage of diuretics and after 2 consecutive days of plasma volume expansion with albumin with no intrinsic renal disease and a normal renal ultrasound [1,35]. An acute tubular necrosis (ATN) patient is unlikely to recover with medical management and may require a simultaneous liver and kidney transplant (SLK) [1,37]. During the treatment, the resolution of AKI has a good prognosis (38–40%), and the progression of AKI has a mortality rate of approximately 75% [37,40]. Biomarkers like NGAL, KIM-1, and IL-18 can be of some help in predicting renal failure and the development of ATN [37,41].
Cerebral failure
It is usually defined using West Haven criteria; grades III–IV are considered to be cerebral failure. Its pathophysiology is very complex, with neuroinflammation and impairment of brain energy metabolism resulting in cerebral oedema [42]. The arterial ammonia can be measured, and it is a good surrogate marker for the severity of HE in the advanced stages of ACLF, i.e., grades III–IV. The ammonia level above 140 mg/dL at baseline or at any time point in the first week with grades III–IV HE is considered a poor prognostic marker for 28- and 90-day survival (P<0.001), as demonstrated in a study done in India [1,40].
Coagulation failure
SIRS and sepsis have a significant role in inducing and progressing the coagulation defect [43]. The main mechanism of the defect is due to systemic inflammation and endothelial dysfunction, which is well demonstrated in the study by Premkumar et al. [44] In this study, it showed that a hypo-coagulable thromboelastography (TEG) at baseline was a predictor of bleeding (hazard ratio [HR] 2.1; 95% confidence interval [CI] 1.6–4.9; P=0.050) and mortality (HR 1.9; 95% CI 1.3–7.9; P=0.043) [42]. This can also be because ACLF has increased heparinoids, which affect coagulability [45]. Dynamic coagulation parameters are measured by TEG or other point-of-care (POC) tests and determine the likelihood of bleeding and mortality in ACLF. Studies have shown that parameters like activated clotting time (ACT), clot rate, platelet function (PF), time to peak, peak amplitude, and fibrinogen levels are important predictors. Using these, a score was developed called the “bleeding risk score,” which was validated in the study. ACT >190 seconds, PF 1.25, and fibrinogen <1.2 g/L could predict coagulopathic bleeding. The bleeding risk score ranges from 3 to 9. The coagulopathic bleeding increases as the score increases from 3 to 9 [44]. The POC tests can be helpful in situations where correction is required, and they also reduce unnecessary transfusion and can be helpful in guiding liver transplantation (LT) or major surgery [1].
Circulatory failure and respiratory failure
EASL defines circulatory failure as hypotension requiring vasopressors [2,46]. The dysfunction is considered when mean arterial pressure (MAP) <70 mm of Hg, while North American Consortium for the Study of End Stage Liver Disease (NACSELD) defines failure as MAP <60 mm Hg or reduction of systolic blood pressure of 40 mm of Hg from baseline [2,5,47]. Sepsis and SIRS play an important role. Respiratory failure is defined as the need for mechanical ventilation, or PaO2/FiO2 <200 or SpO2/FiO2 <214 [5,48]. Table 2 shows organ failure definitions according to various societies. Various definitions are tabulated in Table 2.
ASSESSMENT OF OUTCOME
The outcome of ACLF is poor, especially when organ failures are present at presentation or develop in the early weeks of ACLF, and mortality is as high as 40–50% [49]. Thus, the early part of the week provides a “golden window of opportunity,” as discussed earlier, with effective therapy showing improved clinical outcomes. As for the other causes of CLD, common scores like MELD-Na scores or CTP scores are used for prognosticating and for allocating organs for liver transplantation as for the other causes of CLD. Since ACLF is a progressive disease that warrants continuous monitoring, no single score can assess and predict the outcome or suggest treatment options as seen in other diseases.
CTP/MELD/Lille’s score
Chen et al. [33] showed that CTP >12 and MELD >28 were very independently predictive of mortality in ACLF patients. Not only does static score help us to predict, but also during the course, if there was no improvement in the MELD score after treatment on day 7, like in patients of autoimmune hepatitis (AIH), suggests urgent expedition for transplantation [50]. The Lille model was used in the same way after corticosteroid use for alcoholic hepatitis and can help in predicting steroid nonresponders and advising LT as a treatment option. This becomes very important as Mathurin et al, showed that early LT improved survival with cumulative survival (77±8% vs. 23±8%, P<0.001) with acceptable low alcohol relapse [26].
CLIF-C ACLF and CLIF-SOFA scores
If we look at the overall picture, the above scores lack a good predictive ability for survival among ACLF patients. These scores do not include organ failures like encephalopathy, vasopressor support, etc., which can predict poor outcomes. So other scoring systems, which include dynamic parameters have been proposed by various societies like chronic liver failure sequential organ failure assessment (CLIF-SOFA), chronic liver failure consortium (CLIF-C OF score), AARC score, etc [1]. About 11.3% of patients with acute decompensation developed ACLF within 28 days of enrolment in the CANONIC study, and 50% of these patients died within 3 months [5]. The CLIF-C acute decompensation score was developed based on independent predictors of mortality (age, white blood cell count, serum sodium, creatinine, and INR) in patients with acute decompensation but without ACLF [46]. A score of 45 or less was associated with a 3-month mortality rate of 1.8%, representing a low-risk group that may be discharged early with de-escalation of care. A score of 60 or more represents a high-risk group with a 3-month mortality rate of 31 percent and a greater likelihood of progression to ACLF, hence requiring escalation of care [4].
Similarly, a CLIF-C ACLF score ≥70 at 48 hours predicts mortality more accurately, with an area under receptor operative curve (AUROC) of 0.643 (95% CI 0.505–0.781; P=0.046), which is significantly higher than MELD scores at 48 hours. In another study, it was shown that if LT is contraindicated or cannot be available for patients with ≥4 organ failures or CLIF-C ACLFs >64 at days 3–7 after the diagnosis of ACLF grade 3, intensive organ support can be discontinued owing to futility (Table 3) [51].
The AARC score
This score uses five parameters like serum bilirubin, lactate, creatinine, INR, and HE. Each parameter scored 1–3, with these parameters, the total score is later calculated and it is classified into different grades like grade I (5–7), grade II (8–10), and grade III (11–15). The analysis of the data showed 28-day mortality rates of 12.7%, 44.5%, and 85.9%, respectively, among the 3 groups. Its dynamicity is excellent; it is accurate in the prediction of survival on days 7 and 28; scores <10 have better survival, and for each unit raised above 10, the day 7 mortality increases by about 20%. AARC score >11 at baseline or persisting after 1 week of treatment is associated with poor survival (P<0.001) [33]. Similarly, when compared to baseline AARC scores, any shift from grade I to III on day 4 or day 7 has higher mortality. If the grade III ACLF state persists at day 7, the prognosis is poor, and early liver transplantation should be considered [1,52,53]. Various scores are tabulated in Table 3.
TREATMENT: AN ALGORITHMIC APPROACH
As ACLF patients often suffer from underlying chronic liver disease, they need holistic care. Moreover, these patients have a rapid downward course with high short-term mortality rates; treating such patients requires careful assessment, monitoring, and proper intensive care unit (ICU) care. In view of the short window of opportunity, they need continuous and dynamic monitoring with the backup of a liver transplant unit. Additionally, facilities for organ support and bridging therapies should also be available. Management of ACLF needs a proper multidisciplinary team approach with the hepatologist, intensivist, infection control team, and transplant team for optimal management. Figure 5 gives a simple algorithm for approaching the management.
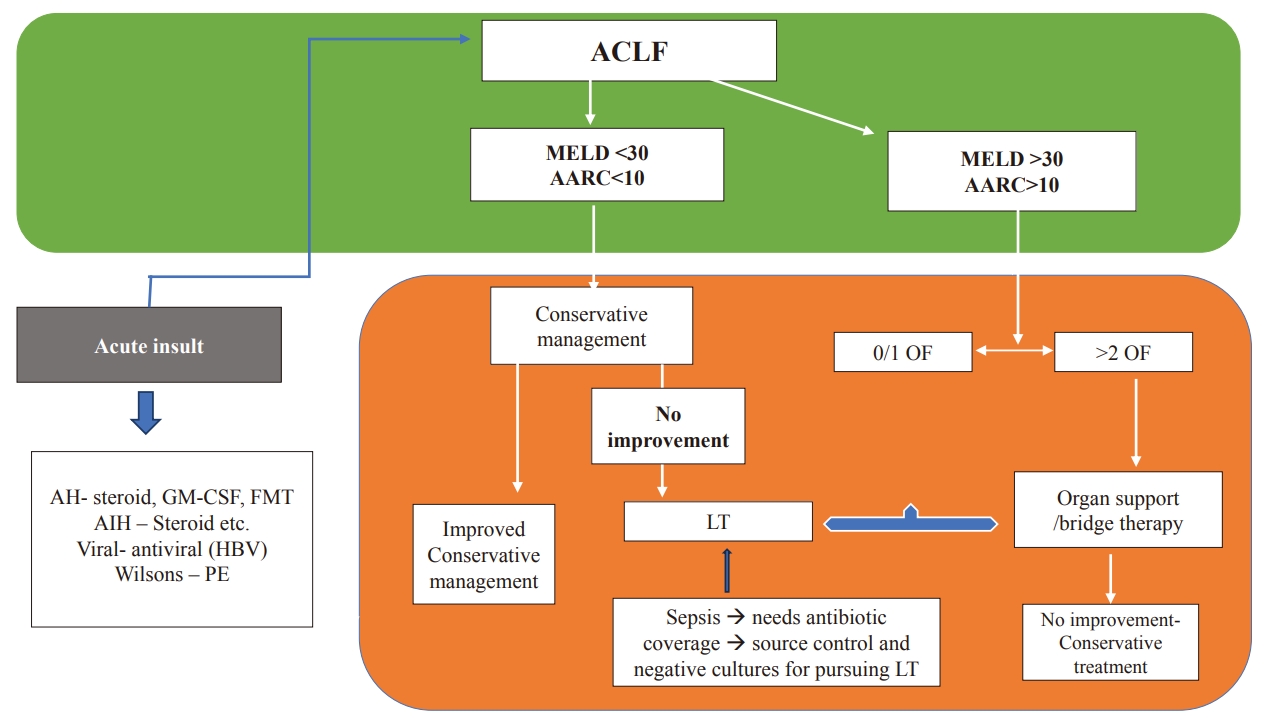
Algorithmic approach for treatment. AH, alcoholic hepatitis; AIH, auto-immune hepatitis; FMT, fecal microbiota transplant; LT, liver transplantation; GM-CSF, granulocyte and monocyte colony stimulating factor; MELD, Model for End-Stage Liver Disease; APASL, Asian Pacific Association for Study of Liver; ACLF, acute-on-chronic liver failure; AARC, APASL ACLF Research Consortium; OF, organ failures; CST, continue same treatment; PE, plasma exchange.
The basics of management include:
· Treating the precipitating causes, which are usually correctable. Usually, grade I and II ACLF patients will respond well.
· Identification of the complications and their management
· Identifying organ dysfunction, preventing organ failures, and, if needed, providing prompt organ support [33].
· For proper assessment, dynamic monitoring of the patients and the use of ACLF-specific scores are necessary.
Nutrition
ACLF patients usually present early and have, clinically, a better nutritional status in comparison to decompensated cirrhosis patients with worsening clinical status. However, during the illness, especially in these patients with SIRS, the provision of high calorie and protein intake, oral or parenteral, is essential [54]. These patients require nutrition of approximately 35–40 kcal/kg/day with a protein intake of 1.2–2 g/kg (ideal body weight, IBW)/day [48,55]. In some cases, enteral feeding (through nasogastric [NG] feeding with 1.5–2.0 kcal/mL feed) along with enriched intake of the omega fatty acid may be beneficial, especially among those, who cannot take the appropriate amount orally, especially those who are critically ill or comatose and admitted in the ICU. The use of branchedchain amino acid supplements may be beneficial [56].
Infections
Up to 50% of patients with ACLF in general and more than 80% of critically ill patients with ACLF grade 3 develop infections during their hospital stay [57-59]. Fungal infections are also very common and can be seen in patients with risk factors like diabetes, AKI, ICU admission, prolonged hospitalisation, prior and prolonged antibiotic usage, etc. These fungus infections can be proven, probable, or possible [60].
Septic shock is identified by the requirement of vasopressors to maintain a MAP of ≥65 mmHg and a serum lactate level >2 mmol/L. Organ dysfunction is defined as an increase in the SOFA score of ≥2 points [58].
The choice of antibiotics depends on the type, severity, and origin of the infection (community-acquired or nosocomial) and on local epidemiological data on antibiotic resistance. Empirical antibiotics are started early, as every hour without adequate treatment, mortality rates increase by about 3.3%. In a recent randomised controlled trial of 143 ACLF patients, norfloxacin was shown to prevent bacterial infections and improve transplant-free survival at day 90 as compared to placebo (58.3% [95% CI, 46.11–69.84] vs. 43.7% [95% CI, 31.91–55.95], respectively P=0.058) [17]. Once there is improvement in the condition or cultures are available after 48 hours, suitable antibiotics can be given according to the cultures. Antifungal drugs are also recommended in selected patients. As discussed earlier, echinocandins can be used in resistant infections and in some suspected invasive aspergillosis infections, which are increasing in incidence and may require IV amphotericin B for the treatment [58,59].
Specific treatment of acute insult
Viral causes
Early detection and treatment of viral infections can reduce the progression of hepatic injury. Direct antiviral therapy using antiviral therapy with high resistance barrier drugs which reduce HBV DNA >2 log reduction from the baseline within 2 weeks has shown better survival from 17% to 57% in an randomised contolled trial (RCT) [61]. Dual antiviral therapies, although shown to improve renal functions, did not improve the overall antiviral potency. A recent pilot study published showed a good response to infection in HBeAg+patients with FMT [62]. HCV infection with detectable viral RNA can be effectively treated with new direct-acting antiviral therapy [1,61,63].
Alcoholic hepatitis
Patients with AH need nutrition and psychological support. Among the medical management, tailored nutrition, psychosocial rehabilitation, and treatments aimed at suppressing inflammation or TNF-a production (such as corticosteroids or pentoxifylline) steroids are the best available pharmacological option in severe AH and are the first-line treatment for patients, it is recommended by American association for the study of liver diseases (AASLD), EASL, and American college of gastroenterology (ACG). So, AH patients need assessment for treatment with a corticosteroid. The response is seen in up to 60% of the patients, with better short-term mortality [26]. Response can be assessed with Lille’s model score on day 7, and if there is no improvement on day 7, they should be evaluated for transplantation. Among the patients receiving steroids, almost one-fourth may develop an infection during the therapy [64].
If liver transplantation is not available in these patients, other therapies can be explored, like granulocyte-colony stimulating factor (G-CSF) therapy [46]. G-CSF was found to mobilise the hematopoietic stem cells and induce liver regeneration. In patients with alcoholic hepatitis, a 5-day G-CSF administration (10 μg/kg/d, subcutaneously) mobilised CD34+ stem cells, increased circulating hepatocyte growth factor, and induced proliferation of hepatic progenitor cells in liver biopsy specimens, which improved survival and reduced renal dysfunction [47]. But in a recent study published, 176 patients with ACLF (EASL-CLIF criteria) were randomly assigned to receive G-CSF (5 μg/kg daily for the first 5 days and every third day thereafter until day 26) plus standard medical therapy (SMT) (n=88) or SMT alone, which showed no survival benefit with a 90-day transplant-free survival rate of 34.1% compared to 37.5% in the SMT group (HR 1.05; 95% CI 0.711–1.551; P=0.805) [65]. Antibody against IL-2 receptor antibody also is a promising therapy, in a study, where in vitro usage of Basiliximab showed improvement in steroid resistance [46].
Pentoxifylline, anti-TNF-a therapy, N-acetylcysteine, and s-Adenosyl methionine or recently TLR 4 antagonist or IL-1 antagonist have been variously used. Recently, the modulation of the gut microbiome has been assessed for treatment, as it seems very important in altering the resident microbiome and thus plays an important emerging role [66]. If no options are available, one can use artificial liver support systems (ALSS) although significant beneficial effects are not demonstrated, nevertheless, one can always use it as bridging ther apy if required, especially among those with renal impairment [1,33].
AIH
Patients with AIH can present with an ACLF, or acute failure-like presentation, in up to 20 percent of cases. Steroid therapy in these patients can be tried, but it is controversial. Some studies favour the therapy as having a better outcome, but it’s still controversial [62]. For the therapy with other immunosuppression like tacrolimus or mycophenolate mofetil the data is inconclusive, although some studies also support these drugs [67]. Continuous assessment of these patients is necessary; if there is no improvement in the MELD scores within 7 days of treatment, they should be evaluated for LT.
DILI
Drugs causing liver injury can act as the acute insult that can precipitate ACLF with underlying NAFLD; the incidence can be up to 11.6%, and they have a very high mortality rate of 57 percent. In India anti-tubercular treatment forms a very important cause of DILI [63]. However, in most of the patients, no identifiable cause for acute insult can be present, so in that case, these patients need monitoring, identification, treatment of the complications, and transplant evaluation as indicated, along with organ support for the organ failures [68,69].
Treatment of complications
Hepatic encephalopathy
HE is seen in up to 40% of the cases associated with high mortality. Arterial ammonia >140 mcg/dL with grade III–IV HE has a high mortality. Ammonia-lowering therapy using lactulose therapy and Rifaximin therapy has shown significant improvement in HE scores. Mechanical ventilation may be required for an advanced grade of HE [69,70].
AKI
AKI is diagnosed as discussed above has increased mortality by up to 38% at day 7 [70-72]. Adequate fluid resuscitation, avoiding and withdrawing the nephrotoxic agents, and targeting the PIRO (predisposition, infection, inflammation, response, and organ failure) by using anti-inflammatory strategies like albumin are important [1]. Albumin (SLED). (20%), IV infusion at a dose of 1 g/kg followed by 20–40 g/day [66] and N-acetyl cysteine infusion at 100 mg/kg/24 hours can be useful in the patients [1,37]. Volume expansion with albumin, either alone or along with vasoconstrictors, may be used as an excellent treatment option [73-76]. Terlipressin, along with albumin infusion, is the standard of care in patients with ACLF who develop Hepatorenal syndrome (HRS). Studies have shown that a reduction in the serum creatinine has better 90-day survival, especially among those with grade III ACLF, or can help as a bridge to survival after transplant [76]. The dosage can vary, as demonstrated by various trials. Various studies have used terlipressin at a dose varying from 0.5–1 mg every 4–6 hours to as high as 2–12 mg of total dose per day and shown clinical improvement [72]. Another role of terlipressin is in patients with SBP, prevention of paracentesis induced circulatory dysfunction (PICD), variceal bleeding, and perioperative management, especially in the setting of LT. HRS reversal is defined as a decrease in serum creatinine to ≤1.5 mg/dL (≤133 μmol/L), which is reported in 35–80% of patients with terlipressin therapy [63,67]. It should be noted that non-responders have very high mortality [1,3,38]. ACLF patients requiring renal replacement therapy (RRT) have a severe grade of ACLF. Mortality in critically ill patients with a need for RRT is substantially high, independent of the LT options [77]. Upon failure of the medical treatment, RRT should be offered. Common indications where it should be considered are severe volume overload, hyperkalaemia, hyponatremia, and severe metabolic acidosis [78-80]. Especially the sicker group of patients benefits from continuous venovenous hemodialysis (CVVHD) over conventional hemodialysis or slow low-efficient dialysis (SLED). CVVHD is probably safe because it reduces the fluctuations in the mean arterial pressure and cerebral perfusion pressure [70]. The postoperative mortality of liver transplant recipients requiring RRT is increased compared with those not needing renal support (15% vs. 4% at 3 months and 30% vs. 10% at 1 year, respectively), but similar to that observed in patients requiring the initiation of RRT post-transplant (21% and 33%, respectively) [77,81].
Respiratory failure
Respiratory failure requires urgent care in the ICU. Initially, non-invasive ventilation strategies are to be considered except in cases of uncooperative or encephalopathic patients. Common indications like pneumonia, volume overload, ARDS (acute respiratory distress syndrome), or advanced encephalopathy for airway protection are also important and should be kept in mind. Intubation before transplantation increases the incidence of postoperative pneumonia (15% vs. 5%, P=0.02) as well as post-operative mortality (38 vs. 23%; P<0.01) [1,82].
Circulation failure and vasopressors
Early goal-directed fluid therapy should be taken care of within the first 6 hours, similar to the surviving sepsis campaign guidelines. The goal of such therapy is to maintain MAP >65 mm of Hg [54,69]. The common resuscitation fluids used are crystalloids and 5% albumin [83] and the preferred vasopressors commonly used are norepinephrine, epinephrine, or terlipressin. IV fluids with balanced solutions improve renal function [73-75]. Use of IV hydrocortisone in refractory shock at a dose of 200 mg IV (4 divided doses) [73] can also be beneficial, as these patients have been demonstrated to have a low adrenal reserve [84].
Definitive Liver transplant as option-sickest first
LT in ACLF needs to be prioritised if required. When the united network for organ sharing (UNOS) database analysed patients with ACLF-III and those with the status-1a listing, it was demonstrated that patients with ACLF-III showed significantly greater 14-day mortality (sub-distribution HR of 1.45, 95% CI 1.31–1.61) compared with status-1a candidates; these results were independent of MELD-Na score [85]. At 30 days, mortality was high in patients on the waiting list without LT with the increasing number of OFs, and liver transplantation could be done in only about 25% of the patients. Recent studies by Thuluvath et al. [6] demonstrated that, with LT, the 90-day patient survival was 90% and the one-year survival was 81% even in the presence of 5–6 OFs [86]. The futility of a liver transplant should also be well assessed over time. A liver transplant is considered less beneficial if 5-year survival is 50%. Patients should be considered for early transplant with MELD score >28, AARC score >10, high-grade HE, and in the absence of >2 organ failure or overt sepsis as there is a high chance of failure of conservative management and high mortality [33,87,88].
Overall, some ACLF patients may show improvement with conservative treatment, but even after recovery from the initial insult, there is an increased likelihood of decompensation in the future and an inherent high mortality rate in the recovered patients of up to 40–50% at 6 months [89]. But as shown in the Canonic study, transplanted ACLF patients had a very good long-term survival rate of up to 80% [82].
RELATIVE CONTRAINDICATIONS FOR LT
The relative contraindications for LT for other causes also hold good for LT in ACLF patients. Active alcoholism is a contraindication; most countries need at least 6 months of abstinence, but this can be relative and has been challenged by a study by Mathurin et al. [90] Respiratory failure or active lung infection can have poor survival which can be present at the time of presentation or during the care and can be considered a contraindication [91]. In patients with respiratory failure, the best time for LT is when improvement is seen in PaO2/FiO2 >150 [86]. Systemic infections can be one of the precipitating causes or initial events, as defined in the western definition, at the time of presentation. So, this can be a general contraindication if active, especially with a culture-positive infection or fungal sepsis [92]. However, after control of infection, or in the case of special infections like cholangitis in primary sclerosing cholangitis, it is not considered a contraindication [93]. Uncontrolled HIV infection is also a contraindication. A psychiatric condition is also contraindicated as it may hamper the patient’s self-care.
Transplantation in alcoholic hepatitis
Since the outcome in these patients is very poor, especially with severe AH or steroid non-responders. LT becomes a useful salvage option for improving survival. But the problem is, these patients would have actively taken alcohol even just before presentation [94]. Many transplant centres recommend a minimum of six months of abstinence from alcohol so that recovery can be given a chance even before considering an evaluation, so LT for severe AH has remained a controversial subject [95,96]. Dom and Peuskens [97] showed that longer periods of abstinence from alcohol beyond six months can have a stronger prognostic value for a low risk of post-transplant relapse at 5% per month. But in a landmark case-control prospective study, Mathurin et al. [90] challenged the “six-month rule.” In this study, they selected 26 patients with severe AH who did not respond to steroid therapy to receive LT (6-month survival: 77% vs. 23%, P<0.0001) [90,98]. Similarly, in the UNOS dataset comparing alcoholic cirrhosis vs. AH, the five-year graft survival was 73% and 75% (P=0.97), and the five-year patient survival was 78% and 80% (P=0.90), respectively [96]. So currently, patients with severe alcoholic hepatitis who are non-responders to the medical therapy, with the presence of a strong social support network, insight into the disease process, the absence of co-existing psychiatric disorders, agreement with adherence to lifelong abstinence, and complete agreement by the liver transplant committee, Recently, the mean rate of adding patients with acute alcoholassociated hepatitis to the liver transplant waiting list was 2.3% (0.7%), and their rate of receiving liver transplants was 4.4% (1.9%) [91].
ALSS AS BRIDGING THERAPY
Bridging devices fall into one of two categories: liver support devices (biological and nonbiological) and hepatocyte transplantation. Extracorporeal liver-support systems have shown a good safety profile. However, they have not shown major improvements in synthetic function. Plasma exchange improves the biological response. Multiple randomised controlled trials in patients with ACLF have shown better improvement in circulatory dysfunction, HE, hepatorenal syndrome, and immune dysfunction without much significant improvement in transplant-free survival [95].
ALSS are safe and have demonstrated the following benefits: improvement of biochemistry, hemodynamic status, and hepatic encephalopathy. The thinking behind its usage is that the cellular damage in ACLF is extensively driven by an increased “cytokine burst”, with an accumulation of cytokines and vasoactive substances in the blood [12,95]. Artificial extracorporeal liver support systems remove water-soluble and albumin-bound toxins to maintain normal serum chemistry, prevent further hepatic and organ system damage, and create an environment for potential hepatic regeneration and recovery.
Hepatocyte transplantation with liver progenitor cells is another emerging bridging therapy under study. However, current studies lack data on long-term safety and effectiveness. Various cell-based therapies, augmentation of hepatic regeneration, and gut modulation by faecal microbiota transplantation are interesting but still far from being recommended as an alternative to LT [12].
WAY FORWARD
· ACLF is a serious complication with high mortality.
· There is a need for a universally acceptable definition.
· Early diagnosis and management are the keys to survival.
· Early intervention at the stage of organ dysfunction can improve patient outcomes.
· The need to search for better biomarkers to detect organ failure early is an important unmet need.
· Management currently largely relies on early identification and the provision of a liver transplant. However, quite often, this is not feasible.
· There is a need for the development of effective nontransplant medical therapies such as liver regeneration and cell-based therapies.
· Bridge therapies like plasma exchange, bio-artificial liver support systems, and hemoperfusion systems are promising.
Notes
Authors’ contribution
Prof S K sarin: Conceptualisation, writing and editing of the manuscript. Dr vinay: compilation and writing of the manuscript.
Conflicts of Interest
The authors have no conflicts to disclose.
Abbreviations
ACLF
acute-on-chronic liver failure
LPS
lipopolysaccharide
DAMPS
damage-associated molecular patterns
AARC
APASL ACLF research consortium
MELD
model for end stage liver disease
APASL
Asian Pacific Association for Study of Liver
HE
hepatic encephalopathy
NACSELD
North American Consortium for the Study of End-Stage Liver Disease
cACLD
compensated advanced chronic liver disease
LSM
liver stiffness measurement
CSPH
clinically significant portal hypertension
HBV
hepatitis B virus
HCV
hepatitis C virus
AH
alcohol-associated hepatitis
DILI
drug-induced liver injury
CLD
chronic liver disease
HBsAg
hepatitis B surface antigen
FasL
FAS ligand
AD
acute decompensation
OF
organ failure
SIRS
systemic inflammatory response
SBP
spontaneous bacterial peritonitis
UTI
urinary tract infection
CAMs
complementary and alternative medicines
TB
treatment of tuberculosis
ATN
acute tubular necrosis
SLK
simultaneous liver and kidney transplantation
TEG
thromboelastography
POC
point-of-care
ACT
activated clotting time
MAP
mean arterial pressure
AIH
autoimmune hepatitis
CLIF-SOFA
chronic liver failure sequential organ failure assessment
CLIF-C
chronic liver failure consortium
G-CSF
granulocyte-colony stimulating factor
SMT
standard medical therapy
AKI
acute kidney injury
HRS
Hepatorenal syndrome
RRT
renal replacement therapy
CVVHD
continuous venovenous hemodialysis