| Clin Mol Hepatol > Volume 29(3); 2023 > Article |
|
ABSTRACT
Background/Aims
Methods
Results
ACKNOWLEDGMENTS
FOOTNOTES
SUPPLEMENTAL MATERIAL
Supplementary┬ĀMaterial┬Ā1.
Supplementary┬ĀTable┬Ā2.
Supplementary┬ĀTable┬Ā3.
Supplementary┬ĀTable┬Ā4.
Supplementary┬ĀFigure┬Ā1.
Supplementary┬ĀFigure┬Ā2.
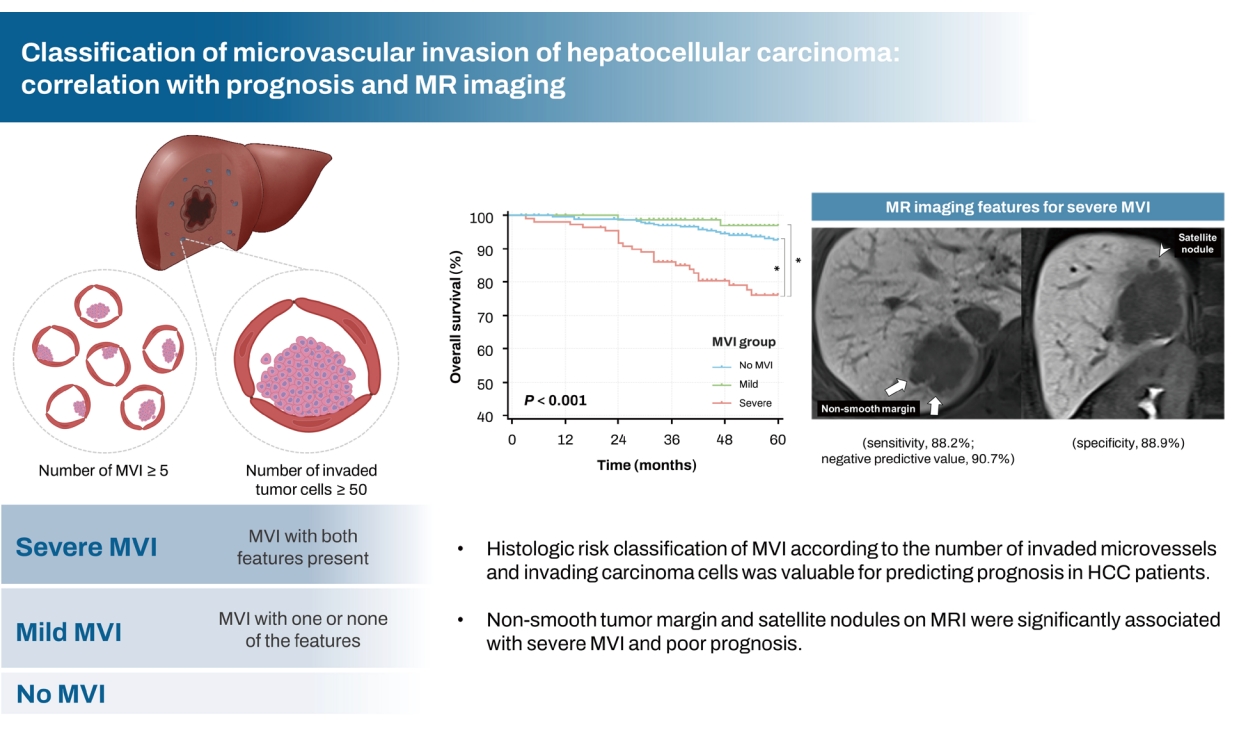
Figure┬Ā1.
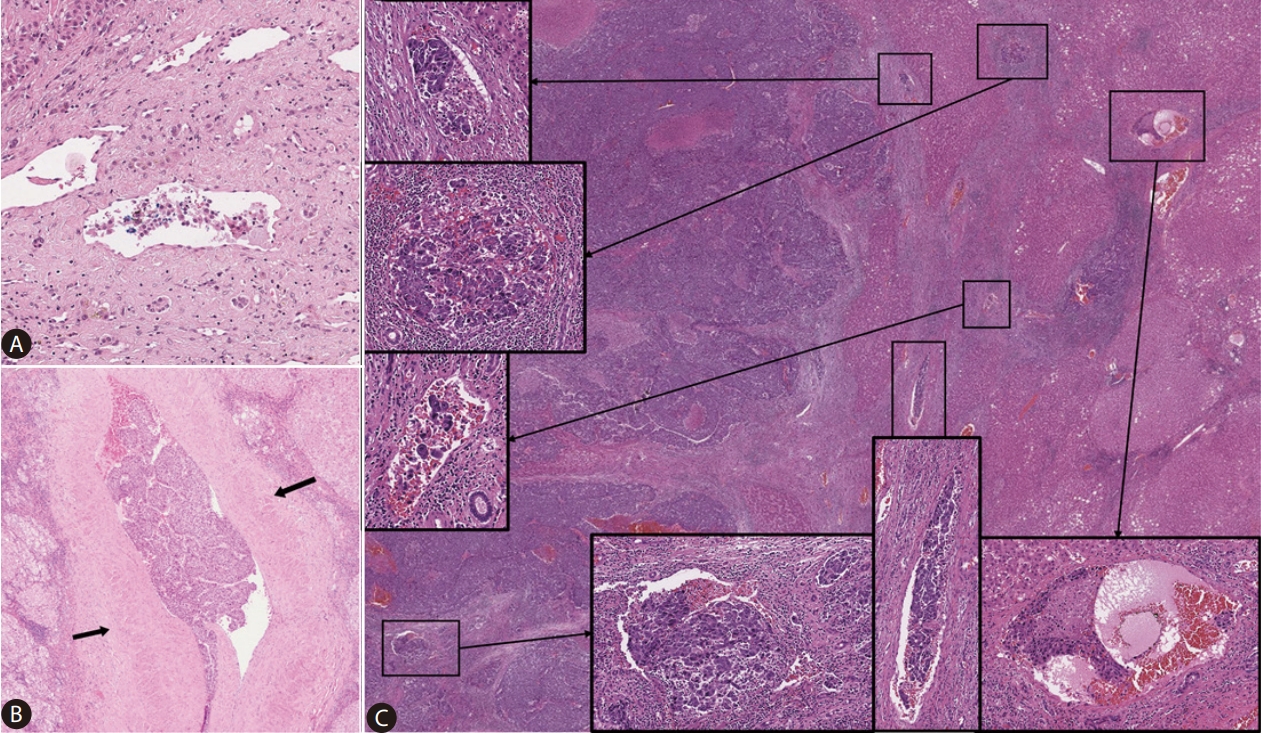
Figure┬Ā2.

Figure┬Ā3.
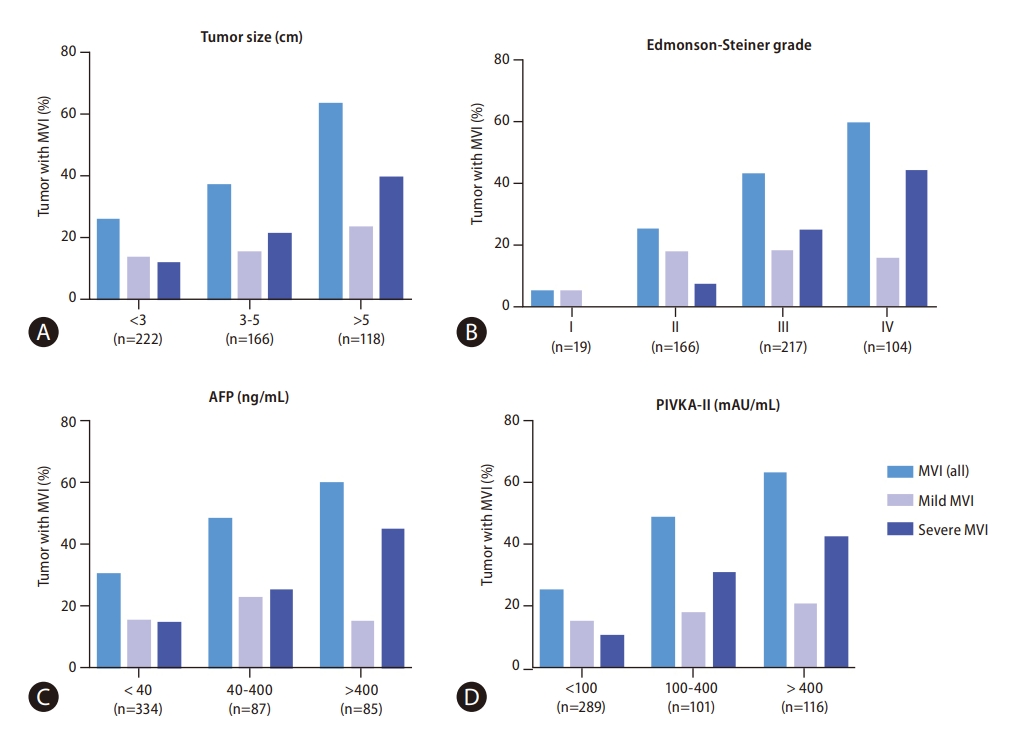
Figure┬Ā4.

Figure┬Ā5.
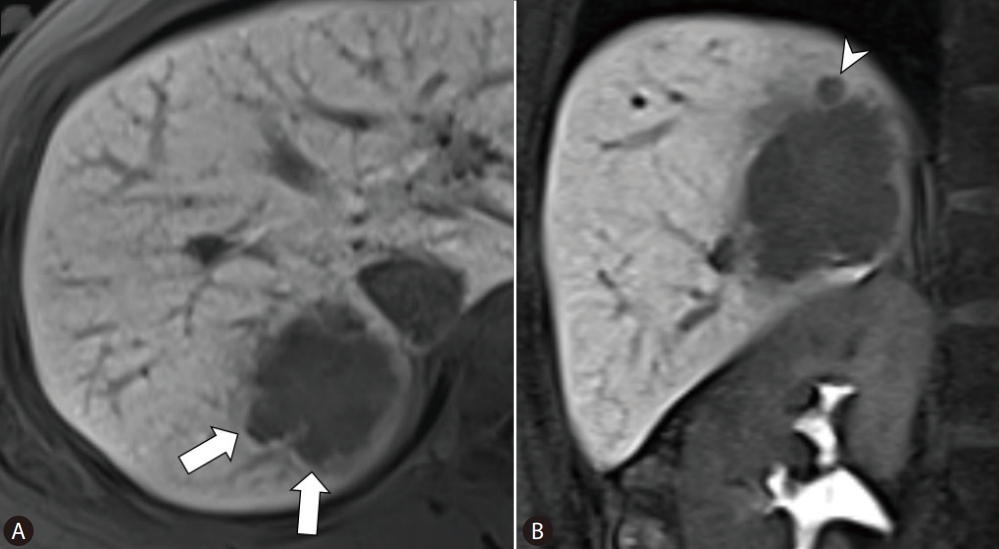
Table┬Ā1.
| Variable | Total (n=506) | No MVI (n=311, 61%) | Mild MVI (n=85, 17%) | Severe MVI (n=110, 22%) | P-value | ||
|---|---|---|---|---|---|---|---|
| Clinical feature | |||||||
| Age (yr) | 62(55ŌĆō69) | 63 (56ŌĆō70) | 60 (52ŌĆō66) | 63 (54ŌĆō70) | 0.043* | ||
| Sex (male/female) | 410 (81)/96 (19) | 253 (81)/58 (19) | 66 (78)/19 (22) | 91 (83)/19 (17) | 0.651 | ||
| Etiology | 0.001* | ||||||
| Hepatitis B | 399 (79) | 248 (80) | 70 (82) | 81 (74) | |||
| Hepatitis C | 37 (7) | 14 (5) | 5 (6) | 18 (16) | |||
| Alcohol | 29 (6) | 24 (8) | 2 (2) | 3 (3) | |||
| Unknown | 41 (8) | 25 (8) | 8 (9) | 8 (7) | |||
| Child-Pugh score | 0.316 | ||||||
| 5 | 468 (93) | 292 (94) | 77 (91) | 99 (90) | |||
| 6 | 38 (7) | 19 (6) | 8 (9) | 11 (10) | |||
| Platelet (├Ś109/L) | 166 (133ŌĆō203) | 166 (130ŌĆō200) | 172 (141ŌĆō218) | 158 (139ŌĆō202) | 0.227 | ||
| PT-INR | 1.04 (0.99ŌĆō1.09) | 1.04 (1.00ŌĆō1.09) | 1.05 (1.00ŌĆō1.10) | 1.03 (0.97ŌĆō1.09) | 0.092 | ||
| Albumin (g/L) | 4.2 (3.9ŌĆō4.4) | 4.2 (3.9ŌĆō4.3) | 4.2 (4.0ŌĆō4.4) | 4.2 (3.8ŌĆō4.4) | 0.798 | ||
| Bilirubin (mg/dL) | 0.8 (0.6ŌĆō1.0) | 0.8 (0.6ŌĆō1.0) | 0.8 (0.6ŌĆō1.0) | 0.8 (0.6ŌĆō1.1) | 0.497 | ||
| AST (U/L) | 32 (24ŌĆō42) | 31 (23ŌĆō41) | 29 (23ŌĆō38) | 36 (27ŌĆō48) | 0.067 | ||
| ALT (U/L) | 33 (22ŌĆō47) | 33 (22ŌĆō47) | 31 (20ŌĆō45) | 35 (23ŌĆō48) | 0.499 | ||
| AFP (ng/mL) | 11 (4ŌĆō115) | 6 (3ŌĆō41) | 11 (4ŌĆō96) | 69 (7ŌĆō1036) | 0.005* | ||
| PIVKA-II (mAU/mL) | 64 (28ŌĆō357) | 40 (25ŌĆō154) | 106 (37ŌĆō498) | 322 (89ŌĆō1722) | <0.001* | ||
| Pathological finding | |||||||
| Liver cirrhosis | 146 (29) | 93 (30) | 23 (27) | 30 (27) | 0.805 | ||
| Tumor size (cm) | 3.2 (2.2ŌĆō4.9) | 2.8 (2.0ŌĆō4.0) | 3.5 (2.5ŌĆō5.2) | 4.5 (3.0ŌĆō6.3) | <0.001* | ||
| Tumor differentiation (Edmondson-Steiner grade) | <0.001* | ||||||
| I | 19 (4) | 18 (6) | 1 (1) | 0 | |||
| II | 166 (33) | 125 (40) | 29 (34) | 12 (11) | |||
| III | 217 (43) | 125 (40) | 39 (46) | 53 (48) | |||
| IV | 104 (21) | 43 (14) | 16 (19) | 45 (41) | |||
| Capsule formation | 0.044* | ||||||
| Absent | 122 (24) | 85 (27) | 18 (21) | 19 (17) | |||
| Partial | 212 (42) | 130 (42) | 29 (34) | 53 (48) | |||
| Complete | 172 (34) | 96 (31) | 38 (45) | 38 (35) | |||
| Satellite nodule | 29 (6) | 12 (4) | 2 (2) | 15 (14) | <0.001* | ||
Numbers are presented as medians (interquartile ranges) or values (percentages).
MVI, microvascular invasion; PT-INR, prothrombin time-international normalized ratio; AST, aspartate aminotransferase; ALT, alanine aminotransferase; AFP, alpha fetoprotein; PIVKA-II, prothrombin induced by vitamin K absence-II.
Table┬Ā2.
| Variable |
Overall survival |
Recurrence-free survival |
Recurrence beyond Milan |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Univariable analysis |
Multivariable analysis |
Univariable analysis |
Multivariable analysis |
Univariable analysis |
Multivariable analysis |
||||||||
| Hazard ratio (95 % CI) | P-value | Hazard ratio (95 % CI) | P-value | Hazard ratio (95 % CI) | P-value | Hazard ratio (95 % CI) | P-value | Hazard ratio (95 % CI) | P-value | Hazard ratio (95 % CI) | P-value | ||
| Clinical feature | |||||||||||||
| Age (Ōēź60 years) | 0.996 (0.580ŌĆō1.710) | 0.988 | 0.989 (0.751ŌĆō1.302) | 0.938 | 0.811 (0.535ŌĆō1.229) | 0.323 | |||||||
| Sex (male) | 1.509 (0.682ŌĆō3.340) | 0.310 | 1.438 (0.982ŌĆō2.107) | 0.062 | 1.534 (0.835ŌĆō2.817) | 0.168 | |||||||
| Etiology (hepatitis B virus) | 0.543 (0.305ŌĆō0.964) | 0.037* | 1.075 (0.764ŌĆō1.512) | 0.680 | 0.916 (0.552ŌĆō1.521) | 0.735 | |||||||
| Platelet (<100├Ś109/L) | 0.767 (0.277ŌĆō2.125) | 0.610 | 1.656 (1.105ŌĆō2.482) | 0.015* | 1.775 (1.164ŌĆō2.708) | 0.008* | 1.584 (0.862ŌĆō2.908) | 0.138 | |||||
| PT-INR (>1.0) | 1.090 (0.601ŌĆō1.979) | 0.776 | 1.420 (1.038ŌĆō1.943) | 0.028* | 1.561 (0.949ŌĆō2.566) | 0.079 | |||||||
| Albumin (<4.0 g/dL) | 1.720 (0.995ŌĆō2.974) | 0.052 | 1.523 (1.146ŌĆō2.024) | 0.004* | 1.744 (1.142ŌĆō2.666) | 0.010* | 1.549 (1.006ŌĆō2.3387) | 0.047* | |||||
| Bilirubin (>1.0 mg/dL) | 1.440 (0.816ŌĆō2.542) | 0.209 | 1.273 (0.938ŌĆō1.728) | 0.121 | 1.620 (1.044ŌĆō2.514) | 0.032* | 1.479 (0.948ŌĆō2.308) | 0.085 | |||||
| AST (>30 U/L) | 2.138 (1.192ŌĆō3.835) | 0.011* | 1.711 (0.943ŌĆō3.104) | 0.077 | 1.661 (1.259ŌĆō2.193) | <0.001* | 1.510 (1.139ŌĆō2.002) | 0.004* | 1.955 (1.261ŌĆō3.031) | 0.003* | |||
| ALT (>30 U/L) | 1.672 (0.941ŌĆō2.969) | 0.080 | 1.360 (1.029ŌĆō1.797) | 0.031* | 1.725 (1.109ŌĆō2.685) | 0.016* | 1.567 (1.005ŌĆō2.443) | 0.048* | |||||
| AFP (Ōēź400 ng/mL) | 1.558 (0.834ŌĆō2.908) | 0.164 | 1.001 (0.696ŌĆō1.441) | 0.994 | 1.280 (0.763ŌĆō2.147) | 0.350 | |||||||
| PIVKA-II (Ōēź400 mAU/mL) | 2.204 (1.275ŌĆō3.810) | 0.005* | 1.575 (1.165ŌĆō2.128) | 0.003* | 1.832 (1.182ŌĆō2.841) | 0.007* | |||||||
| Pathological finding | |||||||||||||
| Cirrhosis | 1.272 (0.722ŌĆō2.242) | 0.405 | 1.291 (0.966ŌĆō1.723) | 0.084 | 0.884 (0.554ŌĆō1.412) | 0.606 | |||||||
| Tumor size (Ōēź3 cm) | 3.103 (1.632ŌĆō5.897) | 0.001* | 2.193 (1.133ŌĆō4.244) | 0.020* | 1.676 (1.270ŌĆō2.211) | <0.001* | 1.485 (1.115ŌĆō1.979) | 0.007* | 2.578 (1.625ŌĆō4.091) | <0.001* | 1.992 (1.242ŌĆō3.195) | 0.004* | |
| Tumor differentiation (E-S grade III or IV) | 1.681 (0.914ŌĆō3.090) | 0.095 | 1.266 (0.951ŌĆō1.686) | 0.106 | 1.476 (0.935ŌĆō2.328) | 0.094 | |||||||
| Complete tumor capsule | 0.917 (0.524ŌĆō1.603) | 0.760 | 0.973 (0.732ŌĆō1.293) | 0.973 | 0.899 (0.580ŌĆō1.394) | 0.635 | |||||||
| Satellite nodule | 4.961 (2.551ŌĆō9.648) | <0.001* | 2.832 (1.415ŌĆō5.667) | 0.003* | 4.325 (2.814ŌĆō6.647) | <0.001* | 3.377 (2.172ŌĆō5.251) | <0.001* | 5.404 (3.134ŌĆō9.318) | <0.001* | 3.464 (1.958ŌĆō6.126) | <0.001* | |
| Severe MVI (vs. mild or no MVI) | 4.030 (2.357ŌĆō6.890) | <0.001* | 2.962 (1.686ŌĆō5.205) | <0.001* | 1.863 (1.377ŌĆō2.519) | <0.001* | 1.638 (1.192ŌĆō2.252) | 0.002* | 3.694 (2.425ŌĆō5.628) | <0.001* | 2.797 (1.803ŌĆō4.340) | <0.001* | |
Table┬Ā3.
| Variable |
Univariable analysis |
Multivariable analysis |
|||
|---|---|---|---|---|---|
| Odds ratio (95 % CI) | P-value | Odds ratio (95 % CI) | P-value | ||
| Clinical feature | |||||
| Age (Ōēź60 years) | 0.990 (0.644ŌĆō1.521) | 0.962 | |||
| Sex (male) | 1.156 (0.665ŌĆō2.011) | 0.608 | |||
| Etiology (hepatitis B virus) | 0.685 (0.419ŌĆō1.120) | 0.131 | |||
| Platelet (<100├Ś109/L) | 0.424 (0.163ŌĆō1.101) | 0.078 | |||
| PT-INR (>1.0) | 0.837 (0.534ŌĆō1.314) | 0.440 | |||
| Albumin (<4.0 g/dL) | 1.423 (0.903ŌĆō2.242) | 0.128 | |||
| Bilirubin (>1.0 mg/dL) | 1.662 (1.038ŌĆō2.661) | 0.035* | 1.254 (0.728ŌĆō2.159) | 0.415 | |
| AST (>30 U/L) | 1.590 (1.034ŌĆō2.446) | 0.035* | 1.460 (0.888ŌĆō2.401) | 0.136 | |
| ALT (>30 U/L) | 1.458 (0.943ŌĆō2.255) | 0.090 | |||
| AFP (Ōēź400 ng/mL) | 3.919 (2.384ŌĆō6.443) | <0.001* | 2.749 (1.560ŌĆō4.844) | <0.001* | |
| PIVKA-II (Ōēź400 mAU/mL) | 3.945 (2.493ŌĆō6.240) | <0.001* | 2.377 (1.348ŌĆō4.193) | 0.017* | |
| MRI finding | |||||
| Tumor size (Ōēź3 cm) | 3.233 (2.033ŌĆō5.141) | <0.001* | 1.576 (0.892ŌĆō2.786) | 0.118 | |
| Arterial peritumoral enhancement | 2.455 (1.548ŌĆō3.892) | <0.001* | 1.156 (0.664ŌĆō2.013) | 0.609 | |
| Non-smooth tumor margin | 4.139 (2.190ŌĆō7.825) | <0.001* | 2.224 (1.115ŌĆō4.434) | 0.023* | |
| Peritumoral hypointensity on HBP | 3.201 (2.061ŌĆō4.973) | <0.001* | 1.321 (0.761ŌĆō2.291) | 0.323 | |
| Satellite nodule | 5.290 (2.897ŌĆō9.660) | <0.001* | 3.264 (1.622ŌĆō6.567) | <0.001* | |
Abbreviations
REFERENCES
- TOOLS
-
METRICS

- ORCID iDs
-
Dong Ho Lee

https://orcid.org/0000-0001-8983-851XHaeryoung Kim

https://orcid.org/0000-0002-4205-9081 - Related articles



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement1
Supplement1 Print
Print



