The evolving role of lenvatinib at the new era of first-line hepatocellular carcinoma treatment
Article information
Abstract
Emergence of multi-targeted kinase inhibitors (MTIs) and immune checkpoint inhibitors (ICI) have changed the landscape of management in hepatocellular carcinoma (HCC). Combination therapy involving ICI has superseded sorafenib as the first-line treatment option for advanced HCC due to their superior response rates and survival benefits based on recently published phase III trials. However, the role of first-line lenvatinib remains uncertain as no prospective trials have compared its efficacy with ICI in advanced HCC. Several retrospective studies have shown that first-line lenvatinib may not be inferior to ICI combination. Indeed, a growing body of evidence suggests that ICI treatment is associated with inferior treatment outcome in non-viral HCC patients, questioning the supremacy of ICI treatment in all patients and rendering first-line lenvatinib as a potential preferred treatment option. Furthermore, in high-burden intermediate-stage HCC, accumulating evidence supports first-line lenvatinib, or in combination with transarterial chemoembolization (TACE), as a preferred treatment option over TACE alone. In this Review, we describe the latest evidence surrounding the evolving role of first-line lenvatinib in HCC.
INTRODUCTION
Hepatocellular carcinoma (HCC) is a huge global healthcare burden according to the latest GLOBOCAN statistics [1]. In 2020, primary liver cancer (with HCC representing ~75–85% of cases) ranked the sixth most commonly diagnosed cancer with approximately 906,000 new cases, and the third leading cause of cancer mortality worldwide resulting in 830,000 deaths [1]. Despite improvement in surveillance strategies, many HCC patients present at an advanced stage where systemic therapy is a central component of treatment.
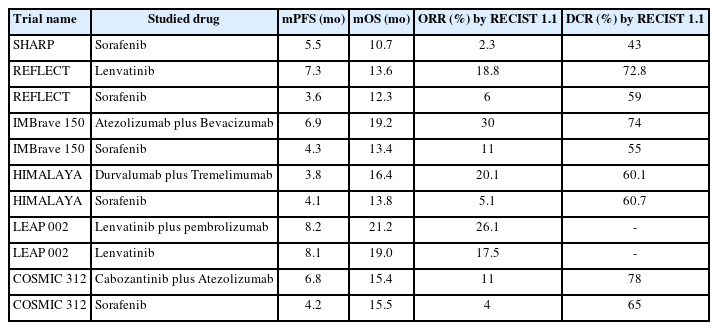
Systemic treatment has been limited for HCC. Sorafenib was the first multi-targeted kinase inhibitor (MTI) approved for the treatment of advanced HCC. It was approved in 2007 based on the SHARP trial, in which sorafenib improved progression-free survival (PFS) from 2.8 months to 5.5 months (hazard ratio [HR]: 0.58; P<0.001), and overall survival (OS) from 7.9 months to 10.7 months (HR: 0.69; P<0.001) [2]. Despite these statistically significant findings, the objective response rate (ORR) of sorafenib was only 2%, and majority of patients treated with sorafenib achieved stable disease only (Tables 1 and 2). Unfortunately, a number of subsequent trials testing other MTIs had failed to demonstrate superiority compared to sorafenib [3-6], and sorafenib remained as the only systemic treatment option for advanced HCC for the next ten years.
Patients’ characteristics in major positive phase III trials of first-line MTI or ICI combination in advanced HCC
In the recent five years, systemic treatment of HCC was met with an expansion of treatment options including both MTIs [7-10] and immune checkpoint inhibitors (ICI) [11,12]. The approval of lenvatinib as the first-line treatment in advanced HCC in 2018 based on the noninferiority REFLECT trial marked the turning point of systemic treatment options in advanced HCC (Tables 1 and 2) [7]. Currently, OS for HCC patients with advanced disease have become more than doubled from a few months only in the era of SHARP trial to more than one and a half year in the immunotherapy era [2,13,14]. In particular, the introduction of ICIs as a treatment strategy has revolutionized the treatment paradigm of many cancers, including HCC. Combination of atezolizumab (anti-PD-L1) plus bevacizumab (anti-VEGF), or durvalumab (anti-PD-L1) plus tremelimumab (anti-CTLA4), has demonstrated unprecedented high ORR in the range of 20% to 30%, and OS in the range of 16 to 19 months unseen in the history of HCC (Tables 1 and 2). These ICI combinations have now become the recommended first-line treatment options for advanced HCC [11,12].
With these rapid developments of systemic treatment options for HCC, there is much ambiguity on the role of lenvatinib in the first-line setting. In particular, given the remarkable clinical outcomes offered by ICI combinations, should we cast away lenvatinib as a treatment option in the first-line setting in advanced disease? Alternatively, are there situations where lenvatinib may be a reasonable, or perhaps a more suitable, first-line treatment option in the management of HCC? In this Review, we will address these controversies and discuss about the evolving role of lenvatinib as a first-line treatment option for HCC.
LENVATINIB MONOTHERAPY AS FIRST-LINE TREATMENT IN ADVANCED HCC
Patients who are not suitable for ICI therapy
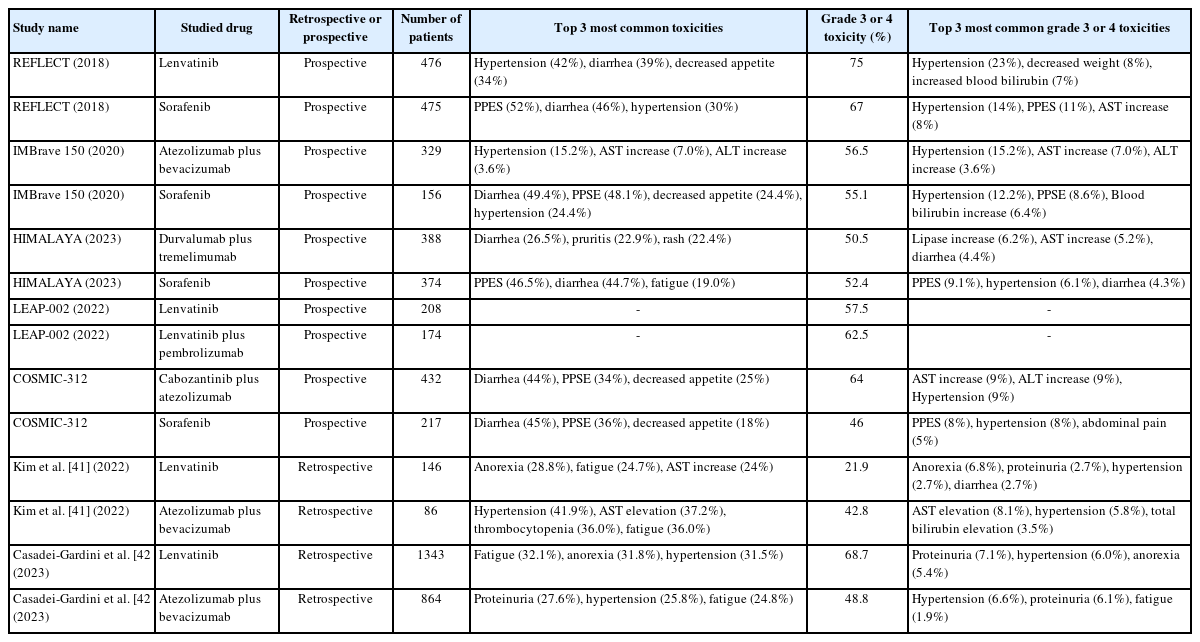
Lenvatinib is an oral MTI that targets the VEGF receptors 1–3, FGF receptors 1–4, PDGF receptor alpha, RET, and KIT [15]. Lenvatinib monotherapy was approved for advanced HCC based on the REFLECT study which showed noninferiority of lenvatinib compared to sorafenib. In the trial, patients who received lenvatinib had a median OS of 13.6 months compared to 12.3 months for patients who received sorafenib (HR 0.92, 95% confidence interval [CI] 0.79–1.06). Patients treated with lenvatinib also had longer PFS (7.4 vs. 3.7 months; HR: 0.66, 95% CI 0.57–0.77) and higher ORR (24.1% vs. 9.2%; OR: 3.13, 95% CI 2.15–4.56) compared to sorafenib. Treatment emergent adverse events were similar between the two drugs. In a post-hoc analysis of patient-reported outcomes (PRO) of the REFLECT trial, most PRO scales generally favoured the lenvatinib group. Patients treated with lenvatinib experienced statistically significant delays in fatigue, pain and diarrhea compared to sorafenib [16]. In the real world setting, the similar OS observed between sorafenib and lenvatinib have also been demonstrated in a meta-analysis including 15 studies containing 3,908 patients from both Asian and Western populations, with consistent findings of higher ORR and prolonged PFS with lenvatinib compared to sorafenib [17]. Furthermore, lenvatinib was associated with higher incidence of asymptomatic adverse events such as hypertension, proteinuria and hypothyroidism, whereas sorafenib was associated with higher incidence of symptomatic adverse events such as palmar-plantar erythrodysaesthesia and diarrhea (Table 3) [17]. Therefore, lenvatinib might be preferable over sorafenib in clinical practice if MTI monotherapy is prescribed as systemic treatment.
Grade 3 or 4 adverse events of lenvatinib and ICI combinations in landmark phase III studies and large retrospective studies
However, the role of MTI monotherapy as first-line treatment in advanced HCC has diminished since the introduction of ICI combinations. The current recommended first-line treatment for advanced HCC is either atezolizumab plus bevacizumab based on the IMBrave 150 trial [11,14], or durvalumab plus tremelimumab based on the HIMALAYA trial [18]. These ICI combinations have demonstrated superior ORR and OS benefits over sorafenib. In the updated analysis of IMBrave 150, atezolizumab plus bevacizumab prolonged PFS for 2.6 months, from 4.3 months to 6.9 months, and prolonged OS for 5.8 months, from 13.4 months to 19.2 months compared to sorafenib. Higher ORR was observed in the atezolizumab plus bevacizumab group (30% and 11%) (Table 2). Incidence of grade 3 to 4 treatment-related adverse events were similar between the two treatment arms (Table 3). In the HIMALAYA trial, durvalumab plus tremelimumab was associated with improved OS at 16.4 months compared to sorafenib at 13.8 months, and a higher ORR with 20.1% for the durvalumab plus tremelimumab group compared to 5.1% for the sorafenib group [12]. But the PFS was similar between durvalumab plus tremelimumab and sorafenib (3.8 vs. 4.1 months) (Table 2). Notably, the survival curve for patients treated with durvalumab and tremelimumab plateau at around 30%, implying a significant proportion of patients were long-term survivors.
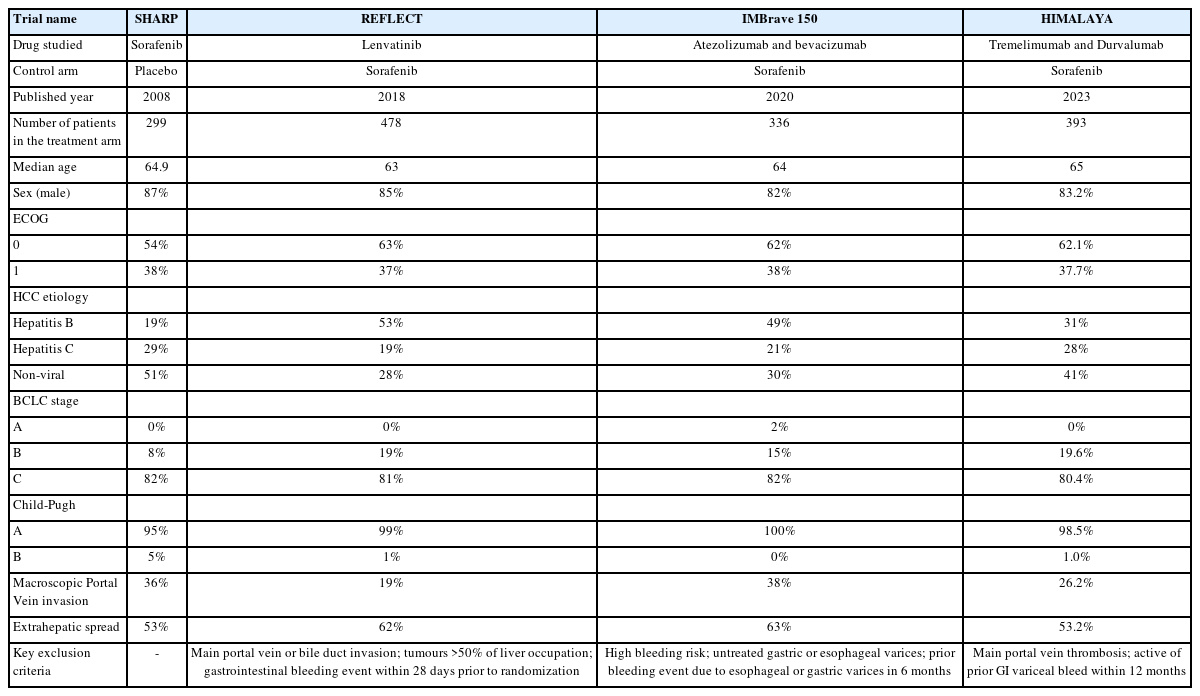
Despite the higher ORR and survival offered by ICI combinations, there are scenarios in which clinicians may consider lenvatinib over ICI combinations, considering patients’ comorbidities, physical conditions and preferences. For instance, patients with untreated or incompletely treated esophageal or gastric varices with signs of portal hypertension should avoid atezolizumab plus bevacizumab, because of the significant bleeding risk associated with high dose bevacizumab (15 mg/kg). Patients with underlying autoimmune diseases are at risk of disease flare (up to 50%) or the occurrence of other immune-related adverse events if given ICIs. Indeed, this group of patients is usually excluded from clinical trials testing ICIs [19,20]. Furthermore, the management of immunosuppressive drugs at the beginning of ICI therapy in patients with pre-existing autoimmune disease remains a question in clinical practice [21].
Importantly, a minority group (~10–16%) of HCC patients developed disease recurrence after liver transplantation [22]. Liver transplantation is a potential curative treatment option for selected HCC patients who fulfilled the Milan criteria [23]. Patients with liver transplantation require long-term immunosuppressive drugs to avoid acute or chronic rejection. The use of ICIs in HCC recurrence post liver transplantation is controversial due to the risk of enhancing alloimmunity and inducing rejection, as well as concerns of the efficacy of ICIs under the background of immunosuppressants [24]. Indeed, evidence on this topic is scarce. A recent literature review including 27 cases of liver transplants with HCC recurrence treated with ICIs, 8 (29.6%) patients had disease control, but 6 (22.2%) patients developed acute graft rejection [25]. Therefore, the most suitable systemic therapy for HCC recurrence post liver transplant is still MTI. Sorafenib, being the MTI with the longest history in the treatment for HCC, has accumulated the highest amount of evidence in this group of patients [26-28]. Recently, more evidence is also available for lenvatinib. In a retrospective case-control study in Taiwan, 10 patients were identified to have received lenvatinib after disease recurrence post liver transplantation. The median PFS and OS were 3.7 and 16.4 months respectively [29]. In this small cohort of patients, 20% of patients achieved partial response and 50% of patients achieved stable disease. Adverse events were predominantly grade 1 to 2, with only 1 patient developed grade 3 hypertension. Compared to the control group which were 25 HCC patients without liver transplantation who received second-line lenvatinib, there were no difference in PFS, OS or pattern of adverse events observed [29]. In another multinational, multicenter, retrospective study evaluating 45 patients with recurrent HCC after liver transplantation, lenvatinib achieved a median PFS and OS of 7.6 months and 14.5 months respectively [30]. The most common grade 3 adverse event was hypertension, which developed in 20% of patients. There were no grade 4 toxicity observed. In another case series conducted in Milan of 9 HCC recurrence post liver transplantation, lenvatinib was associated with a median PFS of 321 days and 1 patient experienced grade 3 adverse event (nephrotic syndrome) requiring drug withdrawal. Comparing with a matched cohort of patients treated with sorafenib, lenvatinib was associated with a better median PFS and OS [31]. Overall, lenvatinib is also an effective treatment option for recurrent HCC post liver transplantation, with no new toxicity signal seen.
Patients with severe portal hypertension or main portal vein thrombosis
Patients with severe portal hypertension or main portal vein thrombosis (Vp4) represent a group with particularly poor prognosis and at high risk of treatment-related adverse events. Extra considerations are needed when choosing systemic therapy for them. Severe portal hypertension is associated with high risk of variceal bleeding. Surveillance with endoscopy or prophylactic treatment with beta-blocker is advocated in the latest Baveno VII consensus [32]. The use of agents with anti-VEGF properties such as lenvatinib and bevacizumab in patients with severe portal hypertension has raised concerns of increased variceal bleeding risk and mortality secondary to exacerbation of portal hypertension [33]. In a prospective cohort study on the portal hemodynamic effects of lenvatinib in 28 advanced HCC patients, lenvatinib reduced the portal venous flow velocity, increased congestion index, and aggravated portal hypertension after 2 weeks of administration [34]. However, bleeding events related to portal hypertension with the use of MTIs (including lenvatinib) were consistently reported to be lower than 2% in the recent published phase 3 trials [33,35]. In a prospective multicenter study of 93 patients treated with lenvatinib, in which 37 patients had advance portal hypertension, OS did not seem to be compromised by advanced portal hypertension [36]. On the contrary, the risk of variceal bleeding was elevated in IMbrave 150 at 2.4% in the atezolizumab plus bevacizumab group compared to 0.6% in the sorafenib group. Of note, this was a group of well-selected patients benefited from optimal portal hypertension prophylaxis and patients with bleeding esophageal or gastric varices have been excluded from the study already [11,33]. In unscreened patients, the use of bevacizumab has been associated with 10% risk of bleeding varices based on systematic review on phase II trials [37].
Patients with main portal vein thrombosis (Vp4) were excluded from both the REFLECT and HIMALAYA trial. A retrospective study of 20 patients with Vp4 advanced HCC demonstrated efficacy and safety of lenvatinib, with ORR of 20% by mRECIST criteria and median OS of 6.7 months [38]. Variceal bleed was seen in 2 (10%) patients. In another retrospective study included 41 HCC patients with major portal vein tumor thrombosis (Vp3/4) treated with sorafenib or lenvatinib, lenvatinib treatment was the only significant predictor of better OS (HR 0.19, 95% CI 0.06–0.68; P=0.0106) and time to tumour progression (HR 0.16, 95% CI 0.05–0.56; P=0.004) [39]. Worsening of liver function was noted in the first 2 weeks in the lenvatinib group but improved afterwards. The study did not report any incidence of variceal bleeding in the adverse events. On the contrary, in an exploratory analysis of IMbrave 150 evaluating the efficacy and safety of atezolizumab plus bevacizumab in patients with Vp4 portal vein invasion, OS was numerically higher in the atezolizumab plus bevacizumab group compared to the sorafenib group (7.6 vs. 5.5 months; HR 0.62; 95% CI 0.34–1.11) but the incidence of variceal bleeding was higher with atezolizumab plus bevacizumab group (13.6% vs. 0%) [40].
Therefore, for patients with severe portal hypertension, durvalumab plus tremelimumab may be considered as first-line treatment as it has the least bleeding risk compared to atezolizumab plus bevacizumab and lenvatinib. Unfortunately, despite tremelimumab has been approved for use in combination with durvalumab by the Food and Drug Administration, and recently by the European Medicines Agency, it is still under regulatory review in many places worldwide (e.g., United Kingdom, Australia, Hong Kong). The cost of tremelimumab is also prohibitive and so it is not accessible to many patients. Between atezolizumab plus bevacizumab and lenvatinib, lenvatinib is preferred if timely pre-treatment screening for variceal bleeding is not available. Similarly, for patients with main portal vein thrombosis, given the finding of 13.4% of patients developed variceal bleeding with atezolizumab plus bevacizumab in the exploratory analysis of IMBrave 150, lenvatinib might be considered safer if a timely screening of esophageal/gastric varices is not available.
Is lenvatinib inferior to ICI combination?
The current recommendation of ICI combinations as first-line treatment in advanced HCC is based on their superior response rates and survival compared to sorafenib. There is no prospective data to compare lenvatinib with ICI combinations. In fact, it is logical to expect lenvatinib to be inferior to ICI combination as lenvatinib was shown to be noninferior to sorafenib based on the REFLECT trial [7]. Recently, evidence has emerged to suggest that first-line lenvatinib may not be inferior to first-line ICI combination. In a retrospective study including 232 advanced HCC patients conducted in three academic hospitals in Korea, treatment with either lenvatinib or atezolizumab plus bevacizumab did not result in any statistically significant difference in ORR (32.6% vs. 31.5%, P=0.868), PFS (5.7 vs. 6.0 months; P=0.738) and OS (not reached vs. 12.8 months; P=0.357) [41]. Subgroup analyses showed that OS was comparable between the atezolizumab plus bevacizumab and lenvatinib group according to all strata (e.g., age, sex, performance status, etiology etc.) except for alpha feto-protein (AFP) level, of which AFP<200 was associated with favourable outcome with lenvatinib. In terms of toxicity, more grade 3 or 4 adverse events were observed in the atezolizumab plus bevacizumab group compared to lenvatinib group, but the difference was not statistically significant (42.8 vs. 21.9%; P=0.141) [41]. In another large international retrospective study including 2,205 patients with advanced HCC, after balancing clinical features using inverse probability of treatment weighting methodology, there was no difference in either time to progression (HR 0.82; P=0.117) or OS (HR 0.97; P=0.739) comparing atezolizumab plus bevacizumab with lenvatinib [42]. But grade 3 or 4 adverse events were more common in the lenvatinib group compared to the atezolizumab plus bevacizumab group (84.9% vs. 69.8%; P=0.009).
These retrospective results might somehow appear puzzling as one would expect lenvatinib to be inferior to ICI combination. In order to interpret these results, a few points should be considered. First, while the primary endpoint was noninferiority in OS in the REFLECT trial, there was a trend towards more favourable outcomes with lenvatinib compared to sorafenib, in terms of OS, PFS and ORR [7]. In fact, several real-world studies have shown that lenvatinib performed much better in clinical practice than in randomized clinical trials. It has been consistently shown that lenvatinib not only offered superior ORR but also survival compared to sorafenib [43-45]. Second, evidence suggested that improved clinical outcomes were linked to more experience in management of adverse events with sorafenib [46,47]. Since sorafenib and lenvatinib belonged to the same drug class and shared many pharmaceutical characteristics, it is plausible that prior experiences with sorafenib resulted in shorter learning curve in managing adverse events during lenvatinib treatment leading to better clinical outcome. Third, it was noted that more patients who had first-line lenvatinib in both retrospective studies cited above received locoregional therapy as a subsequent treatment [41,42]. However, as the authors pointed out, this discrepancy could be related to the earlier approval of lenvatinib compared to atezolizumab plus bevacizumab, leading to a lack of effective second-line treatment (i.e., immunotherapy) in the post-lenvatinib setting [41]. All in all, although many plausible hypotheses exist to explain the similar OS between patients treated with lenvatinib and atezolizumab plus bevacizumab in the real-world setting, one should keep in mind of the limitations of these retrospective studies that they were intrinsically biased and the population studied in the different treatment arms could be unbalanced. Future prospective studies with well-balanced populations comparing lenvatinib and atezolizumab plus bevacizumab, or other ICI combinations, would be needed to understand whether these treatments are indeed similar in efficacy.
In the prospective setting, lenvatinib monotherapy has also been compared to ICI combination. In the LEAP 002 trial, which was a global, randomized, double-blind, phase 3 study evaluating the efficacy and safety of lenvatinib plus pembrolizumab versus lenvatinib in the first-line setting for advanced HCC. This is the first phase 3 study of lenvatinib since the REFLECT study. Lenvatinib plus pembrolizumab failed to demonstrate improved PFS and OS according to the pre-specified statistical significance [48]. Combination of lenvatinib plus pembrolizumab resulted in PFS and OS of 8.2 months and 21.2 months respectively, compared to 8.1 months (HR for PFS: 0.87, 95% CI 0.73–1.02; P=0.047) and 19.0 months (HR for OS: 0.84, 95% CI 0.71–1.00; P=0.0227) respectively with lenvatinib monotherapy. Of note, the lenvatinib arm performed exceptionally well compared to the REFLECT trial (median OS 13.6 months) which included patients of similar characteristics. ORR was improved with lenvatinib plus pembrolizumab at 26.1% as compared to the lenvatinib arm at 17.5%, which was similar to the reported figures in the REFLECT trial. One major reason for the exceptional performance of lenvatinib arm was the availability of second-line treatment. In the LEAP 002 trial, 52.1% of patients on the lenvatinib arm received additional treatment, which was higher than in the REFLECT study at 33% only. Of that 52%, 22.8% received additional immunotherapy (e.g., atezolizumab plus bevacizumab), which is considered very active in HCC [49]. In terms of toxicity, ICI combination was associated with higher toxicity, with grade 3 to 5 treatment related adverse events of 62.5% in the lenvatinib plus pembrolizumab group, compared to 57.5% in the lenvatinib group.
Taken together, it appears that first-line lenvatinib may be non-inferior to ICI combinations. In terms of toxicity, rate of grade 3 or higher toxicity was variable for lenvatinib in published studies, ranging from 20 to 75% (Table 3). In comparison, grade 3 or higher toxicity for ICI combinations was more consistently reported at around 40 to 50% (Table 3). Most common grade 3 or higher adverse events for lenvatinib was hypertension, which could usually be managed with anti-hypertensives, interruptions, and dose reductions. For ICI combinations, the type of grade 3 or higher adverse events was more variable depending on the ICI being used [7,11,12,41,42,48]. Nonetheless, first-line lenvatinib monotherapy may allow for titration of dose according to patients’ performance status and tolerance, which could be a more versatile treatment option for those with borderline fitness to systemic treatment. Indeed, a recent retrospective study including 176 patients with advanced HCC treated with lenvatinib showed that upfront dose reduction of lenvatinib was not associated with inferior survival outcome [50].
Does etiology of HCC have an impact on treatment outcome?
In the past in which treatment for advanced HCC was mainly MTIs [2,7-10], it was thought that the etiology of HCC did not have an impact on HCC. However, after the introduction of ICI in the management of advanced HCC, evidence is accumulating that the etiology of HCC might have an impact on treatment outcomes [14,51]. For example, in the updated analysis of IMBrave 150, atezolizumab plus bevacizumab resulted in improved PFS and OS compared to sorafenib, in hepatitis B HCC (HR for OS: 0.58, 95% CI 0.40–0.83; HR for PFS: 0.51, 95% CI 0.37–0.70) but not in non-viral HCC (HR for OS: 1.05, 95% CI 0.68–1.63; HR for PFS: 0.80, 95% CI 0.55–1.17) [14]. The COSMIC 312 trial was a multi-centre, randomized, phase III trial comparing cabozantinib plus atezolizumab with sorafenib in advanced HCC [51]. Although the trial was negative for its primary endpoint in OS, in the prespecified exploratory subgroup analysis, PFS and OS were longer with the combination treatment versus sorafenib in the hepatitis B HCC subgroup (PFS: HR 0.46, 95% CI 0.29–0.73; OS: HR 0.53, 95% CI 0.33–0.87) but not in the non-viral subgroup (PFS: HR 0.92, 95% 0.60–1.41; OS: 1.18, 95% CI 0.78–1.79) [51]. A recent translational study indicated that the use of anti-PD-1 treatment may paradoxically induces and accelerates carcinogenesis in HCC patients with underlying non-alcoholic steatohepatitis (NASH) [52]. The group found that CD8+PD1+ T-cells were specifically enriched in NASH-HCC both in mouse model and human tumours. Notably, anti-PD-1 treatment promoted tissue damage, resulted in malignant changes, and induced more aggressive behaviour of existing NASH-HCC. Furthermore, in a meta-analysis of 3 published phase III trials (CheckMate 459, Keynote 240, and IMBrave 150) conducted by the same group, they found that patients with non-viral HCC did not derive survival benefits from immunotherapy (HR 0.92, 95% CI 0.77–1.11). Instead, OS was prolonged with immunotherapy in patients with viral HCC (HR 0.64, 95% CI 0.48–0.94) [52].
In light of these interesting data, research has grown to test if HCC patients with non-viral etiology would benefit less from immunotherapy compared to MTIs. In a recently published, multinational, prospectively consecutively enrolled, retrospective study of 759 advanced HCC with non-viral etiology, treatment with lenvatinib was associated with better OS (HR: 0.65, 95% CI 0.44–0.95; P=0.0268) and PFS (HR: 0.67, 95% CI 0.51–0.86; P=0.002) compared to atezolizumab plus bevacizumab [53]. In particular, in the non-alcoholic fatty liver disease (NAFLD)/NASH population, multivariate analysis showed that lenvatinib treatment was associated with longer OS (HR: 0.46, 95% CI 0.26–0.84; P=0.011) and PFS (HR: 0.55, 95% CI 0.38–0.82; P=0.031) compared to atezolizumab plus bevacizumab, but not in the non-NAFLD/NASH patient subgroup [53].
Several factors should be considered before using etiology of HCC to determine the first-line choice of treatment. First, it is evident that non-viral subgroup of HCC is a heterogeneous population, including patients with NAFLD, chronic alcoholism, occult HBV infection (anti-HBc positive but HBsAg negative) or patients with mixed picture from above causes. Analyses of benefits of systemic therapy in each of the above subgroup is required to understand the benefits of each systemic therapy. Second, current supporting data came from subgroup analyses of clinical trials or retrospective series, which was prone to bias. The hypothesis requires validation by prospective clinical trials comparing lenvatinib to ICI-based treatment for HCC of specific etiology subgroup. Third, more informed definitions of non-viral HCC are required as these subgroups were not clearly defined in the reported analyses [14,51,53]. For example, the gold standard of diagnosis of NAFLD is based on histological presence of steatosis in >5% hepatocytes which can only be obtained by invasive procedures such as liver biopsy. Although non-invasive diagnosis is feasible with computed tomography and ultrasonography, the reporting of radiological images is limited by intra-observers discrepancy and the sensitivity of detection by these imaging modalities [54]. Furthermore, concurrent fatty liver disease with viral hepatitis can occur in a high proportion of viral hepatitis patients in the current metabolic liver disease pandemic. For example, in one retrospective cohort study in Hong Kong including 270 HBV-infected patients, histologically confirmed concurrent fatty liver disease was found in 107 (39.6%) patients [55]. Therefore, future trials should clearly define the different etiologies of HCC, and take into account the possibility of concomitant etiologies occurring in the same patient.
First-line lenvatinib with transarterial chemoembolization (TACE)
In addition to first-line lenvatinib monotherapy in advanced HCC, lenvatinib has also been explored in combination with TACE in advanced HCC setting to improve clinical outcome [56]. Insufficient intrahepatic tumour response remains a major problem with repeated TACE [57]. Upregulation of VEGF and other pro-angiogenic factors post TACE induced by the creation of ischemic tumour environment has been implicated as the major mechanism of resistance to treatment [58,59]. Lenvatinib as a potent anti-angiogenic agent could theoretically offer synergism with TACE by inhibiting angiogenesis and tumour growth after TACE.
In the LAUNCH study, 338 Chinese patients with primary treatment-naïve or initial recurrent advanced HCC after surgery were randomly assigned to lenvatinib or lenvatinib plus on-demand TACE (LEN-TACE) [56]. Majority of patients (>85%) had hepatitis B. TACE was given 1 day after oral administration of lenvatinib and then repeated if there were incomplete necrosis or tumour regrowth. After a median follow-up of 17 months, it was shown that the OS was significantly longer in the LEN-TACE group at 17.8 months compared to 11.8 months in the lenvatinib monotherapy group (HR: 0.45; P<0.001). The median PFS was also prolonged in the LEN-TACE group at 10.6 months compared to 6.4 months in the lenvatinib monotherapy group (HR: 0.43; P<0.001). ORR was higher in the LEN-TACE group at 54.1% compared to lenvatinib monotherapy group at 25.0% (P<0.001) by the mRECIST criteria [56]. In terms of safety, more grade 3 or 4 deranged liver enzymes were seen in the LEN-TACE group compared to lenvatinib monotherapy group (~20% vs. 2%), but the frequency of other grade 3 or 4 adverse events such as hand-foot skin reaction, diarrhea, abdominal pain etc. were similar between the two groups [56]. While this study showed promising evidence of first-line lenvatinib combined with TACE in hepatitis B HCC patients of Chinese ethnicity, further studies will be needed to extend this finding to HCC patients with other etiologies and ethnicities.
LENVATINIB AS FIRST-LINE TREATMENT OPTION IN INTERMEDIATE-STAGE HCC
Intermediate-stage HCC represents the most heterogeneous group of patients. Up until 2018, the recommended treatment for intermediate-stage (i.e., Barcelona Clinic Liver Cancer [BCLC]-B) HCC was TACE only [60]. In the 2022 updated version, it was decided that intermediate-stage HCC should be divided into three subgroups according to tumour burden and liver function, to better stratify this heterogeneous patient group and guide treatment [61]. In the subgroup with diffuse, infiltrative, extensive bilobar liver involvement, the recommended treatment is no longer TACE but systemic treatment. TACE is not an effective treatment strategy for high-burden intermediate-stage HCC and can lead to early liver function deterioration [61,62].
Indeed, systemic therapy for intermediate-stage HCC is nothing new. Sorafenib has been shown to be effective in intermediate-stage HCC in three large-scale real-world studies [63-65]. The GIDEON trial was a global prospective observational study performed between 2009 and 2012 to evaluate the safety and efficacy of sorafenib in HCC patients at different BCLC stages. It showed that the median OS was much longer in BCLC-B patients than in BCLC-C patients (OS: 29.5 vs. 11.1 months) [63]. The SOFIA and INSIGHT trial, two similar studies conducted in Europe over similar period, also showed better median OS in BCLC-B patients than in BCLC-C patients when treated with sorafenib (SOFIA, OS: 20.6 vs. 8.4 months; INSIGHT, OS: 19.6 vs. 13.6 months) [64,65].
But which group of patients would benefit from systemic treatment instead of TACE was still largely unknown. To characterize the group of patients who would be better suited for systemic therapy, lenvatinib was evaluated against TACE as first-line treatment for intermediate-stage, TACE-naïve HCC with ‘up-to-7’ out tumor burden and Child-Pugh A liver function [66]. Lenvatinib was chosen over sorafenib by virtue of its higher ORR in the REFLECT trial [7,67]. The ‘up-to-7’ criteria refers to the sum of the number of lesions and the diameters of these lesions being seven or smaller. This was a criteria first developed in extension to the Milan criteria to predict outcomes for liver transplantation [68]. In a proof-of-concept retrospective propensity score-matched study, it was shown that lenvatinib was associated with significantly improved OS (37.9 vs. 21.3 months; P<0.01), PFS (16.0 vs. 3.0 months; P<0.001) and ORR (73.3% vs. 33.3%; P<0.001). The study also showed that hepatic function deteriorated with repeated TACE (baseline ALBI score from –2.66 to –2.09; P<0.001) but was maintained in the group treated with lenvatinib (baseline ALBI score from –2.61 to –2.61; P=0.254). Of note, two patients achieved significant downstaging with lenvatinib enabling subsequent ablation and resection. These encouraging results warrant confirmation of the role of lenvatinib in intermediate-stage HCC with beyond ‘up-to-7’ tumour burden and preserved liver function in a large randomized controlled trial. Nonetheless, those who were thought to be poor responder of TACE should also be considered for lenvatinib upfront.
The TACTICS-L study was a Japanese, phase II, single-arm study to evaluate the efficacy and safety of combination therapy with lenvatinib and TACE in unresectable, intermediate-stage HCC [69]. The study recruited 62 patients who were predominantly of advanced age (≥65 years old: 79%) with BCLC-B stage (59.7%) disease. 64.5% of patients had tumour within up-to-7 criteria. Lenvatinib was given 14 to 21 days then stopped 2 days before TACE and resumed 2 days after, until disease progression. With a median follow-up of 20.3 months, the median PFS was 28.3 months and 2-year PFS was more than 60%. ORR at best response was 88.7% with complete response observed in 66.1% of patients. Around half (50.5%) of the treatment responders (n=55) had sustained response at 1 year. This treatment approach was well tolerated with the most common adverse events being hypothyroidism (58.1%), hypertension (53.2%) and decreased appetite (50.0%). No new safety signal was observed [69]. Therefore, lenvatinib-TACE is another promising first-line strategy for patients with unresectable intermediate-stage HCC despite the study recruited a significant proportion of earlier stage, BCLC-A HCC patients. Further phase III studies would be required to validate this combination approach.
On a different note, a number of phase III trials are ongoing testing the efficacy of atezolizumab plus bevacizumab (or in combination with TACE) versus TACE alone in intermediatestage HCC (NCT04803994, NCG04712643) [70,71]. As the pattern of response differs between patients treated with atezolizumab plus bevacizumab (i.e., induces tumour shrinkage) and lenvatinib (i.e., induces tumour necrosis via reduced blood through) [72], it would be interesting to compare atezolizumab plus bevacizumab-TACE with lenvatinib-TACE in intermediate-stage HCC in the future.
CONCLUDING REMARKS
In the era of effective ICI combination therapy with remarkable response rate and survival, the role of first-line lenvatinib in advanced HCC has diminished. However, not all patients are suitable for ICI therapy due to their underlying medical conditions such as autoimmune disease or on long-term immunosuppressants (Table 2). Lenvatinib in these settings plays an important role and appears to be safe and equally effective. Nevertheless, clinicians should pay attention to the frequent adverse events such as hypertension, proteinuria and hypothyroidism following long-term use of lenvatinib, and it is important to manage these side effects well. In addition, with the increasing number of drugs available for the treatment of advanced HCC, the correct sequence of treatment (e.g., ICI first vs. TKI first) is currently an active area of research. Several retrospective studies have reported efficacy and safety of lenvatinib in the second-line setting post ICI but prospective data is still lacking [73,74].
On a different note, we are now starting to understand that patients with HCC of different etiologies may response to ICI therapies differently, in which some patients may achieve better response with lenvatinib. For example, multiple retrospective analyses have shown that lenvatinib might be more effective than ICI combination in non-viral HCC patients [14,51,53]. This differential response has been attributed to the differences in tumour microenvironments and immune milieu associated with the underlying etiologies [52]. Nonetheless, non-viral HCC is a heterogeneous group of patients, and future studies specifically designed for HCC patients with a specific underlying etiology will be needed to validate these postulations (Table 2).
In addition, first-line lenvatinib has now been evaluated in the intermediate-stage setting, in particular for those patients with high tumour burden, such as beyond the ‘up-to-7’ criteria (Table 2). This group of patients is known to be refractory to conventional treatment like TACE. Several studies involving small number of patients have demonstrated that lenvatinib monotherapy or in combination with TACE is effective and safe for this group of patients, with the additional benefit of preservation of liver function [66,69]. In the neoadjuvant and adjuvant setting, a number of trials are ongoing exploring lenvatinib in combination with immunotherapy and/or locoregional treatments (e.g., TACE, RFA) which are expec ted to repor t outcomes in the nex t few years (NCT05185739, NCT04227808, NCT05113186). Combination of lenvatinib, pembrolizumab and TACE is also currently being explored in the phase III LEAP 012 study [75]. Therefore, the role of lenvatinib continues to evolve in the management of HCC and will remain an important pharmaceutical agent in the years to come.
Notes
Authors’ contribution
Both authors contribute equally to the conceptualization, literature review, drafting and final review of this manuscript.
Conflicts of Interest
S.L. Chan is the advisory for Astra-Zeneca, MSD, Eisai, BMS and Roche. S.L. Chan received research fund from MSD, Bayer, Eisai, Ipsen and SIRTEX. S.L. Chan received Honoraria from Bayer, Astra-Zeneca, Eisai, Roche and MSD. S.L. Chan is the speaker for MSD, BMC, Astra-Zeneca, Eisai, Roche, Ipsen, SIRTEX and Hutchmed.
L.L. Chan has received travel support from Roche.
Abbreviations
MTIs
multi-targeted kinase inhibitors
ICI
immune checkpoint inhibitor
HCC
hepatocellular carcinoma
TACE
transarterial chemoembolization
PFS
progression-free survival
HR
hazard ratio
OS
overall survival
ORR
objective response rate
AFP
alpha feto-protein
NASH
non-alcoholic steatohepatitis
NAFLD
non-alcoholic fatty liver disease
BCLC
Barcelona Clinic Liver Cancer
ECOG
Eastern Cooperative Oncology Group