Transarterial chemoembolization for hepatocellular carcinoma: 2023 Expert consensus-based practical recommendations of the Korean Liver Cancer Association
Article information
Abstract
Transarterial chemoembolization (TACE) was introduced in 1977 with the administration of chemotherapeutic agent to gelatin sponge particles through the hepatic artery in patients with hepatocellular carcinoma (HCC) and was established as conventional TACE using Lipiodol in the 1980s. In the 2000s, drug-eluting beads were developed and applied clinically. Currently, TACE is a commonly used non-surgical treatment modality for patients with HCC who are unsuitable for curative treatment. Considering the vital role of TACE in the management of HCC, it is crucial to organize current knowledge and expert opinions regarding patient preparation, procedural techniques, and post-treatment care in TACE, which can enhance therapeutic efficacy and safety. A group of 12 experts in the fields of interventional radiology and hepatology, convened by the Research Committee of the Korean Liver Cancer Association (KLCA), has developed expert consensus-based practical recommendations in TACE. These recommendations have been endorsed by the Korean Society of Interventional Radiology and provide useful information and direction in performing TACE procedure as well as pre- and post- procedural patient care.
INTRODUCTION
Transarterial chemoembolization (TACE) is an interventional treatment to deliver chemoembolic materials via the tumor-feeding arteries to induce tumor necrosis by selective ischemia and anticancer drug effects. TACE can be classified as conventional TACE (cTACE) using chemoemulsion, a mixture of Lipiodol (Lipiodol Ultra Fluid; Guerbet) and chemotherapeutic agents and drug-eluting bead TACE (DEB-TACE) using microspheres loaded with chemotherapeutic agents [1-4].
The effectiveness of cTACE regarding tumor responses and survival gain in patients with unresectable hepatocellular carcinoma (HCC) was proven by two landmark randomized controlled trials (RCTs) in 2002, Japanese large-scale cohort studies, and meta-analyses of these studies [5-9]. Accordingly, TACE is the most-frequently recommended treatment for patients with large or multiple HCC, and widely utilized as a salvage treatment for recurrence after radical treatment as well as an initial treatment of HCC [10,11].
Considering the vital role of TACE in the management of HCC, it is crucial to organize current knowledge and expert opinions regarding patient selection, pre-treatment management, preparation of chemoembolic materials, procedural techniques, procedure intervals, and post-treatment assessment, which can enhance procedural efficacy, safety, and ultimately patients’ survival and quality of life.
In this regard, the Korean Liver Cancer Association (KLCA) and Korean Society of Interventional Radiology (KSIR) jointly composed a panel of 12 experts, conducted expert surveys regarding the technical aspects of TACE in Korea, and reviewed literature. Subsequently, the expert panel drew the consensus-based practical recommendations for TACE. This work was announced at the 17th Annual Conference of KLCA in March 2023 and has been endorsed by the KSIR.
EXPERT SURVEY
From September to October 2022, online surveys were conducted separately for board-certified interventional radiologists (IRs) and hepatologists. An IR survey was requested for professionals performing at least one case of TACE per month, and 132 of 336 active members (39.3%) of the KSIR answered. With a regard to expertise, 73.5% of respondents had more than five years of experience in interventional radiology, and 90.1% were working in hospitals with more than 500 beds. A hepatologist survey was sent to 63 hepatologists who were active members of the KLCA, had more than eight years of experience in hepatology, and were working in teaching hospitals, and 55 hepatologists responded (response rate, 87.3%).
PATIENT SELECTION
In the international guidelines, including the 2022 KLCA-National Cancer Center (NCC) Korea practice guidelines for the management of HCC, TACE is recommended as the first-line treatment for patients with preserved liver function, good performance status, and no radiologic evidence of vascular invasion and extrahepatic spread when surgical resection, transplantation, or ablation are not viable options [12-16]. Although curative treatments are primarily recommended for early HCC, TACE can be an alternative treatment when curative treatments cannot be conducted considering patients’ liver function, performance status, underlying diseases, portal hypertension, tumor location, or tumor visibility on ultrasonography [17]. In Eastern guidelines, TACE is also performed in HCC with vascular invasion, which may have a survival benefit when it is conducted for selected patients with locally advanced HCC and preserved liver function [12-15,18].
Unlike systemic treatments, the amount of chemotherapeutic agents and extent of treatment depend on tumor size, location, and distribution, which affects liver function as well as tumor responses. In addition, tumor location and vascular anatomy may contribute to technical difficulties in TACE, which may affect the treatment outcomes. Hence, it is important to communicate closely with each other so that the physicians requesting TACE understand the technical aspects of the TACE procedure and the IRs performing TACE understand the clinical situation.
The assessment of procedural safety is crucial in patient selection for TACE. Risk factors of post-procedural liver failure, the most fatal complication following TACE, include main portal vein occlusion, obstructive jaundice, underlying liver function impairment, extensive TACE with massive chemoembolic materials for more than half of the liver, non-selective TACE, and hepatic arterial occlusion due to repetitive nonselective TACE [19,20]. Liver functional reserve should be considered even in patients with well-preserved liver function when the treatment extent is large. On the other hand, TACE can be considered in patients with compromised liver function when tumors are small and superselective TACE is available [21].
Liver abscess is an important complication following TACE, and its risk increases in patients with biliary obstruction, bile duct injury due to previous surgery, bilioenteric anastomosis, and biliary stenting [22-24]. Therefore, operators should address whether TACE can be safely performed considering the tumor size and location and whether patients can tolerate a liver abscess if it develops. Transarterial radioembolization, external beam radiotherapy, systemic therapy, or best supportive care can be considered as alternative options in patients with high risk.
In real-world practice, TACE is widely used for patients with recurrent HCC or HCC previously treated with TACE; however, well-designed studies are limited regarding this clinical situation. Therefore, the decision of TACE is made by adopting the metrics of the HCC guidelines for initial treatment or depending on the physician’s experience and preference, and the medical environment of each hospital. Scoring systems for patient selection are often used: about 60% of the respondents in the hepatologist survey answered that they utilize scoring systems including the Barcelona Clinic Liver Cancer (BCLC) B subclassification, the hepatoma arterial-embolization prognostic (HAP) score, and the Up-to-seven criteria in selective or all cases. In addition, 72.7% and 65.6% of the respondents communicates with IRs and uses multidisciplinary team approaches for patient selection, respectively.
In conclusion, TACE should be performed after careful evaluation of prognostic factors including tumor stage, tumor location, growth pattern, liver function, performance status, underlying disease, complication risks, and alternative options. A personalized approach through the multidisciplinary team approach may assist this process.
[Recommendations]
1. Indications of TACE follow the 2022 KLCA-NCC Korea practice guidelines for the management of HCC, and a personalized approach regarding tumor location, liver function, and performance status, as well as tumor stage, is required.
PRE-TREATMENT IMAGING
Pre-treatment imaging for TACE is conducted by multiphasic computed tomography (CT) or multiphasic magnetic resonance imaging (MRI). The two modalities are mutually complementary: CT has the advantage of demonstrating the vascular anatomy, calcification, and Lipiodol accumulation after TACE, while MRI enables the detection of small HCCs and the evaluation of residual viable tumor after treatment and tumor characteristics due to the excellent contrast resolution. In the IR survey, 47% of respondents answered that TACE is not disrupted if only one of two imaging (CT or MRI) is preceded in general. However, CT is preferred in cases of re-TACE (25.8% in re-TACE vs. 16.7% in initial TACE), which may be due to the easier identification of Lipiodol accumulation
The time interval between pre-treatment imaging and TACE should not be too long, considering the chance of interval tumor growth and being used as the baseline for future assessment of tumor responses. In an international panel meeting for the standardization of cTACE in 2014, this interval was recommended to be less than one month ideally, and not to exceed two months [25]. The hepatologist and IR surveys showed that 83.6% and 78.8% of respondents preferred imaging within one month, respectively, and all hepatologists agree that it should not exceed two months.
Operators have to check the following on pre-treatment imaging for safe and effective TACE: tumor size, growth pattern, number of tumors, tumor-bearing liver segment, tumor distribution, vascular invasion, arterioportal shunt, portal hypertensive signs (imaging surrogate markers like splenomegaly, ascites, portosystemic collaterals along with laboratory findings), bilioenteric anastomosis or biliary stent (to identify patients at risk of abscess), celiac trunk and hepatic artery anatomy, celiac stenosis, parasitic tumor supply, and non-hepatic arteries from the hepatic artery (e.g., accessory left gastric artery, cystic artery, and falciform ligament artery).
[Recommendations]
1. Dynamic CT or MRI should be performed as a pre-TACE evaluation, and the interval between imaging and the procedure should be within two months.
PROPHYLACTIC MEDICATIONS FOR INFECTION AND POSTEMBOLIZATION SYNDROME
Prophylactic antibiotics
The use of prophylactic antibiotics has been debated. In the hepatologist survey, 49.1% of the respondents used prophylactic antibiotics for all or selected cases. Although small retrospective studies showed negative results [26,27], a recent large-scale cohort with propensity score analysis demonstrated that prophylactic antibiotics reduced the occurrence of liver abscess following TACE by two-thirds [28].
As the risk of liver abscess increases in cases of biliary obstruction, bilioenteric anastomosis, and biliary stent across the ampulla of Vater [22,24], prophylactic antibiotics can be considered in patients with these biliary risk factors [29]. In the hepatologist survey, 60.9% of the respondents answered that patients with the biliary risk factors are indicated for preemptive use of antibiotics. However, a study showed that long-term antibiotic use was not needed as prolonged use over two weeks did not make any difference in prevention of liver abscess compared to short-term use [30]. In a retrospective study, moxifloxacin monotherapy prevented liver abscess by 100% [29], and another RCT showed that levofloxacin is non-inferior to cefazolin [31]. Therefore, 1st-generation cephalosporin or fluoroquinolone can be used as prophylactic antibiotics for TACE.
Prophylaxis of postembolization syndrome
The most common adverse events following TACE is postembolization syndrome, which involves non-infectious fever, pain, nausea, and vomiting [32]. In the hepatologist survey, 43.6% of the respondents used anti-emetics preemptively. However, only 18.2% of the respondents in the hepatologist survey considered the use of prophylactic steroid; although recent RCTs consistently showed that preemptive use of steroids reduces the occurrence of postembolization syndrome [33-35]. Due to their reluctance to use preemptive steroids, 56.3% of the respondents worried about adverse events related to steroids, and 25% regarded that steroids were not necessarily needed considering the individual difference in severity of postembolization syndrome. Considering the low fatality of postembolization syndrome and underlying liver disease, this may reflect the trend that the clinical importance of postembolization syndrome was low and the concern about the side effects of steroid in these patients was high.
Some RCTs show that non-steroid anti-inflammatory drugs such as parecoxib can reduce pain intensity and duration, and the need for narcotics [36,37]. However, caution is needed while using these drugs as they may incite kidney failure, considering most patients with HCC have underlying liver cirrhosis [38].
[Recommendations]
1. Prophylactic antibiotics can be considered when TACE is performed in patients with biliary risk factors.
INTERVENTIONAL TECHNIQUES FOR TACE
Angiography
Anatomical variations of the celiac trunk and hepatic arteries are common, and many patients also have celiac stenosis [39-41]. Therefore, it is critical to recognize the anatomy of the celiac trunk and superior mesenteric artery (SMA), as well as tumor locations and tumor-feeding arteries, by performing angiography. Celiac angiography should include the left gastric artery, and SMA angiography needs to depict its ostium so that anatomical variations of the hepatic artery, if any, can be identified. The advancement of spatial and temporal resolutions of multidetector CT (MDCT) facilitates identification of vascular anatomy, which causes debate in the routine conduct of SMA angiography. According to the IR survey, however, 81.8% of the respondents routinely performed SMA angiography in cases of initial TACE.
SMA angiography provides useful information about arterial hemodynamic, especially in cases of celiac artery stenosis and occlusion which cause flow inversion in the common hepatic artery [42]. In addition, SMA angiography, which has better spatial resolution than MDCT, can identify very small deformed hepatic or collateral arteries that develop after repeated TACE [43]. Therefore, SMA angiography needs to be conducted with celiac angiography at the first TACE session so that operators can clearly identify arterial anatomy and refer to the images in subsequent TACEs.
Extrahepatic collateral supply of HCC can be suspected when the tumor is unidentified or partially identified on hepatic arteriography. It frequently develops in cases of HCC abutting the liver capsule, recurrence of previously TACE-treated HCC, and hepatic arterial stenosis or occlusion [44-46]. Selective angiography for suspected extrahepatic collateral arteries should be performed after recognizing the typical situations and presence of hypertrophied extrahepatic collateral arteries on pre-treatment imaging [44].
Non-hepatic arteries such as the accessory left gastric artery, cystic artery, and falciform artery, can arise from the hepatic artery [47]. Infusion of chemoembolic materials into the non-hepatic arteries can cause serious adverse events, including gastric ulcer, cholecystitis, and supraumbilical skin rash. Therefore, it is crucial to identify the anatomy of non-hepatic arteries on angiography, and if the origin of the non-hepatic artery is unclear, selective angiography of the suspected hepatic arterial branch should be performed.
In cTACE, transarterially delivered Lipiodol can pass through the hepatic sinusoid and vein, and ultimately get impacted in the peripheral pulmonary arteries. The use of a small amount of Lipiodol is not clinically problematic, but the use of excessive Lipiodol can cause symptomatic pulmonary oil embolism. In particular, when there is a shunt between the tumor vessel and hepatic vein, Lipiodol can induce pulmonary or systemic embolism without the operator being aware of it. Therefore, it is necessary to closely check the presence of a hepatic arteriovenous shunt on angiography.
Cone-beam CT
Cone-beam CT (CBCT) utilizes a smaller focal spot and a larger matrix than CT, which leads to better spatial resolution [48]. Because contrast medium is directly injected into the hepatic artery for CBCT scanning, it can provide pure hepatic arterial phase images like a CT hepatic arteriography. Thus, it can demonstrate fine hepatic arteries accurately and has high sensitivity to detect hypervascular tumors [49]. Therefore, the use of CBCT during TACE is highly recommended in the international guidelines [12-14,25,50]. In the IR survey, 52.3% of the respondents performed CBCT in most TACE cases, and 32.5% used it only when necessary (Fig. 1).
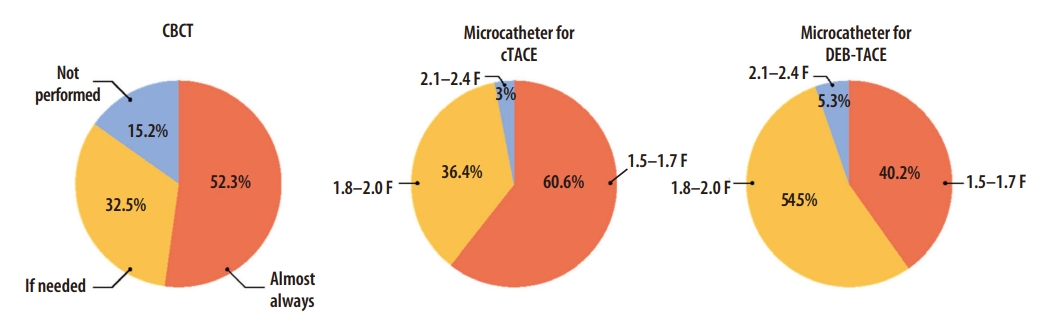
Interventional radiologist survey on the use of cone-beam computed tomography (CBCT) and microcatheter. cTACE, conventional TACE; DEB-TACE, drug-eluting bead TACE.
CBCT can potentially enhance treatment efficacy by more precisely depicting hepatic arterial anatomy, tumors, tumor-feeding arteries, extrahepatic supplies, and Lipiodol accumulation at the tumor during the procedure and also by detecting occult lesions that were not detected on pre-treatment imaging [51-55]. Moreover, CBCT facilitates selective catheterization and reduces procedure time by providing three-dimensional hepatic arterial anatomy. Furthermore, CBCT shows the exact location of the accessory left gastric artery, cystic artery, and falciform artery, preventing nontarget embolization. When CBCT is conducted at the extrahepatic artery supplying HCC, it allows operators to detect must-avoid arterial branches as well as tumor-feeding branches. Moreover, post-procedural CBCT can be used to evaluate Lipiodol accumulation in target tumors, which helps determine the need for any immediate additional treatments in the same session or in a future treatment plan. In the IR survey, the most common reason for CBCT imaging use is identification of the tumor-feeding arteries (78.7%), followed by evaluation of chemoembolic material accumulation (65.2%), and exact localization of target tumors (64.4%) (multiple-choice allowed). On the other hand, IRs who did not perform CBCT reported their reluctance to utilize CBCT due to the adequate information obtained from angiography alone (43.3%), concerns about increased radiation exposure (28.3%), and the additional time required for the procedure (26.7%).
CBCT is susceptible to motion artifacts from cardiac and respiratory motions because of its longer scan time compared to MDCT [56]. Although motion artifact correction software has been recently introduced, cooperative respiratory motion control remains a key to obtaining high-quality CBCT images and highly deteriorated CBCT images need caution during interpretation.
CBCT generally uses more radiation compared to digital subtraction angiography, multiple CBCT scans may induce overexposure in patients. However, a single CBCT scan can prevent multiple digital subtraction angiographies by providing three-dimensional anatomic information, and effective TACE using CBCT can reduce the number of TACE sessions in the future, which may ultimately reduce radiation exposure to patients [57]. Furthermore, operators can minimize the radiation dose by using proper collimation and up-to-date technology such as rapid scan and low-dose mode. Moreover, information from CBCT facilitates safe and effective TACE, which provides more benefits than disadvantages to patients.
Superselective TACE
Liver function preservation as well as tumor control should be achieved to increase survival in patients with HCC. Therefore, TACE should be conducted as selectively as possible to the tumor-feeding arteries (Fig. 2) [58]. Matsui et al. [59] reported that superselection of the peripheral part of the segmental hepatic artery induced complete tumor necrosis without hepatic functional damage in two-thirds of the cases. Miyayama et al. [60] proposed ultraselective TACE, infusion of chemoemulsion when a microcatheter is semi-wedged at the tumor-feeding artery for nodular HCC <5 cm; accumulation of chemoemulsion in the peritumoral portal vein as well as the tumor using this method reduces a local tumor recurrence. However, there is no consensus regarding which level of catheterization can be considered superselective or what stages of the tumor should be targeted by superselective TACE.
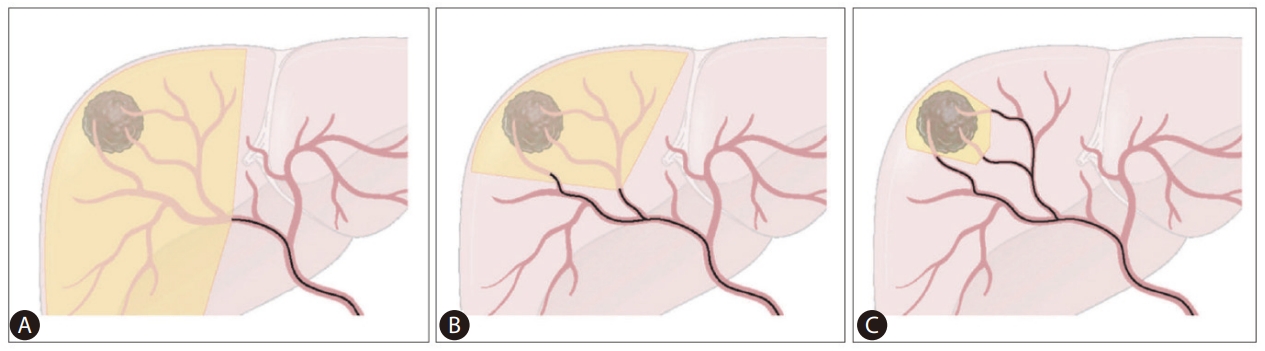
Extent of treatment depending on the microcatheter position in superselective transarterial chemoembolization (TACE). (A) Nonselective TACE at the right hepatic artery. (B) Superselective TACE at A7 and less selective TACE at the right anterior hepatic artery. (C) Superselective TACE at every tumor-feeding artery.
Although it is difficult to clearly define superselection, it generally means catheterization of at least the segmental or more distal hepatic artery. The use of smaller caliber microcatheter is a crucial part of superselective TACE. In the IR survey, ≥95% of the respondents used 1.5–2.0 F microcatheters (Fig. 1). 1.5–1.7 F microcatheters are primarily used in cTACE (60.6%), while 1.8–2.0 F are mainly used in DEB-TACE (54.5%). This may reflect the concern of microsphere clumping in DEB-TACE. The expert panel drew the conclusion that 1.5–1.7 F microcatheters should be primarily used for superselective TACE considering that small caliber microcatheters are widely distributed in the Korean medical environment, and 2.0 F is the upper limit for superselective TACE.
There are multiple reports showing the superiority of selective TACE compared to non-selective TACE. According to a Japanese cohort study that analyzed 4,966 patients with HCC, selective TACE yielded significantly higher survival rates than non-selective TACE (P<0.001) [8]. Golfieri et al. [61] evaluated 67 explanted livers and reported that selective TACE were related to a higher tumor necrosis rate than non-selective TACE (P=0.002). In a retrospective study with 43 institutes in Japan, selective TACE significantly enhanced patients’ survival compared to non-selective TACE (hazard ratio 0.68; 95% confidence interval 0.48–0.97; P=0.033) [62]. Although most studies were non-comparative and retrospective, it should be considered that a RCT is practically and ethically impossible due to the absence of controversy regarding the theoretical background and current reports.
Although selective TACE is ideal, it can be impractical or meaningless depending on tumor stages. In the IR survey, superselective TACE is conducted by 72.0% of the respondents for single HCCs ≤3 cm, 69.7% for HCC within the Milan criteria, and 34.8% for multinodular HCCs ≤5. HCC within the Milan criteria is commonly treated with curative intent, therefore, superselective TACE should be attempted in such cases. There is a report that superselective TACE for HCC patients beyond the Milan criteria but within the Up-to-seven criteria yielded similar survival outcomes compared to that of early HCC [63]. Superselective TACE should be performed when a locally complete response of the target tumors can be expected after TACE such as single HCC <7 cm and oligonodular (2–5 nodules) HCC <5 cm.
It has been reported that repetitive TACE for the tumor burden beyond the Up-to-seven criteria has limited effectiveness and puts the patient at risk of liver damage [64]. This may be a consequence of repetitive non-selective TACE. Even in cases of high tumor burden, liver damage can be minimized by combining superselective TACE for the main lesions and less selective/less intense TACE for the remaining lesions. Non-selective and aggressive TACE should be avoided when it fails to perform selective catheterization of the tumor-feeding artery in centrally located HCC, and alternative modalities, including ablation and external beam radiotherapy, can be considered.
Intra-arterial drug administration during TACE
It is reported that intra-arterial injection of lidocaine can reduce pain during TACE [65,66]. In the IR survey, 55.3% of the respondents used intra-arterial lidocaine during TACE, and 18.2% answered that they always administered lidocaine prior to chemoembolic agent injection. In an RCT with 113 patients, intra-arterial lidocaine prior to chemoembolic agent injection significantly reduced the need for narcotics after TACE, whereas intra-arterial lidocaine injection after chemoembolic agent injection did not [66]. As patients requiring high doses of chemoembolic agents, with young age, or without chronic liver disease are likely to have severe abdominal pain after TACE [67], preemptive use of intra-arterial lidocaine needs to be considered for such patients. Although it is known that intra-arterial lidocaine up to 100 mg (i.e., 10 mL of 1% lidocaine) is safe [66], caution is needed because excessive amounts of lidocaine may incite serious cardiac arrythmia.
Hepatic arterial flow can be diminished or blocked when the microcatheter stimulates the artery and induces vasospasm, which hampers the delivery of chemoembolic agents. Nitroglycerin is a commonly used vasodilator, and preemptive intra-arterial administration may prevent vasospasm [68], which can be especially useful during the infusion of particulate embolic materials such as DEB-TACE. Although preemptive intra-arterial use of nitroglycerin ≤100 g per tumor-feeding artery is recognized as safe, caution is needed because excessive amounts of nitroglycerin may cause serious adverse events such as hypotension.
[Recommendations]
1. Both celiac and SMA angiographies can be performed during the initial TACE session.
2. The utilization of CBCT is recommended for TACE to enhance the therapeutic efficacy and safety.
3. 1.5–2.0 F microcatheters are preferred and recommended for superselective TACE.
4. Superselective TACE should be performed when a locally complete response of target tumors is aimed (e.g., single HCC <7 cm and oligonodular [2–5 nodules] HCC <5 cm).
cTACE
Chemoembolic agents
There is a controversy over whether chemoembolization is superior to bland embolization without chemotherapeutic agents [69]. This is because large tumors rarely undergo complete necrosis, and the embolic effect overwhelms the effect of chemotherapeutic agents in many cases. In addition, TACE may incite more liver damage than nonselective bland embolization once conducted in a nonselective manner, which ultimately neutralizes the advantage of chemotherapeutic agents. Moreover, prospective comparative studies are difficult to be justified in Korea and Japan, where superselective TACE is recognized as a semi-curative treatment for small HCCs. Nonetheless, recent studies from Korea and Japan may provide evidence to end this debate. In these retrospective and prospective studies, cTACE yielded higher complete response rates compared to DEB-TACE, and the difference was even more significant in small HCCs ≤3 cm [70,71], suggesting that chemotherapeutic agents contribute to local tumor responses and superselective cTACE is more beneficial than bland embolization for small HCCs.
Excessive use of Lipiodol (≥20 mL) in cTACE potentially causes pulmonary embolization and dyspnea [72]. In the IR survey, the maximum amount of Lipiodol per session was ≤10 mL in 40.2%, and ≤15 mL in 34.8%. The expert panel agreed to limit the maximum dose to 15 mL.
In terms of chemotherapeutic agents, doxorubicin, cisplatin, epirubicin, or idarubicin is frequently used worldwide. Little is known about the differences in TACE outcomes among these drugs. According to the IR survey, 92.4% of the respondents used doxorubicin, while the remaining utilized cisplatin (Fig. 3). The dose of doxorubicin and cisplatin per session should be ≤75 mg, more ideally ≤50 mg, and 2 mg/kg (maximum 200 mg), respectively. Cisplatin should be diminished in cases of renal impairment.
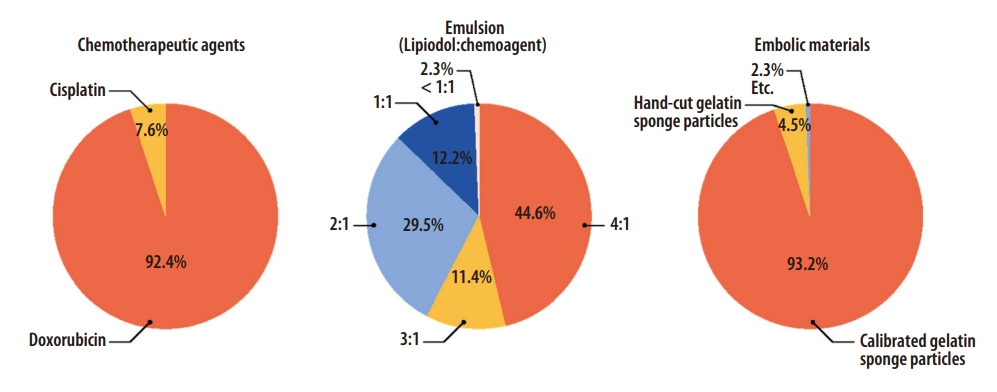
Interventional radiologist survey on chemoembolic agents in conventional transarterial chemoembolization.
A water-in-oil chemoemulsion can be made when the chemotherapeutic agent dissolved in a hydrophilic solvent is mixed with a larger amount of Lipiodol. Since blood is hydrophilic, water-in-oil chemoemulsion is not rapidly mixed with blood; instead, it becomes a drug carrier that delivers chemotherapeutic agents along the blood flow to the tumor. Several manipulations are needed to increase its stability because emulsion is a simple mixture of water and oil. Chemotherapeutic agents should be dissolved in iodinated contrast agents instead of normal saline so that the specific gravities of water and oil become similar. The volume of Lipiodol should be 2- to 4-times larger than that of the chemotherapeutic agent and iodinated contrast agent mixture [73]. Stable chemoemulsion shows a favorable pharmacokinetic profile, and less frequently induces systemic toxicity such as bone marrow suppression [74,75]. According to the IR survey, 85.5% of the respondents used chemoemulsion with Lipiodol to a chemotherapeutic solvent volume ratio of ≥2:1, and 44.6% utilized chemoemulsion of 4:1 volume ratio (Fig. 3). This suggests that the concept of stable chemoemulsion is widely accepted among Korean IRs [76].
As the amount of Lipiodol per session is limited and the volume of chemotherapeutic solution should be less than that of Lipiodol to make a stable emulsion, a highly concentrated chemotherapeutic solution is needed. Therefore, it is recommended to use powdered-form chemotherapeutic agents in cTACE. As of 2023, doxorubicin and idarubicin powders are available in Korea. In doxorubicin-based cTACE, the amount of doxorubicin dissolved in the contrast agent is 10 mg per 0.5 mL. Idarubicin has been studied mainly in France and could be an alternative to doxorubicin in cTACE [77,78].
Because only liquid-form cisplatin is available in Korea, it is impossible to make a cisplatin-concentrated chemoemulsion. Therefore, centers conduct hepatic arterial infusion of cisplatin in conjunction with cisplatin-based cTACE [79,80]. Hepatic arterial infusion of cisplatin is sometimes added to doxorubicin-based cTACE in cases of HCC with vascular invasion [81,82]. According to the IR survey, 37.2% of the respondents considered cisplatin at the hepatologists’ or operators’ discretion in selected patients.
Embolization of tumor-feeding artery
Embolization following the infusion of chemoemulsion increases therapeutic effectiveness compared to chemoemulsion monotherapy [83,84]. Gelatin sponge particles, non-spherical poly-vinyl alcohol particles, and spherical embolic particles are widely used, but little is known about the differences in outcome depending on the types of particles. Gelatin sponge powder is not used any more, as it substantially increases the risk of biliary injury [85]. Fine and tortuous collateral channels can develop when the hepatic artery is damaged due to previous TACE, which hampers superselective TACE. Therefore, non-selective infusion of small and permanent embolic agents that can cause biliary, hepatic arterial, and parenchymal injury should be avoided. Typical gelatin sponge particles allow vascular recanalization about two weeks after embolization, which facilitates catheterization of the embolized artery in the next TACE [86].
Unlike in Western countries, gelatin sponge particles have been widely used in Korea [76]. In the IR survey, 93.2% of the respondents used commercially available, calibrated gelatin sponge particles (Fig. 3), and the most-preferred sizes were 100–350 μm in 56.8% and 351–500 μm in 33.8%. The smaller and more spherical particles can be delivered to the more distal arteries, which can potentially enhance the treatment efficacy. However, small and spherical particles can increase the risk of biliary injury, liver parenchymal damage, and systemic embolization, especially when the tumor is large. The expert panel meeting drew the conclusions that the sizes and types of embolic agents cannot be unified but should be carefully determined by operators considering tumor size, vascularity, size of the tumor-feeding artery, location of the microcatheter, and safety, and that the embolic agents should be delivered as selectively as possible.
Embolization endpoint
Chemoemulsion, as a liquid agent, can be delivered to the portal vein through the venous drainage route and peribiliary plexus, and local tumor progression can be minimized when superselective TACE is performed until a peritumoral oily portogram is obtained in small HCCs [60,87]. However, it may be difficult to achieve an oily portogram even after the use of the maximum amount of chemoemulsion when the tumor burden is large or multinodular tumors exist in both lobes of the liver. In addition, excessive use of chemoemulsion in large HCC to obtain an oily portogram may induce severe liver damage as well as postembolization syndrome. Therefore, the endpoint should be adjusted to avoid overtreatment in patients with advanced age, poor performance status, and a high risk of liver abscess.
It is difficult to determine the universal endpoint for cTACE. If non-selective cTACE is unavoidable, the procedure should be stopped when tumor staining disappears and hepatic arterial flow becomes sluggish. In contrast, if a microcatheter is advanced in the vicinity of the tumors, complete stasis should be targeted to prevent local tumor progression. In large HCC, tumor-feeding arteries are often rapidly recanalized after embolization as embolic agents migrate to the distal parts. Therefore, delayed angiography five to ten minutes after complete stasis needs to be considered to enhance the efficacy. Thus, the embolization endpoint should be tailored to the patient and tumor conditions.
[Recommendations]
1. The maximum doses of Lipiodol, doxorubicin, and cisplatin are 15 mL, 75 mg (50 mg, preferably), and 2 mg per kg (maximum 200 mg) per session of cTACE, respectively.
2. Chemotherapeutic agents should be dissolved in iodinated contrast agents and then mixed with a two- to four-times larger volume of Lipiodol.
3. Embolic agents (type, size) should be determined based on the size of the tumor-feeding artery and the location of the microcatheters and delivered as selectively as possible.
4. The embolization endpoint should be determined based on tumor size, location, vascularity, patient’s condition including performance status and risks of complications, and the location of the microcatheter.
DEB-TACE
Patient selection based on pros and cons
DEB has a pharmacokinetic advantage over Lipiodol chemoemulsion. As microspheres impacted in tumor-feeding arteries slowly release chemotherapeutic agents, drugs can be concentrated in tumors while minimizing the systemic circulation of the drugs [4]. Although this benefit was initially expected to improve effectiveness and safety, RCTs have shown no significant differences in tumor response, time to progression, survival, and liver toxicity between cTACE and DEB-TACE [88-90]. However, patients treated by DEB-TACE experienced milder pain, less postembolization syndrome, and shorter hospitalization [90]. Although there were concerns that DEB, as a small and permanent embolic agent, could cause significant damage to the bile duct and liver parenchyma, this may result from non-selective infusion of DEB [91]. According to a later study, DEB-TACE appeared to have little difference in liver toxicity and biliary injury from cTACE when conducted in a superselective manner [71].
In the surveys, hepatologists mainly considered mild postembolization syndrome (63.6%) while IRs considered tumor size (59.1%) when they chose DEB-TACE instead of cTACE (dual-choice allowed). It appears that IRs have considered the suboptimal local tumor response rates associated with DEB-TACE when treating small HCCs. In a prospective multicenter study from Korea, DEB-TACE yielded the best responses in HCC 2–5 cm, and poorer responses in HCC ≤2 cm [92]. Furthermore, a retrospective multicenter study from Korea and a RCT from Japan consistently reported that DEB-TACE showed poorer objective response rates than cTACE in HCC ≤3 cm [70,71]. This may be because small HCCs have fine tumor-feeding arteries and intratumoral delivery of relatively large microspheres is limited compared to liquid embolic agents (e.g., Lipiodol chemoemulsion) (Fig. 4). However, it should be considered that the aforementioned studies utilized DEBs 100–300 μm, and further investigation is needed for recently-used microspheres ≤150 μm.

Drug-eluting bead transarterial chemoembolization (DEB-TACE) vs. conventional transarterial chemoembolization (cTACE). (A) DEBTACE: DEBs cannot reach to the intra-tumoral fine arteries in small hepatocellular carcinoma, and blood supply from the portal venule can remain after DEB-TACE. (B) cTACE: Chemoemulsion can be accumulated in the peri-tumoral portal venules as well as intra-tumoral fine arteries.
Therefore, DEB-TACE provides similar survival outcomes compared to cTACE in general. It has advantages such as milder postembolization syndrome and shorter hospitalization, which are potentially beneficial for patients with poor performance status or old age. However, it should be considered that DEB-TACE may be less effective in treating small HCCs (≤3 cm) compared to cTACE.
Size of DEBs
As of 2023, DC-beadTM (Biocompatibles UK Limited) and HepaSphereTM (Merit Medical System, Inc.) are available in Korea. According to the IR survey, DEBs 100–300 μm were the most preferred and DEBs ≥300 μm were rarely used. In retrospective studies, DEBs 100–300 μm yielded better outcomes and lower complication rates compared to DEBs 300–500 μm and 500–700 μm [93,94]. Recently, DEBs ≤150 μm were used to enhance intratumoral accumulation of the microspheres, but further investigation is warranted regarding the efficacy and safety [95,96].
Data regarding the complications depending on DEB size are limited and controversial. Non-selective DEB-TACE was performed in studies that reported the relationship between small particles and high biliary complication rates [95]. The use of small DEBs may require a large particle load especially in large HCC, while the use of large DEBs may result in insufficient intratumoral accumulation and damage to the arteries and biliary tract due to particles stagnating in the proximal part of the tumor-feeding arteries. Therefore, DEB sizes should be properly determined depending on tumor sizes. Irie et al. [97] reported that there was a positive correlation between the tumor size and tumor-feeding arteries. In the study, most tumor-feeding arteries in HCC ≥3 cm were larger than 300 μm, which potentially allows intratumoral accumulation of DEBs 100–300 μm, whereas the mean diameter of tumor-feeding artery in HCC ≤2 cm was 200 μm, which potentially limits penetration of DEBs 100–300 μm. These findings allow interpretation of previous studies showing poor local tumor responses of DEB-TACE in HCC ≤3 cm. DEBs ≥300 μm can be selected in large HCC regarding the amount of doxorubicin required and embolization endpoint. In particular, large HCC frequently has arterioportal or arteriovenous shunts, which limits the use of too small DEBs that can potentially cause liver and lung damage [98]. Embolization of the shunts using large particles prior to DEB-TACE may be considered if it is feasible.
Drug loading and delivery of DEBs
Drug loading methods follows the instruction of use by each manufacturer. As highly concentrated chemotherapeutic agents shorten drug loading times, powdered form of doxorubicin is widely utilized.
Based on the doxorubicin dose of systemic therapy, 50–75 mg of doxorubicin can be loaded in a vial of DEBs and the maximum dose in one session of DEB-TACE is limited to 150 mg in two vials [4]. However, in the IR survey, 80% of the respondents loaded 50 mg of doxorubicin in a vial of DEBs, and only 5.6% of the respondents used doxorubicin larger than 100 mg per session of DEB-TACE. Regarding the role of chemotherapeutic agents in DEB-TACE, a RCT in 2010 reported that DEB-TACE showed better local tumor responses than bland embolization [99], another RCT in 2016 showed no differences in local tumor responses and survival between them [100]. Recent studies using DEBs ≤150 μm demonstrated no dose-response relationships depending on the amount of doxorubicin [101], the procedure-related complications mainly occur in the high dose group (100–150 mg) [96]. Therefore, the expert panel agreed to limit the maximum dose of doxorubicin to 100 mg per session, considering the lack of a dose-response relationship and relatively clear dose-complication relationship.
Doxorubicin-loaded DEBs are diluted with a mixture of iodinated contrast media and normal saline prior to administration. According to the IR survey, more than 80% of the respondents used more than 10 mL of the liquid mixture for dilution. The expert panel also suggested 30–50 mL of the liquid mixture as an appropriate amount. Although diluted DEBs require more longer injection times, these may allow for effective DEB-TACE by limiting particle clumping and occlusion of the proximal artery.
A superselective manner is recommended in DEB-TACE as well as cTACE for both efficacy and safety. According to the IR survey, 94.7% of the respondents used ≤2.0 F microcatheters (Fig. 1), suggesting the popularity of superselective DEB-TACE in Korea. However, advancing a microcatheter until it is wedged should be avoided. Flow-directed delivery of DEBs is hampered by the wedged status, which increases the risk of particle reflux [102]. As DEBs are invisible on X-ray fluoroscopy and non-target embolization during DEB-TACE may cause more serious problems compared to that of cTACE, the non-hepatic arteries (i.e., accessory left gastric artery, cystic artery, and falciform artery) from the hepatic artery should be identified prior to deciding injection points in every case.
A vascular lake refers to an intratumoral pseudoaneurysm developed during DEB-TACE. This may be a consequence of intratumoral microvessel rupture and is frequently observed in DEB-TACE for HCC with pseudocapsule and DEB-TACE using small particles [103]. Once a vascular lake appears, it is usually impossible to achieve the proper embolic endpoint. Therefore, an angiographic evaluation should be performed when vascular lake is identified. Bland embolization using large gelatin sponge particles or cyanoacrylate should be considered when tumor parenchymal staining disappears and only vascular a lake is visible, as embolization of vascular lakes can potentially enhance treatment outcomes [104,105]. In particular, if serial hepatic arteriography shows an increase in the size of a vascular lake, it must be embolized as the finding suggests a high risk of tumor rupture.
Embolization endpoint
Embolization endpoints can be graded as “complete stasis”, “near stasis”, and “stasis”. When superselective DEB-TACE is feasible for small HCCs, complete stasis can be the endpoint. In general, however, “near stasis”, where contrast agents are slowly washed out during 2–5 heartbeats, or “stasis”, where antegrade flow is preserved but tumor staining disappears, are recommended as ideal endpoints [102,106]. “Complete stasis” in non-selective DEB-TACE increases the amount of doxorubicin administered, liver toxicity, arterial damage, biliary complication rate, and chance of non-target embolization [107,108].
Once DEB-TACE reaches a planned endpoint, the infusion of DEBs should be stopped, irrespective of the amount of residue. On the other hand, excessive use of DEBs when an endpoint is not achieved can increase the risk of complications. Bland embolization using large particles to achieve “near stasis” can be considered in this case. In the IR survey, 10.4% of the respondents stopped the procedure when the embolization endpoint was unachievable. Because additional bland embolization may induce postembolization syndrome and liver dysfunction, short-term re-treatment can be planned instead of additional bland embolization at the same session.
[Recommendations]
1. DEB-TACE provides a similar survival, but milder postembolization syndrome and less hospitalization compared to cTACE.
2. DEB-TACE shows a lower complete response rate than cTACE in small HCCs ≤3 cm.
3. The size of DEBs should be determined depending on the tumor size and the diameter of the tumor-feeding artery.
4. The maximum dose of doxorubicin is 100 mg per session of DEB-TACE.
5. Superselective DEB-TACE should be pursued to enhance the therapeutic efficacy and safety.
6. “Near stasis” is commonly the embolization endpoint of DEB-TACE, but “complete stasis” can be targeted in cases of superselective catheterization of the tumor feeding artery.
POST-TREATMENT CARE
Although diverse adverse events may occur, the most frequent is postembolization syndrome with a reported prevalence of 36.1–41.0% [109]. Liver enzyme levels increases, sometimes along with hyperbilirubinemia, due to hepatocyte damage, but these usually normalize within 10 to 14 days [32]. Serious adverse events were reported in less than 10% of patients who underwent TACE [110]. These included liver infarction, biloma, cholecystitis, gastrointestinal ulcer or hemorrhage, and vascular dissection (less than 1% each). Liver failure occurred in 3–5% of patients, and mortality within 30 days after the procedure occurred in 0–4%. Biliary stenosis usually does not cause clinical problems when it occurs in the liver periphery. However, central biliary stenosis, due to excessive embolization of the caudate or medial segmental hepatic arteries, can have catastrophic consequences such as extensive liver damage [111].
According to the IR survey, 35.6% of the respondents made a direct order or request to hepatologists if necessary, and 16.7% were actively involved in patient care. Active communication with the physician regarding anticipated results following TACE is helpful for post-treatment care and the establishment of future treatment plans.
Postembolization syndrome
Major symptoms include nausea, vomiting, right upper quadrant pain, and fever, which are derived from liver parenchyma ischemia and necrosis, capsular extension, gall bladder ischemia in cases of cystic artery embolization, necrotic substrates, and inflammatory materials. The frequency and severity of postembolization syndrome depends on the tumor size, liver function, presence of portal vein invasion, treatment extent, and amount of chemotherapeutic agents [32]. Although most cases develop within 72 hours after the procedure and disappear spontaneously, they may increase the patient’s physical and psychological stress, medical costs, and hospitalization [32,112,113]. As postembolization syndrome shares its symptoms with conditions requiring immediate management such as infection and tumor lysis syndrome, it should be carefully differentiated from other conditions [114]. Patients with postembolization syndrome are treated with conservative management including painkillers, antiemetics, gastrointestinal medications, and fluid administration, and are usually discharged within 24–48 hours after the symptoms are controlled by oral medications [110].
Most patients complain of post-procedure pain, and more than a quarter of them have moderate to severe severity (visual analogue scale ≥4/10) [115]. According to the hepatologist survey, the most commonly used painkiller was tramadol or tramadol+acetaminophen (72.7%), and opioid, acetaminophen, and non-steroid anti-inflammatory drugs were used by a 50.9%, 43.6%, and 23.6% of the respondents, respectively.
Because the prevalence of nausea and vomiting is reported to be 40.3–52.5% and severe symptoms can cause dehydration, electrolyte imbalance, extended hospitalization, and increased medical cost, nausea and vomiting should be properly managed by antiemetics [109]. Dexamethasone, 5-HT3 receptor antagonists, and NK-1 receptor antagonists are recommended medications from the American Society of Clinical Oncology [116]. According to the hepatologist survey, metoclopramide, 5-HT3 receptor antagonists, steroid, and NK-1 receptor antagonists were used by an 80.0%, 70.9%, 10.9%, and 5.5% of the respondents, respectively.
Infection
Many patients treated by TACE are immunocompromised or vulnerable to invasive procedures [24]. Spontaneous bacterial infections (prevalence, 4%) and liver abscesses (0.1–4.5%) may develop mainly due to Escherichia coli, Enterobacter cloacae, Enterococcus faecalis, and Klebsiella pneumoniae [24,117,118]. Because liver abscess results in a mortality rate of 11.8–13.3% if untreated, active use of antibiotics is required when post-procedural infection is suspected or identified, and percutaneous drainage is also considered in some cases [22,23]. In the hepatologist survey, 3rd-generation cephalosporine and piperacillin-tazobactam were utilized by 96.4% and 16.4% of the respondents, respectively (multiple-choice allowed).
To minimize liver abscess following TACE, operators and hepatologists should recognize patients at higher risk prior to the procedure, avoid excessive TACE in such patients, and administer timely management such as antibiotic use. In patients with a history of biliary intervention or surgery, the underlying causes of infection has not been resolved even with prophylactic antibiotics, which requires careful patient care and follow-up after TACE.
[Recommendations]
1. Postembolization syndrome should be managed based on specific symptoms using painkillers and antiemetics such as 5-HT3 receptor antagonists.
2. Post-TACE infection can be primarily managed by 3rd-generation cephalosporine or piperacillin-tazobactam TACE, and the development of liver abscess, liver failure, and sepsis should be carefully monitored.
FOLLOW-UP
Follow-up imaging should be performed to address tumor responses and occurrence of procedure-related adverse events. Although the timing of first imaging has been recommended from 2–8 weeks [102,106], it may be difficult to directly adopt foreign guidelines considering that heterogeneous patterns of practice. According to the hepatologist survey, 98.2% (54/55) of the respondents requested a follow-up imaging and outpatient visit to their patients after approximately a month. Since the mechanism of TACE is to induce devascularization of hypervascular HCC, the modified Response Evaluation Criteria in Solid Tumors (mRECIST) that evaluates the size of contrast-enhanced tumor parts as viable HCC is recommended to address tumor responses [119,120]. As one month is approximately regarded as a long enough duration for the tumor to undergo devascularization and for the liver to recover from ischemia, the initial follow-up of 4–8 weeks after TACE is adequate to evaluate the tumor response and determine whether additional treatment is needed.
As with pre-TACE imaging, multiphasic CT or multiphasic MRI are recommended as follow-up imaging modalities. In the IR survey, 47.7% preferred CT over MRI, while 35.6% had no preference between the two. Because Lipiodol used in cTACE is a lipid agent that can show diverse signal intensities on MRI but distinct high attenuation on CT, CT also has an advantage of evaluating Lipiodol accumulation in tumors and may be beneficial in addressing potential complications in other abdominal organs. For these reasons, CT can be preferred over MRI as the first imaging modality after TACE. On the other hand, MRI can be primarily considered in patients with hypersensitivity to iodinated contrast agents or when CT-based evaluation of residual tumor is limited.
TACE can be reconsidered when a residual tumor is identified on follow-up imaging, and subsequent procedures can be performed at interval of 4–8 weeks. On-demand TACE is highly recommended over scheduled TACE regardless of tumor responses that can cause a functional loss in the liver [12-14].
TACE refractoriness is a concept that describes the condition of disease progression despite repetitive TACE [121,122]. Although there were no alternatives in such cases in the past, recent developments in systemic treatments permit chances for second-line treatments for the patients. In both the IR and hepatologist surveys, most respondents answered that TACE refractoriness should be determined for at least two consecutive TACE sessions (IR, 69.7%; hepatologist, 72.7%). This result may reflect the common idea that a single session of TACE is insufficient to treat large HCC and that superselective TACE can be achieved with one more try as the tumor and tumor-feeding artery grow together. In 2022 KLCA-NCC Korea practice guidelines defined TACE refractoriness as the absence of an objective response (complete or partial response) or with stage migration owing to new vascular invasion or extrahepatic spread after two sessions of on-demand TACE within 6 months [12-14]. According to a retrospective study from Korea, switching to other treatments was recommended when HCC beyond the Milan criteria did not show an objective response after two consecutive TACE [123]. With regard to the pathological aspect, HCC with K19 expression reflects a progenitor cell phenotype, which suggests a high chance of poor response after TACE. Therefore, this pathological information can be considered to predict tumor responses and to guide treatment plan in patients who underwent biopsy prior to TACE [124].
Thus, TACE refractoriness can be judged after the second TACE because a single session of TACE may be insufficient in cases of large HCC and selective TACE may be possible in the second attempt. However, if the reasons for the low initial responses are derived from the tumor location or technical problems that cannot be overcome (e.g., a very tortuous hepatic artery, parasitic tumor supply from dangerous arteries such as the colic artery), an early switch to other treatments should be considered.
[Recommendations]
1. Follow-up imaging should be conducted 4–8 weeks after TACE to evaluate tumor response and complications.
2. CT can be preferred over MRI as it has advantages in evaluating Lipiodol accumulation in tumors and potential complications in the abdominal organs. MRI can be primarily considered when patients have hypersensitivity to iodinated contrast agents or kidney failure, or when CT-based evaluation of residual tumor is limited.
3. TACE refractoriness can be judged when patients fail to show objective responses or when new vascular invasion or extrahepatic spread occur after two consecutive on-demand TACEs.
Notes
Authors’ contribution
Conceptualization: In Joon Lee, Hyunchul Rhim. Data curation: all authors. Formal analysis: all authors. Funding acquisition: Yuri Cho, In Joon Lee, Hyunchul Rhim. Methodology: Yuri Cho, Jin Woo Choi, In Joon Lee. Project administration: Yuri Cho, Jin Woo Choi, In Joon Lee. Supervision: In Joon Lee, Hyunchul Rhim. Validation: Gyoung Min Kim, Jung Suk Oh, Dongho Hyun, In Joon Lee, Hyunchul Rhim. Visualization: Yuri Cho, Jin Woo Choi, In Joon Lee. Writing—original draft: all authors. Writing— review & editing: Yuri Cho, Jin Woo Choi, In Joon Lee.
Conflicts of Interest
Jin Woo Choi reports sponsored lecture for Philips Healthcare; consultant/advisory roles for Philips Healthcare, Taewoong; research grants from Nextbiomedical. Gyoung Min Kim reports sponsored lectures for Boston Scientific. In Joon Lee reports sponsored lectures for Guerbet Korea, Boston Scientific Korea, Cook. Hyunchul Rhim reports a sponsored lecture for STARmed; research grants from Johnson & Johnson. All remaining authors have declared no conflicts of interest.
Acknowledgements
This study was supported by Korea Liver Cancer Association.
The authors would like to thank to the advisory committee members for this work. The advisory committee members are as follows:
Chang Won Kim (President of KSIR, Radiology, Pusan National University)
Jong Young Choi (The Next President of KLCA, Hepatology, The Catholic University of Korea)
Kwang Cheol Koh (Former President of KLCA, Hepatology, Sungkyunkwan University)
Young Nyun Park (Former President of KLCA, Pathology, Yonsei University)
Jin Wook Chung (Former President of KLCA, Radiology, Seoul National University)
Seung Kew Yoon (Former President of KLCA, Hepatology, The Catholic University of Korea)
Joong-Won Park (Former President of KLCA, Hepatology, National Cancer Center)
Jinsil Seong (Former President of KLCA, Radiation Oncology, Yonsei University)
Kyung-Suk Suh (Former President of KLCA, Surgery, Seoul National University)
Hee Chul Yu (Vice President of KLCA, Surgery, Jeonbuk National University)
Ju Hyun Shim (Secretary General of KLCA, Hepatology, University of Ulsan)
Dong Hyun Sinn (Financial Director of KLCA, Hepatology, Sungkyunkwan University)
Hyeong Joon Kim (Insurance Director of KLCA, Hepatology, Chung-Ang University)
Hwan Jun Jae (Former Secretary General of KSIR, Radiology, Seoul National University)
Ho Jong Chun (Member of KSIR, Radiology, The Catholic University of Korea)
Abbreviations
TACE
transarterial chemoembolization
HCC
hepatocellular carcinoma
cTACE
conventional TACE
DEB-TACE
drug-eluting bead TACE
RCTs
randomized controlled trials
IRs
interventional radiologists
HAP score
hepatoma arterial-embolization prognostic score
BCLC
the Barcelona Clinic Liver Cancer
CT
computed tomography
MRI
magnetic resonance imaging
SMA
superior mesenteric artery
MDCT
multidetector CT
CBCT
cone-beam CT
mRECIST
modified Response Evaluation Criteria in Solid Tumors
