Critical appraisal of metabolic dysfunction-associated steatotic liver disease: Implication of Janus-faced modernity
Article information
See the commentary-article "Adding to the confusion in more than just the name" on page 973.
Abstract
The existing term non-alcoholic fatty liver disease (NAFLD) has raised substantial concerns due to its inherent disadvantages of using exclusionary diagnostic criteria and the stigmatizing word ‘fatty.’ Three pan-national liver associations set out to explore a new nomenclature to replace both NAFLD and its suggested alternative, metabolic (dysfunction)-associated fatty liver disease (MAFLD). They surveyed if a change in nomenclature and/or definition is favored and which nomenclature best communicates disease characteristics and increases awareness. In lieu of NAFLD/MAFLD, metabolic dysfunction-associated steatotic liver disease (MASLD) has been chosen, and an umbrella term, steatotic liver disease (SLD), encompassing the whole spectrum of liver disease, has been proposed. It has been suggested that cardiometabolic risk factors should be considered when categorizing SLD patients. Furthermore, a new subcategory, MASLD with increased alcohol intake (MetALD), casts light on a neglected group of patients with moderate or more alcohol consumption. The importance of metabolic dysfunction was acknowledged in this new nomenclature, but the precise contribution of metabolic dysfunction and alcohol consumption to the development and progression of SLD remains unclear. Herein, we review hepatologists’ and endocrinologists’ perspectives on the new nomenclature, along with its possible impact on clinical practice. Although it is premature to predict the settlement of the new nomenclature, this review may help build more evidence for a soft landing of it in the future.
INTRODUCTION
The term non-alcoholic fatty liver disease (NAFLD) was first introduced in 1986 [1] and was defined as hepatic steatosis affecting at least 5% of hepatocytes in those who consume little or no alcohol without any secondary causes such as viral hepatitis, relevant medications, and lipodystrophy [2-4]. Thereafter, it has served as the anchor point for established clinical practice (i.e., diagnosis and treatment) as well as exploratory research seeking a better understanding of the disease and the development of biomarkers and drugs [2-4]. However, its inherent drawbacks of being exclusionary with the use of ‘non-alcoholic’ and stigmatizing with the use of ‘fatty’ prompted a search for an alternative nomenclature [5,6].
In recent years, researchers proposed metabolic dysfunction-associated fatty liver disease (MAFLD) as an alternative nomenclature for NAFLD [7,8]. MAFLD eliminates exclusionary diagnostic criteria and incorporates metabolic risk factors, making it possible to include patients with concomitant liver disease. Nonetheless, it is critiqued for solely relying on metabolic risk factors and not considering alcohol consumption [9,10]. Moreover, the oversight of non-alcoholic steatohepatitis (NASH), the progressive form of NAFLD, challenged its widespread application in practice [9-11].
Consequently, a new nomenclature that overcomes the exclusionary and stigmatizing nature of NAFLD and the limitations of MAFLD neglecting both alcohol consumption and NASH was required. Three large pan-national liver associations, including the American Association for the Study of Liver Disease (AASLD), the European Association for the Study of the Liver (EASL), and the Latin American Association for the Study of the Liver, embarked on a modified Delphi process to find a new nomenclature and its definition [12-14]. Finally, metabolic dysfunction-associated steatotic liver disease (MASLD) was chosen to replace NAFLD and MAFLD, and steatotic liver disease (SLD) was suggested as an umbrella term. In this new nomenclature, patients with SLD are classified into two separate subcategories depending on the presence and abscence of a cardiometabolic risk factor (CMRF), and the subcategory with CMRF is further classified into MASLD and MetALD based on the etiology and alcohol consumption.
Hence, this review aimed to examine the suggested nomenclature and its potential impact on screening, diagnosis, treatment, and future drug development. The critical perspectives of the hepatologists and the endocrinologists will be addressed, casting light on the merits and demerits that might arise from this change. Since the new nomenclature has just been proposed, there is a mixture of hope and anxiety, and now may not be the right time to predict its settlement. In that context, this review will contribute to building more evidence for the adoption of the new nomenclature in the future.
THE NEW NOMENCLATURE
MASLD vs. NAFLD/MAFLD: Difference in the diagnostic criteria
MASLD is defined as hepatic steatosis and one or more of the five CMRFs: i) body mass index (BMI) ≥25 kg/m2 (≥23 kg/ m 2 for Asians) or waist circumference >94 cm for males and >80 cm for females or ethnicity adjusted; ii) fasting serum glucose ≥5.6 mmol/L (100 mg/dL) or 2-hour post-load glucose levels ≥7.8 mmol/L (≥140 mg/dL) or glycated haemoglobin ≥5.7% (39 mmol/L) or type 2 diabetes or treatment for type 2 diabetes; iii) blood pressure ≥130/85 mmHg or specific antihypertensive drug treatment; iv) plasma triglycerides ≥1.70 mmol/L (150 mg/dL) or lipid-lowering treatment; and v) plasma high-density lipoprotein (HDL) cholesterol ≤1.0 mmol/L (40 mg/dL) for males and ≤1.3 mmol/L (50 mg/dL) for females or lipid-lowering treatment. Patients with SLD and at least one of the CMRFs are categorized as MASLD when they have no other causes of steatosis (Fig. 1) [12,13].
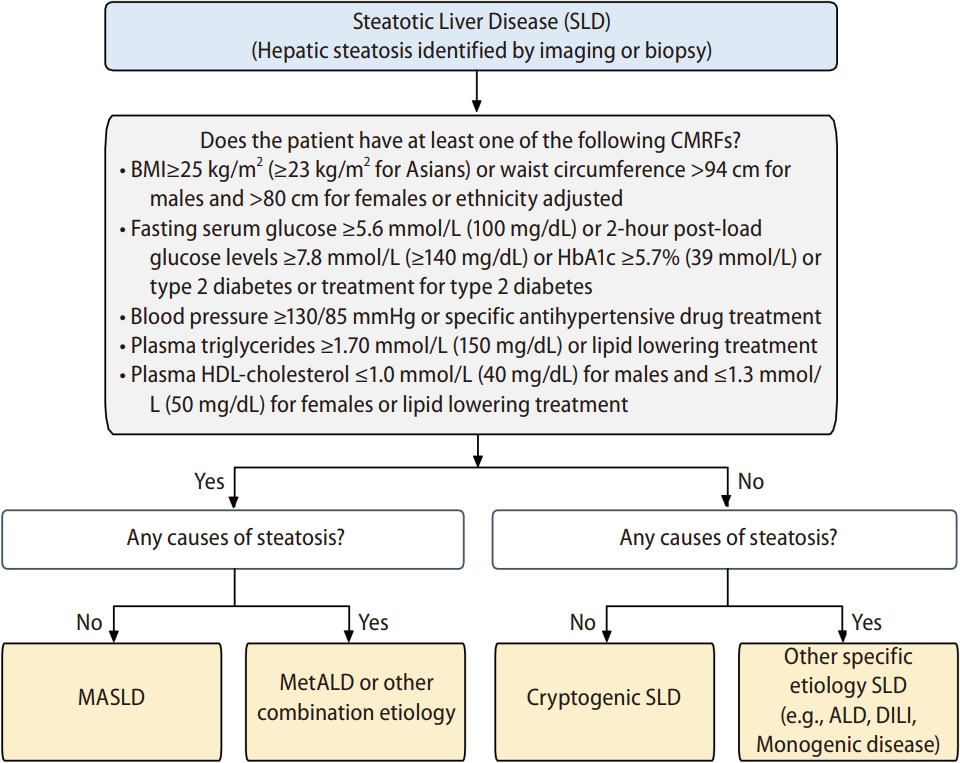
MASLD diagnostic criteria. This figure was adapted from ‘A multi-society Delphi consensus statement on new fatty liver disease nomenclature’ and was modified in the interest of this review [12,13]. ALD, alcohol-associated liver disease; CMRF, cardiometabolic risk factor; DILI, drug-induced liver injury; MASLD, metabolic dysfunction-associated steatotic liver disease; SLD, steatotic liver disease.
NAFLD, on the other hand, is diagnosed when there is hepatic steatosis of ≥5% evident on imaging or histology without concurrent liver diseases such as significant alcohol consumption, use of medications that can cause steatosis, or monogenic hereditary disorders [2,3]. MAFLD shares the same standard with hepatic steatosis in terms of definition but allows for more liberty in its detection. It can be detected by either imaging techniques, serologic biomarkers, or liver histology. Being overweight/obese, having type 2 diabetes mellitus, or having at least two of the metabolic risk abnormalities is a requirement for MAFLD diagnosis [7]. The most significant difference between NAFLD and MAFLD is the recognition of metabolic risk factors for the disease onset and progression. MAFLD adopts a positive criterion rather than a negative one but fails to incorporate alcohol consumption into its diagnostic criteria.
While searching for a new nomenclature, a general consensus was reached that the term ‘metabolic’ should be included and ‘non-alcoholic’ label should be removed [12,13]. MAFLD first captures patients who are overweight/obese and have type 2 diabetes mellitus and then applies other metabolic risk factors to those with normal weight and normoglycemia. In contrast, the new nomenclature SLD applies the five CMRFs to all patients for classifying its subcategories, including MASLD, MetALD, cryptogenic SLD, and other specific etiology SLD. NAFLD and MASLD differ because SLD adopts a positive criterion and incorporates metabolic risk factors while striving to retain the existing understanding of NAFLD. Furthermore, the new nomenclature pays more sophisticated attention to alcohol consumption, creating the new subcategory, MetALD (Table 1).
The umbrella term SLD
SLD was suggested as an overarching term encompassing a broad spectrum of causes contributing to hepatic steatosis [12,13]. It is defined as hepatic steatosis identified by imaging or biopsy regardless of etiology. The SLD patients with one or more of the five CMRFs are further categorized into MASLD or MetALD/other combination etiology (Fig. 1). Those without any CMRFs are further categorized into cryptogenic SLD or SLD with other specific etiology. Other specific etiology includes alcohol-associated liver disease (ALD), drug-induced liver injury, and monogenic disease.
The adoption of an umbrella term allows for a more intuitive and precise classification of patients. Patients not recognized in the previous nomenclature can be recognized and categorized with this new term SLD. The once-neglected disease awareness can be enhanced, and treatment strategies for those patients can be pursued with more precision. With the existing disease staging and severity not being altered by the new nomenclature, a more coherent and straightforward explanation of the disease can be expected. The leverage of SLD also allows physicians and healthcare providers to better communicate with patients about their conditions and possible therapeutic actions.
CMRFs
Although concerns over the precise meaning of ‘metabolic’ and the varying understanding of the term had been raised, a near-universal agreement was made that considering ‘metabolic disease or dysfunction’ would promote a better understanding of the disease and increase disease awareness since NAFLD has a strong epidemiological and pathogenic link with metabolic diseases such as obesity, diabetes, and insulin resistance. The five CMRFs were selected to align with already well-established and validated risk factors and share their criteria significantly with those of metabolic syndrome. While metabolic syndrome merely considers waist circumference, the CMRF for obesity considers both BMI and waist circumference [12,13]. As for type 2 diabetes, the CMRF suggests more specific and detailed standards as its criteria. In the pediatric context, although the five CMRF criteria remain the same, the application varies depending on patients’ age [12,13].
The new category of MetALD
A new subcategory, MetALD was suggested for the previously neglected group of patients who consume greater amounts of alcohol (20 to 50 g/day for females and 30 to 60 g/day for males) compared to MASLD [12,13]. Studies have highlighted the need for creating a distinct category of MetALD from MASLD due to the added pathogenic value of alcohol consumption and the prognostic implications that follow [15]. Although there is no explicit reference to the amount of alcohol consumption to be considered significant [16], EASL and Korean Association for the Study of the Liver guidelines require the exclusion of daily alcohol consumption of >30 g for men and >20 g for women for the diagnosis of NAFLD [3,17]. The consensus process for this new nomenclature asked questions regarding the alcohol consumption limit for this subcategory [12,13], and it was generally agreed that 30–60 g of daily alcohol consumption among NAFLD patients could affect the natural history of the disease and may alter the response to therapeutic interventions [12,13]. Two acronyms, MetALD and MAASLD, were suggested for this group of patients. MetALD was chosen in the interest of avoiding possible confusion and perception associated with the acronym AASLD [12,13].
With the introduction of this separate subcategory of MetALD from MASLD, there is an opportunity to generate new knowledge about patients who have both metabolic and alcohol-related risk factors. A recent study that analyzed the UK Biobank data using this new nomenclature found that MetALD patients are more likely to be males and have higher liver enzymes but lower levels of HDL cholesterol compared to MASLD patients [18]. MetALD as a separate category helps define the natural history better and promotes the development of novel biomarkers and new drugs targeting this selected group of patients. MetALD should not be interpreted as a binary category that separates MASLD and MetALD. Instead, it should be understood as a category with a continuum across MASLD and ALD [12,13].
HEPATOLOGIST PERSPECTIVE
Implications for enhanced disease awareness
NAFLD is a treatable and preventable disease only if it is diagnosed promptly. Once it progresses to cirrhosis, it is irreversible, and patients suffer inevitable complications related to NAFLD [2,19]. However, the terminology “NAFLD” misleads patients to believe that there is little potential harm, and finding the real cause of their suffering is challenging. Studies have reported that more than 95% of subjects with suspected NAFLD were unaware of liver disease, and more than 75% did not realize they were at risk of developing NAFLD [20-22]. There is a growing concern among non-hepatologists that NAFLD is an important liver disease as it often co-exists in patients with diabetes and metabolic syndrome [10].
Even at the risk of confusing practice and the public with suggestions from NAFLD to MAFLD and yet another change from NAFLD/MAFLD to MASLD, an appropriate term that can increase disease awareness and accelerate biomarker and drug development is critical. A new nomenclature has been announced after a thorough sharing of opinions among experts and relevant stakeholders, and the introduction of MASLD and its simple criteria is expected to bring many positive changes that unify terminology and contribute to the mitigation of disease progression.
Implications for clinical practice: diagnosis and treatment
The suggested new nomenclature, such as SLD and MASLD, is expected to affect clinical practice positively. Unlike MAFLD, which does not consider NASH [7], the new nomenclature maintains NASH, but under a different term, metabolic-associated steatohepatitis (MASH), limiting the confusion in practice. The consensus process has diligently considered preserving existing data and causing limited hindrances to ongoing trials. An analysis of the LITMUS consortium European demonstrated that there is a 98% overlap between patients with conventional NAFLD and those with the newly suggested MASLD [12,13,23]. However, further research is needed to adapt this new nomenclature to a specific group with lean NAFLD or SLD without any metabolic risk factors. As for the prevalence, a recent meta-analysis involving 17 studies revealed that MAFLD has a higher prevalence compared to NAFLD (33.0% vs. 29.1%), and future studies should compare the prevalence of MASLD with that of NAFLD [24].
With the suggested new nomenclature [12,13], the diagnostic process is expected to become easier and more intuitive. The shift from a negative to a positive diagnostic criterion means that the excruciating exclusion process can be avoided and diagnoses can be made based on hepatic steatosis and the presence or absence of CMRF. This straightforward diagnosis method can substantially reduce the burden on clinical practice. With the new category, MetALD, and the inclusion of other etiologies, such as viral hepatitis, the range of patients covered by the term SLD is expected to be expansive.
NAFLD is one of the most common liver diseases, affecting millions of patients worldwide. With the overwhelming prevalence reaching almost 30%, identifying at-risk individuals is pivotal [25]. The risk of disease progression to cirrhosis and hepatocellular carcinoma (HCC) along with cardiovascular disease (CVD) and extrahepatic cancer is particularly high among those with NASH [2]. Identifying at-risk individuals is critical to efficiently treat such a prevalent condition with a heterogeneous nature. Recent studies have demonstrated that MAFLD includes more at-risk individuals than NAFLD, showing it has more metabolic comorbidities, elevated liver enzymes, and higher non-invasive liver fibrosis scores [26]. Under the new definition of MASLD, more at-risk patients are expected to be recognized. Additional efforts should be made to identify patients at high risk for MASH or its progression using non-invasive tests or genetics since the current practice of having an invasive biopsy for diagnosing NASH/MASH is rather burdening.
Concerning treatment, the new nomenclature can benefit more patients as individuals with hepatic steatosis and concurrent liver pathologies can now be recognized and receive timely treatment. Although the current standard treatment for NAFLD stays in lifestyle modifications and weight loss [2-4], several phase 3 trials exploring new medical treatments for NASH are in progress with promising preliminary results [25]. Some treatments are expected to receive Food and Drug Administration (FDA) authorization within a few years, allowing healthcare systems to engage more patients.
Implications for clinical outcomes
Studies have reported that the mortality rate associated with MAFLD is higher than that associated with NAFLD. Previous studies conducted in Korea and the United States showed that MAFLD was associated with an increased risk of all-cause mortality even after adjusting for metabolic risk factors, whereas NAFLD was not [27,28]. As for cardiovascular mortality, a nationwide study conducted in Korea showed that CVD risk in the MAFLD group (hazard ratio [HR] 1.43) was higher compared with the NAFLD group (HR 1.09), although both FLD group (HR 1.56) showed the highest risk [29]. However, metabolically dysregulation features rather than MAFLD may have contributed to this higher mortality [8].
The change from NAFLD to MASLD may help identify a greater number of individuals with metabolic risk factors, and thus, the risk for CVD seems to be higher in MASLD than in NAFLD. Studies comparing all-cause and CVD mortality by SLD subcategory are warranted to validate the prognostic role of each subtype of SLD in predicting CVD. Given that alcohol consumption worsens fibrosis severity in NAFLD [30], stratified analysis by the different amounts of alcohol consumption for each MASLD, MetALD, and ALD will enable the development of fine-tuned strategies depending on the behavior of an individual patient.
Cancers, including HCC, are the second leading cause of mortality in NAFLD [2-4]. A recent population-based cohort study conducted in Sweden showed that biopsy-proven NAFLD carries an increased cancer risk attributable primarily to HCC, in contrast, the contribution of other extrahepatic cancers was modest [31]. This study highlights the need for personalized HCC surveillance schemes across all stages of NAFLD. Future studies are needed regarding which SLD subtype most strongly associated with HCC development. The phenotype of MASLD-HCC in patients at high risk of SLD progression should be further investigated. It also merits further scrutiny of the MASLD-HCC phenotype that best responds to immunotherapy. As HCC surveillance or screening in at-risk populations of NAFLD is a critical issue, the same is expected to extend to MASLD.
Implications for clinical trials and drug development
Clinical trials for developing new drugs consider the presence or absence of NASH as an important eligible criterion [25]. However, the new term MAFLD has introduced confusion since MAFLD abandoned the term ‘steatohepatitis’ and took a rather fluid approach. Concerns have been raised that MAFLD could derail phase 2b and 3 trials designed following the guidance for NASH drug development [10]. This appears problematic because the endpoint of current drug development is the resolution of NASH with no worsening of liver fibrosis. Instead of eliminating the term ‘steatohepatitis’ as a distinguishing subtype, the new nomenclature proposes MASH as the alternative term for NASH, reducing confusion in clinical practice and trials [12,13]. The new nomenclature also allowed for further characterization of fibrosis severity combined with MASH (e.g., MASH with F3 fibrosis).
With the new categories of MASLD and MetALD, a broader spectrum of patients under the influence of alcohol can be considered in future clinical trials. The new nomenclature does not conflict with ongoing clinical trials or studies, and some drugs in late-phase development, such as semaglutide and resmetirom [25], can continue their process with the new nomenclature. The FDA approval decision is not expected to be affected by the new nomenclature.
Challenging issues
The newly suggested nomenclature presents several challenges. First, MetALD proposed as a continuum rather than a clear-cut category may make the development of disease-specific biomarkers or drugs specifically targeting this group of patients difficult [12,13]. Second, the dynamic changes in metabolic health status and alcohol consumption pattern or amount over time per patient may alter the diagnosis depending on the specific time point and should be considered cautiously. Periodic evaluation and repeated monitoring, which are inevitably necessary, may add burdens to clinical practice.
Other challenges may include that using SLD as an umbrella term may result in heterogeneous prognoses by widely encompassing various subcategories of SLD. Lacking a category for SLD patients with CMRFs with alcohol consumption greater than moderate amounts within the four-group classification system can serve as another challenge. Although the varying approaches make the subcategorization different, the discordance between the five-group classification, including separate ALD, and the four-group classification with ALD incorporated in the “other specific etiology SLD” may confuse the application of the new nomenclature [12,13]. While the five-group classification has hepatitis C virus in its specific etiology SLD, both classifications failed to provide guidance on where hepatitis B should be included [12,13]. The lack of a definition or a statement on MASLD-related cirrhosis continues to puzzle some professionals. A statement about where patients with MASLD-related cirrhosis are included would help address this issue.
Special attention should be paid to young MASLD patients without CMRF and those with non-obese or lean patients. A recent meta-analysis showed that the non-obese NAFLD population accounts for approximately 40% of the entire NAFLD population globally, and non-obese and lean NAFLD groups still have substantial long-term liver- and non-liver-related comorbidities [32]. Previous studies using the novel diagnostic approach of combining detailed clinical phenotyping and genomic analysis distinguished two types of lean NAFLD [33,34]. The new nomenclature is void of guidance on where lean NAFLD patients without CMRF fit in. Nonetheless, it should be noted that the suggested nomenclature can provide room for adding new subtypes depending on future research findings. When categorizing patients with or without CMRFs, there may be certain contexts that necessitate the use of a separate name or title. The possible incorporation of SLD and its subcategories into the International Classification of Diseases 10th Revision codes should be explored by referencing the guidance made by previous literature on the coding of NAFLD [35]. Future studies that can help guide on how to code MASLD diagnosis based on the coding in electronic health records should follow. The localization of the terms in countries that do not use English as their mother tongue and possible confusion or distortion of their meaning in translation should be considered carefully.
ENDOCRINOLOGIST PERSPECTIVE
The introduction of MAFLD in 2020 emphasized the role of metabolic imbalance in the etiology of SLD. This paved the way for endocrinologists to play an active role in SLD treatment by seeking to enhance individual metabolic profiles, which would, in turn, potentially improve SLD and its relevant outcomes. While the pathophysiological link between insulin resistance and SLD is well established, it remains unclear whether improving metabolic dysfunction or CMRFs would help treat SLD or prevent its progression to steatohepatitis and fibrosis. From a therapeutic perspective, sodium-glucose cotransporter 2 (SGLT2) inhibitors, commonly used for type 2 diabetes, have improved the prognosis of chronic kidney disease and heart failure in non-diabetic patients. They may also be considered for the treatment of SLD, which reduces intrahepatic fat content and improves liver stiffness, thus expanding their clinical indications. Additionally, thyroid hormone receptor β agonists and glucagon-like peptide-1 (GLP-1)-based therapies, such as GLP-1 receptor agonists, GLP-1/glucose-dependent insulinotropic polypeptide (GIP), GLP-1/glucagon dual agonists, and GLP-1/GIP/glucagon triple agonists, known for their beneficial effects on metabolism, may help reverse SLD. However, their anti-NASH efficacy should be proven in further late-phase clinical trials. These emerging metabolic backbone therapies highlight the therapeutic role of reduced metabolic burden in SLD.
Pathogenesis of MASLD
The release of free fatty acids (FFAs) from adipose tissues play a significant role in the pathogenesis and progression of SLD [36,37]. Adipose tissue serves as a major reservoir of triglycerides, which are composed of FFAs. Under normal physiological conditions, adipose tissue maintains a balance between the storage and release of FFAs depending on the body’s energy demands. However, in pathological conditions such as obesity and insulin resistance, this balance is disrupted. Adipose tissue becomes resistant to the suppressive effect of insulin on lipolysis, leading to an increased release of FFAs into circulation, especially in portal circulation. Elevated levels of circulating FFAs contribute to the development of SLD.
Excessive uptake and accumulation of FFAs in the liver lead to increased triglyceride synthesis and subsequent hepatic steatosis. FFAs modulate the expression of pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and interleukin-1 beta (IL-1β), which induce liver inflammation and injury. Further accumulation of FFAs promotes inflammation, oxidative stress, and mitochondrial dysfunction in hepatocytes, thereby contributing to the progression of isolated steatosis to MASH and fibrosis.
Contribution of CMRFs to the development of SLD
As mentioned above, the primary pathophysiology of MASLD is insulin resistance along with increased release of FFAs. Therefore, the criteria for metabolic dysfunction in patients with MASLD should reflect this pathophysiological condition. As proposed in the new consensus statement for SLD, the definition of metabolic dysfunction in SLD, which relies on the criteria for metabolic syndromes, such as central obesity, high blood pressure, high fasting glucose, high fasting triglycerides, and low HDL cholesterol, may reveal inherent limitations [38]. Metabolic syndrome was initially established to classify individuals at risk of diabetes and/or CVD but not SLD. Thus, the new classification system may not fully reflect the pathophysiological background of fat accumulation in the liver. In particular, diastolic blood pressure and HDL cholesterol are weakly associated with insulin resistance and hepatic steatosis [39]. Without adequate data regarding the predictability of individual metabolic components contributing to hepatic steatosis, it is challenging to establish whether hepatic steatosis at an individual level is linked to corresponding cardiometabolic risk factors. Further research is required to obtain more precise insights into the relationship between the components of metabolic dysfunction in SLD and the development of hepatic steatosis.
Cardiometabolic risk threshold required to diagnose MASLD
The fundamental difference in the diagnostic criteria between MAFLD and MASLD lies in the minimum number of cardiometabolic risk factors required to define metabolic dysfunction. The diagnosis of MASLD requires at least one cardiometabolic risk factor, while the diagnosis of MAFLD requires two or more risk factors. Consequently, significantly more individuals will be classified as having metabolic dysfunction under MASLD than under MAFLD. Metabolic syndrome is typically defined as the presence of three or more cardiometabolic risk factors. Therefore, it is crucial to determine the cardiometabolic risk threshold (i.e., the minimum number of cardiometabolic risk factors needed) to identify metabolic dysfunction in SLD, as it plays a pivotal role in determining the extent to which metabolic syndrome contributes to SLD.
Based on our findings, over 90% of Koreans with SLD had at least one cardiometabolic risk factor (data not shown). This may lead to potential over-classification of MASLD and MetALD but under-classification of pure ALD, cryptogenic SLD, and SLD with specific etiology. Among young overweight or obese individuals, insulin resistance and hepatic steatosis may often exist even without any cardiometabolic risk factors. These young individuals may be misclassified as having cryptogenic SLD despite the presence of insulin resistance because insulin resistance is excluded from the diagnostic criteria of MASLD or MetALD. Classifying >90% of SLD cases as either MASLD or MetALD may mislead patients and clinical practitioners regarding their understanding of the disease.
Implications of metabolic dysfunction and glucose-lowering agents in the treatment of MASLD
When refining the new criteria for MASLD, the clinical implications of this classification system should be carefully considered, particularly concerning therapeutic interventions against SLD and its advanced stages. Since MASLD implies metabolic dysfunction as the primary cause of SLD, we may assume that improving metabolic dysfunction can also reverse MASLD.
In principle, SLD treatment is based on lifestyle modifications, including exercise or calorie restriction, to achieve weight loss and accompanying metabolic improvement [40]. However, most people find it challenging to sustain lifestyle modifications in the real world. Thiazolidinediones and vitamin E have shown efficacy in alleviating SLD [41]. In patients with NASH without diabetes, vitamin E as an antioxidant reportedly improved the histological hallmark of NASH, and both vitamin E and pioglitazone significantly reduced liver enzymes as well as intrahepatic fat content. However, both agents failed to demonstrate antifibrotic efficacy and were not free from safety issues. Other phase 3 agents, including obeticholic acid (farnesoid X receptor agonist) [42], elafibranor (peroxisome proliferator-activated receptor-α/δ agonist) [43], selonsertib (apoptosis signal-regulating kinase 1 inhibitor) [44], and cenicriviroc (C-C motif chemokine receptor 2/5 inhibitor) [45], have undergone testing and have shown improvements in NASH. However, none has been approved by the FDA and European Medicines Agency.
SGLT2 inhibitors and GLP-1 receptor agonists are glucose-lowering agents that have the advantage of lowering body weight, which is not easily achieved by lifestyle interventions alone and has been demonstrated to reduce the risk of CVD, the primary cause of mortality in individuals with SLD. As the liver does not express SGLT2 or GLP-1 receptors, improvement of SLD, if any, may be attributed to the indirect effects of these agents, which include weight loss and anti-inflammatory action. A recent review discussed the beneficial effects and potential mechanisms of SGLT2 inhibitors and GLP-1 receptor agonists in treating and preventing SLD [46].
Studies on SGLT2 inhibitors have reported a reduction in intrahepatic triglycerides and reductions in plasma glucose, triglycerides, and body weight. A recent meta-analysis showed that SGLT2 inhibitors slightly improved hepatic steatosis and fibrosis by 12.8 dB/m of controlled attenuation parameter and 0.82 kPa of liver stiffness measurement [47]. In terms of hepatic steatosis, the beneficial effects of SGLT2 inhibitors were more prominent in longer-duration users, younger patients, those treated with dapagliflozin, and those with worse fibrosis and steatosis.
Among many GLP-1 receptor agonist trials, the LEAN trial was the first randomized controlled trial that showed a higher resolution rate in patients with biopsy-proven noncirrhotic NASH [48]. Semaglutide also showed a higher resolution rate of NASH with no worsening of liver fibrosis [49]; however, both trials failed to demonstrate an improvement in the fibrosis stage. Results from two recent meta-analyses were in line with these findings, demonstrating that GLP-1 receptor agonists lead to significant improvements in hepatic fat content, liver enzymes, and other metabolic profiles, including hyperglycemia, but not hepatic fibrosis [50,51].
These studies or lifestyle intervention trials may provide a more practical definition of metabolic dysfunction in SLD. Examining the inclusion criteria of studies that improved SLD could help identify specific cardiometabolic traits that can benefit from such interventions. To illustrate, studies of SGLT2 inhibitors or GLP-1 receptor agonists are primarily based on individuals with type 2 diabetes and/or obesity, suggesting that these two metabolic components will likely be included in diagnosing MASLD. The methods to ameliorate SLD in individuals with hypertension and low HDL cholesterol are yet to be clarified and require further investigation.
Interaction between insulin resistance and alcohol consumption in the development and progression of SLD
The distinction between MASLD, MetALD, and ALD is not always clear. The overlap subtype, MetALD, indicates the complex interplay between metabolic factors and alcohol as one of the factors contributing to SLD. However, the extent of the contribution of each factor to SLD should be further delineated. Among patients diagnosed with MetALD, there may be some individuals for whom MASLD is considered the primary influencing factor. In contrast, ALD may be the main contributing factor for others.
Metabolic dysfunction and equal or more than moderate consumption of alcohol have additive effects on the progression to advanced fibrosis or severe liver disease, including hospitalization and death [30,52,53]. For example, those with moderate alcohol consumption have a ~5-fold increased risk of severe liver disease in each stratum of BMI (<25, 25–30, and >30 kg/m2), wherein the risk of severe liver disease increases with higher alcohol consumption in a dose-dependent manner [52]. However, the exact threshold for alcohol consumption that may lead to liver damage remains unclear. Although some studies have proposed protective effects of mild alcohol consumption [54,55], others have indicated no safe level of alcohol consumption [30,52], especially among individuals with MASLD. Furthermore, the extent of metabolic dysfunction and the amount of alcohol consumption may vary over time among individuals.
The roles of metabolic dysfunction and alcohol consumption in SLD development and progression are complex and multifaceted. Their relative contributions and interactions remain unclear and are likely to be influenced by other genetic and environmental determinants. Metabolic dysfunction or insulin resistance leads to excessive accumulation of fat in hepatocytes. Furthermore, alcohol increases the level of endotoxins, leading to oxidative stress and endoplasmic reticulum (ER) stress responses, contributing to both steatosis and fibrosis [56]. The independent overlapping mechanisms of metabolic dysfunction and alcohol consumption reciprocally interact and cumulatively contribute to hepatic steatosis, steatohepatitis, and fibrosis progression. An integrated understanding of how these two elements influence the disease trajectory would offer insightful perspectives for more accurate diagnosis and prognostic prediction of SLD subtypes. Ultimately, this would help define and identify SLD subtype-specific biomarkers essential for investigating therapeutic targets.
CONCLUSION
It is generally believed that the term NAFLD poorly communicates its potential harm to patients, making it difficult to confront the real cause of their suffering. With an emphasis on metabolic risk factors, MAFLD has been suggested as an alternative to NAFLD; however, its omission of alcohol consumption and NASH has been raised as a significant concern. Even at the risk of confusing practice and the public, a new term SLD and its four subcategories depending on the presence or absence of CMRF were suggested and MASLD was chosen to replace NAFLD. This new nomenclature is affirmative and non-stigmatizing, and is expected to bring about many changes, increasing disease awareness and involving a broader range of patients. The incorporation of NASH under the new term MASH minimizes confusion in ongoing clinical trials, and the FDA approval decision for promising agents is expected not to be disrupted.
In the context of SLD pathophysiology, insulin resistance and alcohol consumption are significant risk factors contributing to SLD, each with distinct but occasionally overlapping mechanisms of disease progression. An ongoing challenge in hepatology is understanding the complex interplay between metabolic dysfunction and alcohol use in the pathogenesis of SLD. While metabolic interventions may help effectively improve SLD, the potential for over-classification of MASLD due to an overemphasis on metabolic dysfunction must be carefully considered. Similarly, the contribution of less than moderate alcohol consumption to SLD and disease progression necessitates further research. Both insulin resistance and alcohol consumption require careful consideration in terms of their contributions to SLD for the optimal management of patients.
Nietzsche’s theoretical and practical nihilism, which described both the bright and dark sides of modernization, suggests that MASLD may have the same contradictory features as modernization with two faces of Janus–one facing the past and one facing the future. The new SLD nomenclature is more advantageous and reasonable than the existing NAFLD nomenclature. However, it still needs to be supplemented and improved in several ways. Additionally, in the context of the new nomenclature, it is crucial to emphasize the significance of preserving and building upon existing NAFLD research results while developing new biomarkers and drugs to avoid unnecessary waste of research resources. In this context, further research is strongly encouraged to develop new biomarkers and drugs against MASLD and MetALD to ensure patients benefit the most from disease name changes.
Notes
Authors’ contribution
All authors were responsible for drafting and critical revision of the manuscript.
Conflicts of Interest
The authors have no conflicts to disclose.
Acknowledgements
This work was supported by grants from the National Research Foundation of Korea (2022R1F1A1076449 to Gi-Ae Kim), (2021R1C1C1009875 and RS-2023-00222910 to Joon Ho Moon), and (2021R1A2C2005820 and 2021M3A9E4021818 to Won Kim) and the Research Program funded by the Korea Centers for Disease Control and Prevention (2022ER090200 to Won Kim).
Abbreviations
ALD
alcohol-associated liver disease
BMI
body mass index
CVD
cardiovascular disease
CMRF
cardiometabolic risk factor
ER
endoplasmic reticulum
GLP-1
glucagonlike peptide-1
HCC
hepatocellular carcinoma
MAFLD
metabolic (dysfunction)-associated fatty liver disease
MASLD
metabolic dysfunction-associated steatotic liver disease
MASH
metabolic-associated steatohepatitis
NAFLD
non-alcoholic fatty liver disease
NASH
non-alcoholic steatohepatitis
SLD
steatotic liver disease

