| Clin Mol Hepatol > Volume 30(1); 2024 > Article |
|
ABSTRACT
Metabolic dysfunction-associated fatty liver disease (MAFLD) is an increasingly common liver disease worldwide. MAFLD is diagnosed based on the presence of steatosis on images, histological findings, or serum marker levels as well as the presence of at least one of the three metabolic features: overweight/obesity, type 2 diabetes mellitus, and metabolic risk factors. MAFLD is not only a liver disease but also a factor contributing to or related to cardiovascular diseases (CVD), which is the major etiology responsible for morbidity and mortality in patients with MAFLD. Hence, understanding the association between MAFLD and CVD, surveillance and risk stratification of MAFLD in patients with CVD, and assessment of the current status of MAFLD management are urgent requirements for both hepatologists and cardiologists. This Taiwan position statement reviews the literature and provides suggestions regarding the epidemiology, etiology, risk factors, risk stratification, nonpharmacological interventions, and potential drug treatments of MAFLD, focusing on its association with CVD.
Metabolic dysfunction-associated fatty liver disease (MAFLD) and nonalcoholic fatty liver disease (NAFLD) are significant global health issues. In the general population, the incidence of MAFLD ranges from 15% to 30% [1]. The prevalence of NAFLD is approximately 55% in patients with type 2 diabetes mellitus (T2DM) and up to 80% in those with obesity [2,3]. The incidence rates of T2DM, hypertension, low high-density lipoprotein cholesterol levels, and hypertriglyceridemia are 9%, 8.4%, 9.6%, and 23.6%, respectively, in patients with biopsy-proven NAFLD [4].
The prognosis of hepatic outcomes in patients with MAFLD is associated with the severity of liver fibrosis [5]. Studies have demonstrated a significantly higher incidence of cirrhosis, hepatocellular carcinoma (HCC), and liver-related death in patients with NAFLD and fibrosis [6,7]. A study revealed an increase in cardiovascular events in patients with MAFLD [8]. The latest international consensus statements on the association between MAFLD and the risk of cardiovascular disease (CVD), which have been developed by experts from six continents, indicate that patients with MAFLD have higher cardiovascular events and mortality than individuals without MAFLD. In addition, CVD is the leading cause of death in patients with MAFLD [9].
Metabolic comorbidities are the leading risk factors for cardiovascular events and liver-related mortality in patients with MAFLD. T2DM intensifies the risks of CVD and chronic kidney disease due to increased insulin resistance (IR) [10]. The incidence of T2DM and hypertension also increases with the severity of MAFLD [11]. A meta-analysis revealed that T2DM, low high-density lipoprotein cholesterol levels, hypertriglyceridemia, and hypertension are significantly associated with a high risk of severe liver diseases, including cirrhosis, HCC, and liver-related mortality [12].
In 2020, the international expert consensus recommended changing the term NAFLD to MAFLD. Compared with NAFLD, MAFLD adequately reflects similar pathophysiological mechanisms and cardiometabolic risk factors for fatty liver disease and CVDs, such as metabolic dysfunction, obesity, IR, and dyslipidemia [13]. MAFLD is diagnosed based on histological, imaging, or biomarker evidence of hepatic steatosis in patients with overweight/obesity, T2DM, or at least two metabolic risk factors (Fig. 1) [13].
Liver biopsy remains the gold standard for the diagnosis and assessment of histological features in patients with NAFLD. However, the invasiveness of liver biopsy limits its routine use in clinical settings [14]. Ultrasound-based modalities are widely adopted as the first-line screening tools for hepatic steatosis; they have excellent performance for detecting moderate and severe steatosis, with a sensitivity and specificity of 84.8% (95% confidence interval [CI]: 79.5ŌĆō88.9%) and 93.6% (95% CI: 87.2ŌĆō97.0%), respectively [15]. Ultrasound-based transient elastography enables the quantitative evaluation of liver stiffness and steatosis. The area under the receiver operative characteristic curve of the ultrasonic controlled attenuation parameter for the detection of steatosis reached 0.95 in a previous study [16]. Magnetic resonance imaging-derived proton density fat fraction is the most sensitive noninvasive method for quantifying hepatic steatosis, with an area under the receiver operative characteristic curve of 0.95 [17]. Several noninvasive serum biomarkers, including the fatty liver index [18], hepatic steatosis index [19], NAFLD liver fat score [20], and lipid accumulation product [21], can be used to evaluate hepatic steatosis with moderate-to-good diagnostic performance (sensitivity: 86ŌĆō93%, specificity: 40ŌĆō71%) [22].
In 1998, the two-hit theory was proposed for the pathogenesis of NAFLD; it involves increased fat accumulation and the inflammatory cascade in the liver [23]. IR in the adipose tissue, muscle, and liver is a key factor in the first hit [24,25]. It is associated with energy imbalance caused by excessive caloric intake. Hepatic steatosis is caused by an imbalance between hepatic lipid storage and clearance, leading to excessive triglyceride-rich droplets in hepatocytes. In the second hit, the inflammatory cascade is overly activated by inflammatory cytokines, adipokines, lipotoxicity, endoplasmic reticulum stress, oxidative stress, and mitochondrial dysfunction [26-31]. Unresolved hepatic steatosis can progress to nonalcoholic steatohepatitis (NASH), fibrosis, cirrhosis, and even HCC in severe cases [32,33]. Recent research has identified genetic factors, epigenetics, and gut microbiota dysbiosis as other MAFLD-associated molecular and metabolic elements [34-36], resulting in the ŌĆ£multiple-hitŌĆØ pathomechanism [37].
Figure 2 presents the pathophysiological interaction between MAFLD and CVD. The ŌĆ£multiple hitsŌĆØ involved in the pathogenesis of MAFLD converge to a vicious cycle that promotes the development and progression of atherosclerosis and CVD [38,39]. In patients with MAFLD, the severity of hepatic steatosis and fibrosis is correlated with the coronary atheroma burden and atherosclerosis [40,41]. Moreover, inflammation and IR in MAFLD may increase the platelet count and the number of coagulation factors, which are associated with coronary arterial disease (CAD) [42] and venous thromboembolism (VTE).
Metabolic disorders and genetic origins are involved in the development of MAFLD and CVD [43,44]. Multiple hits resulting from the interactions between genetic and environmental risk factors for MAFLD and CVD contribute to the occurrence of MAFLD and CVD (Fig. 3) [43,44].
Risk factors for MAFLD include male sex, advancing age, obesity, IR, T2DM, and hyperlipidemia, which are linked to gut dysbiosis [45]. IR is significantly involved in the pathogenesis of MAFLD and its progression to NASH, with T2DM being strongly associated with MAFLD, NASH, and CVD [46]. Cholecystectomy is an independent risk factor of MAFLD, which is attributable to altered bile acid enterohepatic circulation [47].
Several genetic variants (PNPLA3, TM6SF2, and MBOAT7) can increase the susceptibility to NAFLD [48]. However, a Mendelian randomization analysis revealed no causal relationship between the NAFLD-associated PNPLA3 variant and CVD. Among the NAFLD-related genetic variants, TM6SF2 appears to be protective against VTE, whereas MBOAT7 may exert unfavorable effects [49].
Other risk factors include steatogenic drugs, male sex, and infections. Coronavirus disease 2019; hepatitis C; acquired immunodeficiency syndrome; Helicobacter pylori-induced peptic ulcers; and periodontitis caused by Bacteroidetes, Candidatus Saccharibacteria, Firmicutes, and Proteobacteria worsen MAFLD [50].
Patients with MAFLD who have T2DM, central obesity, a sedentary lifestyle, and metabolic syndrome have a high risk of advanced fibrosis [51]. Moreover, the severity of fibrosis is associated with cardiovascular risk in patients with steatosis or steatohepatitis [52]. Thus, MAFLD surveillance should be considered in patients with CVD. For patients with subclinical atherosclerosis and multiple risk factors for CVD, MAFLD screening may be considered [53].
The screening tool should effectively identify patients with MAFLD who have advanced liver fibrosis. Transient elastography is more cost-effective than magnetic resonance elastography (MRE) for detecting advanced liver fibrosis, although its sensitivity and specificity are compromised [54]. Thus, in patients suspected of having advanced fibrosis or those with inconclusive sonography and transient elastography findings, MRE should be considered. Indirect serological biomarkers include AST levels, AST-to-platelet ratio, fibrosis-4 (FIB-4) score, NAFLD fibrosis score, and AST-to-ALT ratio. Direct serological biomarkers include the enhanced liver fibrosis test score and FibroMeter NAFLD test score [53].
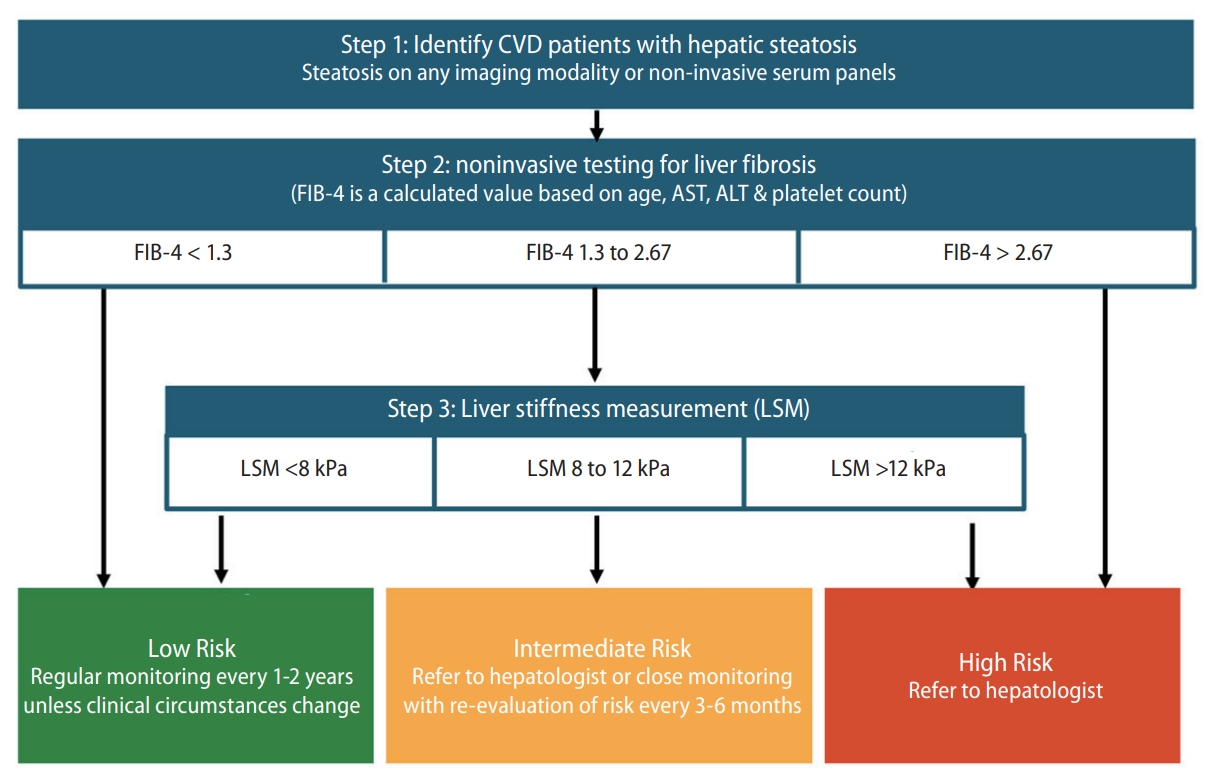
Fibrosis assessment is crucial in patients with MAFLD. Primary care practitioners, gastroenterologists, cardiologists, and neurologists should screen for advanced fibrosis in patients with MAFLD and CVD. The FIB-4 index may be practical, as the calculation is straightforward and is based on widely available, simple, and cost-effective tests. As no single measurement or threshold value has high sensitivity and specificity (Ōēź80%), a sequential algorithm having the FIB-4 index as the first-line test and liver stiffness measurement (LSM) as the second-line assessment is recommended. Figure 4 presents the algorithm recommended for MAFLD screening and liver fibrosis assessment among patients with CVD. The recommended algorithm is based on both clinical evidence and expert consensus. A meta-analysis revealed that a sequential combination of FIB-4 scores of <1.3 and Ōēź2.67 and subsequent LSM scores of <8.0 and Ōēź10.0 kPa could rule-in and rule-out advanced fibrosis, with a sensitivity of 66% (95% CI: 63ŌĆō68%) and specificity of 86% (95% CI: 84ŌĆō87%), respectively [55]. In another study, patients with FIB-4 scores of <1.3 had a low risk of HCC (0.05ŌĆō0.21/1,000 person-years), whereas those with FIB-4 scores of >2.67 had a high risk of HCC (1.9ŌĆō4.56/1,000 person-years) [56]. This sequential algorithm minimizes unnecessary tests and referrals, facilitates the timely identification of advanced fibrosis, and improves cost-effectiveness [53]. Therefore, several clinical guidelines recommended similar algorithms, such as American Gastroenterological Association (AGA)'s NASH Clinical Care Pathway [57], the American Association of Clinical Endocrinology (AACE)/American Association for the Study of Liver Diseases (AASLD) Clinical Practice Guideline for NAFLD [58], and The Japan Society of Hepatology (JSH)-The Japan Society of Gastroenterology (JSG) Clinical Practice Guidelines for NAFLD/NASH 2020 [59].
The association between MAFLD and CVD risk is receiving increasing scientific and clinical research interest, and increasing evidence supports that patients with MAFLD have increased risks of CVD morbidity and mortality [60]. Advanced fibrosis and cirrhosis are associated with high liver-related death rates in patients with MAFLD [61], whereas mild fibrosis predisposes patients with MAFLD to risks of cardiovascular events and nonhepatic malignancies [62]. MAFLD serves as an indicator of high cardiovascular risk and contributes to CVD development. It can therefore be considered an important risk factor for CVD [63-65].
Approximately 10% of patients with MAFLD in primary care facilities have CAD [66]. Chinese and Taiwanese studies have suggested that MAFLD is associated with high risks of cardiovascular events and subclinical CAD, and that the ASCVD burden is substantial in patients with MAFLD [40,67,68]. The extent of steatosis increases the coronary atheroma burden in patients with MAFLD [69]. Moreover, liver fibrosis markers are associated with CAD progression [41]. MAFLD is also associated with worsened outcomes in patients undergoing coronary artery bypass grafting and percutaneous coronary angioplasty [70-72]. In patients with myocardial infarction (MI), concomitant MAFLD exacerbates the risk of cardiovascular events and death [73]. A large biobank analysis reported the association of MAFLD with cardiovascular and all-cause mortality [74]. Patients with both nonŌĆōST-segment elevation MI and MAFLD have a high risk of premature ventricular complexes and ventricular tachycardia [75].
High blood pressure may predict MAFLD onset independently of conventional risk factors [76]. A recent study in Taiwan revealed that patients with fatty liver have a high risk of prevalent and incident hypertension and/or diabetes. Moreover, the risk increases with an increase in the severity of fatty liver [11]. Another study suggested that effective hypertension control reduces the risk of MAFLD [77].
In patients with heart failure (HF) with preserved ejection fraction (HFpEF), the prevalence of MAFLD is approximately 50% [78]. Patients with MAFLD have high left ventricular (LV) filling pressure in addition to a more fibrotic LV myocardium and worse global longitudinal strain [79]. Increased hepatic sinusoid resistance and venous return impairment can lead to a high normal cardiac output and high LV mass, which is characteristic of obstructive HFpEF. MAFLD may affect cardiac metabolism [80,81], and fibrosis may promote the formation of spontaneous portosystemic shunts, altering arterial blood flow and systemic vascular resistance in patients with HFpEF, which are associated with cirrhosis and advanced liver disease [82].
The incidence of QT interval prolongation is high in patients with MAFLD and T2DM [83]. Ventricular arrhythmias, atrioventricular blocks, and atrial fibrillation (AF) are more frequent in patients with MAFLD [75,84]. After catheter ablation, liver fibrosis is linked to adverse atrial remodeling and recurrent AF in patients with MAFLD [85]. The Rotterdam study reported an association between AF and liver stiffness, but not steatosis [86,87]. The conflicting results may be attributed to heterogeneous patient backgrounds.
MAFLD is an independent risk factor for VTE [88], and 81% of patients with VTE have MAFLD [89]. The levels or activities of von Willebrand factor; factors VIIŌĆōIX, XI, and XII [90]; and plasminogen activator inhibitor-1 are high in patients with MAFLD [91]. Patients with NASH have higher anticardiolipin immunoglobulin G levels than those with MAFLD [92], suggesting the association of thrombotic risks with liver fibrosis. Obesity is a VTE-associated risk factor in MAFLD [93,94]. However, the potential benefits of different interventions, such as bodyweight reduction, aerobic exercise, bariatric surgery, and anticoagulation medications, for VTE risk warrant further investigation [95,96].
In patients with MAFLD, CVD risk screening and early management are recommended [97,98]. A regional, validated risk calculator can be used to stratify the 10-year ASCVD or CAD risk in these patients. In patients with a high risk of CAD or angina, stress or imaging tests for CAD should be considered [99]. If risk factors, such as hypertension, obesity, T2DM, and advanced age, are present, referral for echocardiography and natriuretic peptide testing should be considered in symptomatic cases [100]. Early referral to a cardiologist is highly recommended for symptomatic cases or patients with MAFLD who have high cardiovascular risk [99,100].
Lifestyle interventions that reduce bodyweight are crucial for managing NAFLD [101]. Approximately 5% weight loss is required to improve liver steatosis, and >10% weight loss is required for managing both liver steatosis and fibrosis [102,103]. However, sustained weight loss is challenging. Approximately 21.2% of patients with initial weight loss regained weight after a median follow-up of 32.3 months [104]. Thus, a multidisciplinary approach involving physicians, psychologists, behavioral therapists, dieticians/nutritionists, patientsŌĆÖ families, patient support groups, and digital support is pivotal for lifestyle interventions [105,106].
Excessive dietary intake of calories, saturated fats, refined carbohydrates, and sugar-sweetened beverages is common in patients with NAFLD and obesity [107-110]. Dietary macronutrients are involved in the pathogenesis of NAFLD [111]. For instance, fructose promotes hepatic steatosis and inflammatory signaling [107], and polyunsaturated fatty acids exhibit antiinflammatory effects [112]. The current guidelines of the European Association for the Study of the Liver (EASL) and the Asian Pacific Association for the Study of the Liver (APASL) recommend a hypocaloric diet (500ŌĆō1,000 kcal deficit) [113,114]. Several trials support changing the amount and type of dietary carbohydrate/fat or adopting the Mediterranean diet, as both strategies can improve hepatic steatosis, regardless of weight loss [115,116]. Furthermore, the Mediterranean diet is effective in primary CVD prevention [115,117]. Regular coffee consumption is also associated with low risks of NAFLD and liver fibrosis [118,119].
Exercise improves MALFD/NAFLD through various mechanisms, such as the upregulation of several signaling pathways, particularly those involving the peroxisome proliferator-activated receptor gamma (PPAR-╬│) [120,121]. Exercise may downregulate mammalian target of rapamycin complex 1 signaling, further alleviating MAFLD/NAFLD [122]. Exercise training is beneficial for hepatic and cardiometabolic function in patients with MAFLD/NAFLD [123]. It improves vascular stiffness and endothelial dysfunction, thereby decreasing cardiovascular risk [124]. By reducing fibrosis, vigorous exercise improves the histological findings of NASH [125]. Regular and moderate exercise for at least 150 minutes per week or increasing activity levels for >60 minutes per week can ameliorate MAFLD/NAFLD [126].
Aerobic exercise, defined as continuous and rhythmic activities requiring the use of large muscle groups, is the primary training modality assessed in NAFLD exercise studies. By contrast, the benefit of resistance training remains controversial because of the heterogeneity of training intensity and protocols. A combination of aerobic and resistance training is expected to outperform either exercise modality [127,128]. Alternative activities, such as yoga, Pilates, and tai chi, have exhibited beneficial effects in pilot studies [129-131]. Updated guidelines of the AASLD and EASL strongly recommend any type of sustained individualized exercise for patients with MAFLD/NAFLD [126].
Bariatric surgery leads to a sustained weight loss of up to 30% in patients with obesity, in addition to improving T2DM, NASH/NAFLD, morbidity, and mortality [132,133]. Patients undergoing bariatric surgery showed NASH resolution and fibrosis regression 5 years postoperatively [134]. Bariatric surgery also reduced CVD risk and CVD-associated morbidity in patients with obesity and NAFLD [135,136]. In addition, endoscopic bariatric and metabolic therapies (EBMT) improved aminotransferase levels and decreased NAFLD activity scores in patients with obesity and NAFLD [134,137,138]. However, well-designed prospective studies are warranted to assess the hepatic and cardiovascular benefits of EBMT in patients with NAFLD and obesity.
Although no drugs have been approved for MAFLD, the treatment of metabolic conditions closely associated with MAFLD may reverse IR, thereby ameliorating steatohepatitis and preventing fibrosis. Although lifestyle modification and weight loss are recommended as first-line interventions and can effectively reduce steatosis, inflammation, and fibrosis, they are often unsuccessful [102]. Therefore, pharmacological therapy may address the gap in treatments inhibiting MAFLD progression. Table 1 summarizes the investigated drugs for MAFLD. The use of approved antidiabetic drugs, including biguanides, glucagon-like peptide-1 receptor agonists (GLP-1RAs), dipeptidyl peptidase-4 inhibitors (DPP-4is), sodium-dependent glucose cotransporter-2 inhibitors (SGLT-2is), and PPAR agonists, has been investigated in patients with NASH [139,140]. Novel agents for NASH/NAFLD are in different phases of clinical development; their mechanisms of action include participation in de novo hepatic lipogenesis, mitochondrial fatty acid oxidation, inflammation, cell injury, collagen deposition, and fibrinolysis [141].
Oxidative stress plays a key role in the pathogenesis of NASH; thus, vitamin E is justifiable as a therapeutic agent for NASH. Randomized controlled trials (RCTs) have been conducted in nondiabetic adults, children, and adolescents with biopsy-proven NASH [142-144]. Pooled analyses have demonstrated that vitamin E significantly decreases aminotransferase levels and improves the histological characteristics of NASH, except for liver fibrosis [144-146]. In an RCT involving patients with coexisting T2DM and NASH, 18 months of vitamin E supplementation histologically improved steatosis [147]. However, the role of vitamin E in NASH and advanced fibrosis or cirrhosis remains inconclusive.
The safety concerns of vitamin E should be considered. In one study, all-cause mortality was high in patients taking a high dose (>800 IU/day) of vitamin E [148]. Moreover, vitamin E increases the risk of HF in patients with vascular disease or T2DM [149] and the risk of prostate cancer in healthy men [150]. Although a high-vitamin E diet is associated with reduced stroke risk [151], it may significantly increase the risk of hemorrhagic stroke [152]. In summary, vitamin E supplementation at a daily dose of 800 IU may be considered in nondiabetic adults with biopsy-proven NASH. The associated risks and benefits should be fully discussed with each patient before initiating therapy.
The AASLD or EASL does not recommend ursodeoxycholic acid, a natural dihydroxy bile acid, for the treatment of NAFLD or NASH because of insufficient evidence regarding its beneficial effects on liver histology.
Obeticholic acid (OCA) is an analog of the bile acid chenodeoxycholic acid and a potent farnesoid X receptor agonist. Although the primary endpoints were met in the phase 2 FLINT trial and phase III REGENERATE trial of OCA, the U.S. Food & Drug Administration (US FDA) raised safety concerns regarding pruritus, high low density lipoprotein (LDL) levels, and limited changes in cardiovascular risk [153,154]. Consequently, the AASLD, EASL, and APASL do not recommend OCA for offlabel use for the treatment of NASH by [155-157].
Statins may decrease LDL levels and cardiovascular risk in patients with NAFLD and NASH without liver decompensation. However, according to the AASLD and EASL, this treatment does not benefit or harm patients with liver disease [155-157]. Recent study has presented mixed findings regarding the role of PCSK9 inhibitors in managing earlystage NAFLD, emphasizing the need for extensive long-term research to ascertain their efficacy and safety [158].
Metformin is a biguanide with a mild insulin-sensitizing effect. It is traditionally the first-line therapy for T2DM. In patients with NAFLD unresponsive to lifestyle modifications, biochemical improvement was observed after metformin treatment [159]. Hepatic fat reduction with weight loss was also noted in a proportion of patients with NASH who were treated with metformin [160]. In an open-label trial, metformin in combination with rosiglitazone further improved liver histology in patients with NASH [161]. However, a meta-analysis of metformin trials did not reveal improvements in the liver disease activity score or fibrosis stage [162-164]. Overall, insufficient evidence supports the routine use of metformin in patients with NASH [164].
Pioglitazone was found to improve liver function, decrease hepatic fat, and improve NASH features in clinical trials and systemic reviews [165,166], regardless of the diabetic status [167]. Although weight gain was observed after pioglitazone therapy, data on other thiazolidinediones are limited [168]. In patients with T2DM and NASH, pioglitazone reduced CVD events [169].
GLP-1RA is a new class of antidiabetic agents for T2DM that can improve weight loss, glycemic control, and liver enzyme levels by activating the gut-derived incretin pathway [170]. GLP-1RAs exhibit beneficial renovascular and cardiovascular effects on T2DM [171,172]. Histological findings of the phase 2 LEAN RCT revealed that patients with T2DM receiving liraglutide for 48 weeks had higher NASH resolution and lower fibrosis progression than those receiving placebo [173]. In a phase 2 trial of semaglutide, compared with placebo, 72-week semaglutide treatment resulted in significantly higher NASH resolution in patients with biopsy-proven NASH and F1ŌĆōF3 liver fibrosis. However, the semaglutide trial did not reveal beneficial effects in improving the fibrosis stage [174]. In a systematic review and meta-analysis of patients with T2DM and NAFLD, GLP-1RAs effectively improved intrahepatic, visceral, and subcutaneous adipose tissue; liver function; body mass index; waist circumference; and glucose/lipid profiles but did not improve liver fibrosis markers, such as FIB-4 and NAS [175]. The main adverse events were mild-to-moderate gastrointestinal discomfort, such as poor appetite, constipation, diarrhea, and hypoglycemia, which resolved within a few weeks. Although a few small-scale studies have reported that GLP-1RAs are associated with NASH resolution and fibrosis regression, more large-scale studies are warranted.
SGLT-2is are antidiabetic agents that have extended benefits, and they are approved for reducing adverse outcomes in nondiabetic patients with HF and chronic kidney disease [176,177].
An observational study revealed that add-on treatment with 50 mg ipragliflozin for 45 weeks improved glycemic control and normalized ALT levels in patients with T2DM and NAFLD who were unresponsive to incretin-based therapy [178]. SGLT-2is also improved glycemic control and liver function in patients with T2DM and NAFLD and exclusively caused weight loss [179,180]. The efficacy of canagliflozin, dapagliflozin, and empagliflozin for NAFLD or NASH has been investigated in RCTs involving patients with T2DM with or without NAFLD, and the hepatic benefits, including aminotransferase, steatosis, and fibrosis improvements, of SGLT-2is have been noted [175,181-183]. Overall, SGLT-2is have exhibited positive effects on hepatic steatosis in meta-analyses; however, their effect on liver fibrosis requires further investigation [184-186].
DPP-4 inhibition reduces glucagon levels, delays gastric emptying, stimulates insulin release, and augments pancreatic beta-cell regeneration [187]. DPP-4is may alleviate T2DM-related microvascular complications [188].
Early interventions with sitagliptin in patients with T2DM may have long-lasting reno- and islet-protective effects [189]. However, whether sitagliptin increases the risk of hospitalization in patients with HF remains debatable [190,191]. Sitagliptin decreased CVD incidence in patients with T2DM [192]. However, 12-week sitagliptin therapy did not reduce hepatic steatosis or fibrosis in overweight patients with T2DM [193]. Moreover, it did not reduce aminotransaminase levels in patients with NASH [194]. Vildagliptin exhibited a CVD risk comparable to sitagliptin [195], and it prevented the progression of T2DM-related CVD by improving LDL heterogeneity [196].
The prevalence of coexisting MAFLD and chronic hepatitis B (CHB) or chronic hepatitis C (CHC) is 30ŌĆō70%, and MAFLD occurs in 13.6ŌĆō59.3% of patients with CHB [197]. An inverse association has been reported between hepatitis B virus replication and hepatic steatosis [198], as fat deposition in hepatocytes and a related increasing inflammatory status may inhibit or suppress viral replication [199,200]. By contrast, patients with MAFLD and CHB tend to experience accelerated liver disease progression and exhibit more liver-related complications. Furthermore, their death rate is higher than that of patients with CHB or MAFLD [201]. More studies are warranted to explore the effect of coexisting CHB on CVD risk in patients with MAFLD.
Hepatic steatosis, a common histological feature, is detected in 30ŌĆō70% of patients with CHC [202-204]. The coexistence of CHC and MAFLD occurs in 9ŌĆō38% of cases [205]. Data suggest that metabolic disturbances are highly prevalent in patients with CHC, placing them at higher risks of CVD, carotid and coronary atherosclerosis, and myocardial dysfunction [206]. Nevertheless, no direct evidence suggests that MAFLD aggravates CVD risk in patients with CHC.
Delineating the relative contributions of alcohol consumption in patients with MAFLD having metabolic risk factors is challenging. Alcohol consumption may deteriorate liver disease and may lead to CVD development in patients with MAFLD through an additive or synergistic mechanism.
MAFLD has become an important health issue globally. Because of underlying IR or metabolic derangement, substantial cross-talk occurs between hepatic outcomes (steatosis, a hepatic manifestation of metabolic syndrome) and cardiovascular events (CVD, a cardiac manifestation). In this positional statement, 11 important clinical issues regarding the diagnosis, screening, and assessment of MAFLD; the importance of the co-management of MAFLD and CVD; and potential management strategies have been addressed and discussed by both hepatologists and cardiologists. The benefits of various lifestyle modifications and updates on different pharmacological interventions for CVD and steatosis-associated advanced fibrosis have also been briefly reviewed. We hope that these statements simplify the clinical practice of gastroenterologists/hepatologists and cardiologists for treating patients with MAFLD or CVD. These statements also aim to draw the attention of general practitioners to emerging MAFLD, and setting optimal goals for clinical management is crucial.
ACKNOWLEDGMENTS
This work was supported by the National Science and Technology Council, Executive Yuen, Taiwan (MOST 109-2314-B-002 -091 -MY3; NSTC 112-2314-B-002 -205 -MY3).
This work was also partly supported by the ŌĆ£ Center of Excellence for Metabolic Associated Fatty Liver Disease, National Sun Yet-sen University, Kaohsiung, TaiwanŌĆØ from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan, MOHW112-TDU-B-221-124007, NYCUKMU-111-I001 and NYCUKMU-111-I004, and by the Taiwan Association for the Study of the Liver.
FOOTNOTES
Figure┬Ā1.
Definition of metabolic dysfunction-associated fatty liver disease. BMI, body mass index; HDL, high density lipoprotein.
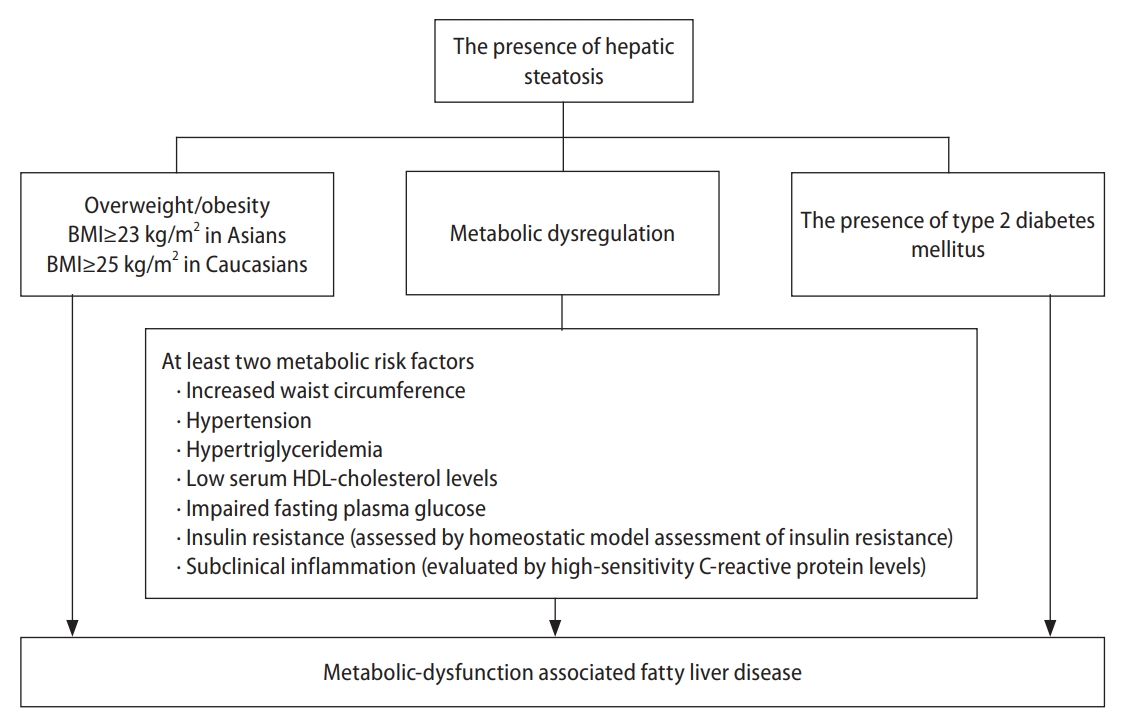
Figure┬Ā2.
Pathophysiological mechanisms underlying the interaction between MAFLD and CVD. MAFLD, metabolic associated fatty liver disease; CVD, cardiovascular disease.

Figure┬Ā3.
Illustration of the risk factors interplaying between the development of MAFLD and CVDs. MAFLD, metabolic associated fatty liver disease; CVD, cardiovascular disease.
Figure┬Ā4.
Algorithm for MAFLD screening and fibrosis assessment among CVD patients. MAFLD, metabolic associated fatty liver disease; CVD, cardiovascular disease; FIB-4, fibrosis-4.
Table┬Ā1.
Summary of the effects of pharmaceutical interventions on liver and CV outcomes in MAFLD patients
Abbreviations
MAFLD
metabolic dysfunction-associated fatty liver disease
NAFLD
nonalcoholic fatty liver disease
T2DM
type 2 diabetes mellitus
HCC
hepatocellular carcinoma
CVD
cardiovascular diseases
IR
insulin resistance
NASH
nonalcoholic steatohepatitis
CAD
coronary arterial disease
VTE
venous thromboembolism
AST
aspartate aminotransferase
ALT
alanine aminotransferase
MRE
magnetic resonance elastography
FIB-4
fibrosis-4
LSM
liver stiffness measurement
MI
myocardial infarction
HF
heart failure
HfpEF
HF with preserved ejection fraction
LV
left ventricular
AF
atrial fibrillation
EASL
European Association for the Study of the Liver
APASL
Asian Pacific Association for the Study of the Liver
PPAR-╬│
peroxisome proliferator-activated receptor gamma
EBMT
endoscopic bariatric and metabolic therapies
GLP-1RA
glucagon-like peptide-1 receptor agonist
DPP-4i
dipeptidyl peptidase-4 inhibitor
SGLT-2i
sodium-dependent glucose cotransporter-2 inhibitor
OCA
obeticholic acid
CHB
chronic hepatitis B
CHC
chronic hepatitis C
REFERENCES
1. Li J, Zou B, Yeo YH, Feng Y, Xie X, Lee DH, et al. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999-2019: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2019;4:389-398.


2. Polyzos SA, Kountouras J, Mantzoros CS. Obesity and nonalcoholic fatty liver disease: From pathophysiology to therapeutics. Metabolism 2019;92:82-97.


3. Younossi ZM, Golabi P, de Avila L, Paik JM, Srishord M, Fukui N, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J Hepatol 2019;71:793-801.


4. Ampuero J, Aller R, Gallego-Dur├Īn R, Crespo J, Calleja JL, Garc├Ła-Monz├│n C, et al. Significant fibrosis predicts new-onset diabetes mellitus and arterial hypertension in patients with NASH. J Hepatol 2020;73:17-25.


6. Dulai PS, Singh S, Patel J, Soni M, Prokop LJ, Younossi Z, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: Systematic review and meta-analysis. Hepatology 2017;65:1557-1565.




7. Sheka AC, Adeyi O, Thompson J, Hameed B, Crawford PA, Ikramuddin S. Nonalcoholic steatohepatitis: A review. JAMA 2020;323:1175-1183.


8. Duell PB, Welty FK, Miller M, Chait A, Hammond G, Ahmad Z, et al. Nonalcoholic fatty liver disease and cardiovascular risk: A scientific statement from the american heart association. Arterioscler Thromb Vasc Biol 2022;42:e168-e185.


9. Zhou XD, Targher G, Byrne CD, Somers V, Kim SU, Chahal CAA, et al. An international multidisciplinary consensus statement on MAFLD and the risk of CVD. Hepatol Int 2023;17:773-791.




10. Davis TME. Diabetes and metabolic dysfunction-associated fatty liver disease. Metabolism 2021;123:154868.


11. Shih CI, Wu KT, Hsieh MH, Yang JF, Chen YY, Tsai WL, et al. Severity of fatty liver is highly correlated with the risk of hypertension and diabetes: a cross-sectional and longitudinal cohort study. Hepatol Int 2023 Sep 25;doi: 10.1007/s12072-023-10576-z.



12. Jarvis H, Craig D, Barker R, Spiers G, Stow D, Anstee QM, et al. Metabolic risk factors and incident advanced liver disease in non-alcoholic fatty liver disease (NAFLD): A systematic review and meta-analysis of population-based observational studies. PLoS Med 2020;17:e1003100.



13. Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J Hepatol 2020;73:202-209.

14. Khalifa A, Rockey DC. The utility of liver biopsy in 2020. Curr Opin Gastroenterol 2020;36:184-191.


15. Hernaez R, Lazo M, Bonekamp S, Kamel I, Brancati FL, Guallar E, et al. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: a meta-analysis. Hepatology 2011;54:1082-1090.



16. Sasso M, Beaugrand M, de Ledinghen V, Douvin C, Marcellin P, Poupon R, et al. Controlled attenuation parameter (CAP): a novel VCTEŌäó guided ultrasonic attenuation measurement for the evaluation of hepatic steatosis: preliminary study and validation in a cohort of patients with chronic liver disease from various causes. Ultrasound Med Biol 2010;36:1825-1835.


17. Middleton MS, Heba ER, Hooker CA, Bashir MR, Fowler KJ, Sandrasegaran K, et al. Agreement between magnetic resonance imaging proton density fat fraction measurements and pathologist-assigned steatosis grades of liver biopsies from adults with nonalcoholic steatohepatitis. Gastroenterology 2017;153:753-761.



18. Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, et al. The fatty liver index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol 2006;6:33.




19. Lee JH, Kim D, Kim HJ, Lee CH, Yang JI, Kim W, et al. Hepatic steatosis index: a simple screening tool reflecting nonalcoholic fatty liver disease. Dig Liver Dis 2010;42:503-508.


20. Kotronen A, Peltonen M, Hakkarainen A, Sevastianova K, Bergholm R, Johansson LM, et al. Prediction of non-alcoholic fatty liver disease and liver fat using metabolic and genetic factors. Gastroenterology 2009;137:865-872.


21. Bedogni G, Kahn HS, Bellentani S, Tiribelli C. A simple index of lipid overaccumulation is a good marker of liver steatosis. BMC Gastroenterol 2010;10:98.




24. Sakurai Y, Kubota N, Yamauchi T, Kadowaki T. Role of Insulin Resistance in MAFLD. Int J Mol Sci 2021;22:4156.



25. Li S, Brown MS, Goldstein JL. Bifurcation of insulin signaling pathway in rat liver: mTORC1 required for stimulation of lipogenesis, but not inhibition of gluconeogenesis. Proc Natl Acad Sci U S A 2010;107:3441-3446.



26. Jorge ASB, Andrade JMO, Para├Łso AF, Jorge GCB, Silveira CM, de Souza LR, et al. Body mass index and the visceral adipose tissue expression of IL-6 and TNF-alpha are associated with the morphological severity of non-alcoholic fatty liver disease in individuals with class III obesity. Obes Res Clin Pract 2018;12(Suppl 2):1-8.


27. DI Maira G, Pastore M, Marra F. Liver fibrosis in the context of nonalcoholic steatohepatitis: the role of adipokines. Minerva Gastroenterol Dietol 2018;64:39-50.

28. Marra F, Svegliati-Baroni G. Lipotoxicity and the gut-liver axis in NASH pathogenesis. J Hepatol 2018;68:280-295.


29. Wu H, Ballantyne CM. Metabolic inflammation and insulin resistance in obesity. Circ Res 2020;126:1549-1564.



30. Parry SA, Rosqvist F, Mozes FE, Cornfield T, Hutchinson M, Piche ME, et al. Intrahepatic fat and postprandial glycemia increase after consumption of a diet enriched in saturated fat compared with free sugars. Diabetes Care 2020;43:1134-1141.




31. Ziolkowska S, Binienda A, Jabłkowski M, Szemraj J, Czarny P. The interplay between insulin resistance, inflammation, oxidative stress, base excision repair and metabolic syndrome in nonalcoholic fatty liver disease. Int J Mol Sci 2021;22:11128.



32. Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology 2006;43(2 Suppl 1):S99-S112.


33. Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology 2010;51:1820-1832.


34. Eslam M, Valenti L, Romeo S. Genetics and epigenetics of NAFLD and NASH: Clinical impact. J Hepatol 2018;68:268-279.


35. Oh JH, Lee JH, Cho MS, Kim H, Chun J, Lee JH, et al. Characterization of gut microbiome in Korean patients with metabolic associated fatty liver disease. Nutrients 2021;13:1013.



36. Hern├Īndez-Ceballos W, Cordova-Gallardo J, Mendez-Sanchez N. Gut microbiota in metabolic-associated fatty liver disease and in other chronic metabolic diseases. J Clin Transl Hepatol 2021;9:227-238.


37. Fang YL, Chen H, Wang CL, Liang L. Pathogenesis of nonalcoholic fatty liver disease in children and adolescence: From ŌĆ£two hit theoryŌĆØ to ŌĆ£multiple hit modelŌĆØ. World J Gastroenterol 2018;24:2974-2983.



38. Stols-Gon├¦alves D, Hovingh GK, Nieuwdorp M, Holleboom AG. NAFLD and atherosclerosis: Two sides of the same dysmetabolic coin? Trends Endocrinol Metab 2019;30:891-902.


39. Umpleby AM, Shojaee-Moradie F, Fielding B, Li X, Marino A, Alsini N, et al. Impact of liver fat on the differential partitioning of hepatic triacylglycerol into VLDL subclasses on high and low sugar diets. Clin Sci (Lond) 2017;131:2561-2573.




40. Zou H, Ge Y, Lei Q, Ung COL, Ruan Z, Lai Y, et al. Epidemiology and disease burden of non-alcoholic steatohepatitis in greater China: a systematic review. Hepatol Int 2022;16:27-37.



41. Tsai TY, Hsu PF, Wu CH, Huang SS, Chan WL, Lin SJ, et al. Association between coronary artery plaque progression and liver fibrosis biomarkers in population with low calcium scores. Nutrients 2022;14:3163.



42. Balta S, Demirkol S, Celik T, Akgul EO. Mean platelet volume as a surrogate marker of long-term mortality in patients undergoing percutaneous coronary intervention. Am J Cardiol 2013;112:142.


43. Dro┼╝d┼╝ K, Nabrdalik K, Hajzler W, Kwiendacz H, Gumprecht J, Lip GYH. Metabolic-associated fatty liver disease (MAFLD), diabetes, and cardiovascular disease: Associations with fructose metabolism and gut microbiota. Nutrients 2021;14:103.



44. Zaiou M, Amrani R, Rihn B, Hajri T. Dietary patterns influence target gene expression through emerging epigenetic mechanisms in nonalcoholic fatty liver disease. Biomedicines 2021;9:1256.



45. Li M, Rajani C, Zheng X, Jia W. The microbial metabolome in metabolic-associated fatty liver disease. J Gastroenterol Hepatol 2022;37:15-23.



46. Guti├®rrez-Cuevas J, Santos A, Armendariz-Borunda J. Pathophysiological molecular mechanisms of obesity: A link between MAFLD and NASH with cardiovascular diseases. Int J Mol Sci 2021;22:11629.



47. Lin YC, Wu CC, Ni YH. New perspectives on genetic prediction for pediatric metabolic associated fatty liver disease. Front Pediatr 2020;8:603654.



48. Dongiovanni P, Paolini E, Corsini A, Sirtori CR, Ruscica M. Nonalcoholic fatty liver disease or metabolic dysfunction-associated fatty liver disease diagnoses and cardiovascular diseases: From epidemiology to drug approaches. Eur J Clin Invest 2021;51:e13519.



49. Meroni M, Longo M, Fracanzani AL, Dongiovanni P. MBOAT7 down-regulation by genetic and environmental factors predisposes to MAFLD. EBioMedicine 2020;57:102866.



50. Boeckmans J, Rombaut M, Demuyser T, Declerck B, Pi├®rard D, Rogiers V, et al. Infections at the nexus of metabolic-associated fatty liver disease. Arch Toxicol 2021;95:2235-2253.




51. Huang JF, Hsieh MY, Dai CY, Hou NJ, Lee LP, Lin ZY, et al. The incidence and risks of liver biopsy in non-cirrhotic patients: An evaluation of 3806 biopsies. Gut 2007;56:736-737.



52. Shiha G, Ibrahim A, Helmy A, Sarin SK, Omata M, Kumar A, et al. Asian-Pacific Association for the Study of the Liver (APASL) consensus guidelines on invasive and non-invasive assessment of hepatic fibrosis: a 2016 update. Hepatol Int 2017;11:1-30.



53. Anstee QM, Castera L, Loomba R. Impact of non-invasive biomarkers on hepatology practice: Past, present and future. J Hepatol 2022;76:1362-1378.


54. Xiao G, Zhu S, Xiao X, Yan L, Yang J, Wu G. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: A meta-analysis. Hepatology 2017;66:1486-1501.



55. M├│zes FE, Lee JA, Selvaraj EA, Jayaswal ANA, Trauner M, Boursier J, et al. Diagnostic accuracy of non-invasive tests for advanced fibrosis in patients with NAFLD: an individual patient data meta-analysis. Gut 2022;71:1006-1019.



56. Balakrishnan M, Li L, El-Serag HB, Kanwal F. Longitudinal changes in fibrosis markers are associated with risk of cirrhosis and hepatocellular carcinoma in non-alcoholic fatty liver disease. J Hepatol 2023;78:493-500.



57. Kanwal F, Shubrook JH, Adams LA, Pfotenhauer K, Wai-Sun Wong V, Wright E, et al. Clinical care pathway for the risk stratification and management of patients with nonalcoholic fatty liver disease. Gastroenterology 2021;161:1657-1669.



58. Cusi K, Isaacs S, Barb D, Basu R, Caprio S, Garvey WT, et al. American Association of Clinical Endocrinology Clinical Practice Guideline for the diagnosis and management of nonalcoholic fatty liver disease in primary care and endocrinology clinical settings: Co-sponsored by the American Association for the Study of Liver Diseases (AASLD). Endocr Pract 2022;28:528-562.

59. Tokushige K, Ikejima K, Ono M, Eguchi Y, Kamada Y, Itoh Y, et al. Evidence-based clinical practice guidelines for nonalcoholic fatty liver disease/nonalcoholic steatohepatitis 2020. J Gastroenterol 2021;56:951-963.




60. Zhou XD, Cai J, Targher G, Byrne CD, Shapiro MD, Sung KC, et al. Metabolic dysfunction-associated fatty liver disease and implications for cardiovascular risk and disease prevention. Cardiovasc Diabetol 2022;21:270.




61. Angulo P. Long-term mortality in nonalcoholic fatty liver disease: is liver histology of any prognostic significance? Hepatology 2010;51:373-375.



62. Wang Y, Yu Y, Zhang H, Chen C, Wan H, Chen Y, et al. Cardiovascular and renal burdens among patients with MAFLD and NAFLD in China. Front Endocrinol (Lausanne) 2022;13:968766.



63. Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol 2013;10:330-344.



64. Kasper P, Martin A, Lang S, K├╝tting F, Goeser T, Demir M, et al. NAFLD and cardiovascular diseases: a clinical review. Clin Res Cardiol 2021;110:921-937.




65. Sumida Y, Yoneda M. Current and future pharmacological therapies for NAFLD/NASH. J Gastroenterol 2018;53:362-376.




66. Godinez-Leiva E, Bril F. Nonalcoholic fatty liver disease (NAFLD) for primary care providers: Beyond the liver. Curr Hypertens Rev 2021;17:94-111.



67. Tsou MT, Chen JY. Gender-based association of coronary artery calcification and framingham risk score with non-alcoholic fatty liver disease and abdominal obesity in taiwanese adults, a cross-sectional study. Front Cardiovasc Med 2022;9:803967.



68. Hsiao CC, Teng PH, Wu YJ, Shen YW, Mar GY, Wu FZ. Severe, but not mild to moderate, non-alcoholic fatty liver disease associated with increased risk of subclinical coronary atherosclerosis. BMC Cardiovasc Disord 2021;21:244.




69. Hsu PF, Wang YW, Lin CC, Wang YJ, Ding YZ, Liou TL, et al. The association of the steatosis severity in fatty liver disease with coronary plaque pattern in general population. Liver Int 2021;41:81-90.



70. Wang L, Li Y, Gong X. Changes in inflammatory factors and prognosis of patients complicated with non-alcoholic fatty liver disease undergoing coronary artery bypass grafting. Exp Ther Med 2018;15:949-953.



71. Ali H, Kazmi M, Choi C, Hashemipour R, Singh I, Pyrsopoulos NT. In-hospital outcomes of patients with non-alcoholic fatty liver disease who underwent percutaneous coronary intervention: A nationwide inpatient sample analysis. Cureus 2021;13:e17338.



72. Shi KQ, Wu FL, Liu WY, Zhao CC, Chen CX, Xie YY, et al. Nonalcoholic fatty liver disease and risk of in-stent restenosis after bare metal stenting in native coronary arteries. Mol Biol Rep 2014;41:4713-4720.



73. Xia W, Yang N, Li Y. Analysis of risk factors for adverse cardiovascular events in elderly patients with acute myocardial infarction and non-alcoholic fatty liver disease (NAFLD). Med Sci Monit 2020;26:e922913.



74. Ma W, Wu W, Wen W, Xu F, Han D, Lyu J, et al. Association of NAFLD with cardiovascular disease and all-cause mortality: a large-scale prospective cohort study based on UK Biobank. Ther Adv Chronic Dis 2022;13:20406223221122478.




75. Chen X, Zhao X, Wu H, Li L, Yang D, Si Y, et al. Association of nonalcoholic fatty liver disease with ventricular tachycardia and sinus arrest in patients with non-ST-segment elevation myocardial infarction. Int Heart J 2022;63:814-820.


76. Ma J, Hwang SJ, Pedley A, Massaro JM, Hoffmann U, Chung RT, et al. Bi-directional analysis between fatty liver and cardiovascular disease risk factors. J Hepatol 2017;66:390-397.



77. Liu P, Tang Y, Guo X, Zhu X, He M, Yuan J, et al. Bidirectional association between nonalcoholic fatty liver disease and hypertension from the Dongfeng-Tongji cohort study. J Am Soc Hypertens 2018;12:660-670.


78. Salah HM, Pandey A, Soloveva A, Abdelmalek MF, Diehl AM, Moylan CA, et al. Relationship of nonalcoholic fatty liver disease and heart failure with preserved ejection fraction. JACC Basic Transl Sci 2021;6:918-932.



79. VanWagner LB, Wilcox JE, Colangelo LA, Lloyd-Jones DM, Carr JJ, Lima JA, et al. Association of nonalcoholic fatty liver disease with subclinical myocardial remodeling and dysfunction: A population-based study. Hepatology 2015;62:773-783.



80. Huang DQ, Downes M, Evans RM, Witztum JL, Glass CK, Loomba R. Shared mechanisms between cardiovascular disease and NAFLD. Semin Liver Dis 2022;42:455-464.



81. Zhang XJ, She ZG, Wang J, Sun D, Shen LJ, Xiang H, et al. Multiple omics study identifies an interspecies conserved driver for nonalcoholic steatohepatitis. Sci Transl Med 2021;13:eabg8117.


82. Chen B, Tang WHW, Rodriguez M, Corey KE, Sanyal AJ, Kamath PS, et al. NAFLD in cardiovascular diseases: A contributor or comorbidity? Semin Liver Dis 2022;42:465-474.


83. Hung CS, Tseng PH, Tu CH, Chen CC, Liao WC, Lee YC, et al. Nonalcoholic fatty liver disease is associated with QT prolongation in the general population. J Am Heart Assoc 2015;4:e001820.



84. Lei F, Qin JJ, Song X, Liu YM, Chen MM, Sun T, et al. The prevalence of MAFLD and its association with atrial fibrillation in a nationwide health check-up population in China. Front Endocrinol (Lausanne) 2022;13:1007171.



85. Decoin R, Butruille L, Defrancq T, Robert J, Destrait N, Coisne A, et al. High liver fibrosis scores in metabolic dysfunction-associated fatty liver disease patients are associated with adverse atrial remodeling and atrial fibrillation recurrence following catheter ablation. Front Endocrinol (Lausanne) 2022;13:957245.



86. van Kleef LA, Lu Z, Ikram MA, de Groot NMS, Kavousi M, de Knegt RJ. Liver stiffness not fatty liver disease is associated with atrial fibrillation: The Rotterdam study. J Hepatol 2022;77:931-938.


87. Long MT, Yin X, Larson MG, Ellinor PT, Lubitz SA, McManus DD, et al. Relations of liver fat with prevalent and incident atrial fibrillation in the framingham heart study. J Am Heart Assoc 2017;6:e005227.



88. Targher G, Byrne CD. Diagnosis and management of nonalcoholic fatty liver disease and its hemostatic/thrombotic and vascular complications. Semin Thromb Hemost 2013;39:214-228.


89. Di Minno MN, Tufano A, Rusolillo A, Di Minno G, Tarantino G. High prevalence of nonalcoholic fatty liver in patients with idiopathic venous thromboembolism. World J Gastroenterol 2010;16:6119-6122.



90. Kotronen A, Joutsi-Korhonen L, Sevastianova K, Bergholm R, Hakkarainen A, Pietil├żinen KH, et al. Increased coagulation factor VIII, IX, XI and XII activities in non-alcoholic fatty liver disease. Liver Int 2011;31:176-183.


91. Targher G, Bertolini L, Scala L, Zenari L, Lippi G, Franchini M, et al. Plasma PAI-1 levels are increased in patients with nonalcoholic steatohepatitis. Diabetes Care 2007;30:e31-e32.



92. Ciavarella A, Gnocchi D, Custodero C, Lenato GM, Fiore G, Sabb├Ā C, et al. Translational insight into prothrombotic state and hypercoagulation in nonalcoholic fatty liver disease. Thromb Res 2021;198:139-150.


93. Ji D, Zhang M, Qin E, Zhang L, Xu J, Wang Y, et al. Letter to the Editor: Obesity, diabetes, non-alcoholic fatty liver disease and metabolic dysfunction associated fatty liver disease are proinflammatory hypercoagulable states associated with severe disease and thrombosis in Covid-19. Metabolism 2021;115:154437.



94. Northup PG, Sundaram V, Fallon MB, Reddy KR, Balogun RA, Sanyal AJ, et al. Hypercoagulation and thrombophilia in liver disease. J Thromb Haemost 2008;6:2-9.


95. Cotrim HP, Daltro C. Liver: Does bariatric surgery reduce the severity of NAFLD? Nat Rev Gastroenterol Hepatol 2010;7:11-13.



96. Zhang HJ, He J, Pan LL, Ma ZM, Han CK, Chen CS, et al. Effects of moderate and vigorous exercise on nonalcoholic fatty liver disease: A randomized clinical trial. JAMA Intern Med 2016;176:1074-1082.


97. Huang PH, Lu YW, Tsai YL, Wu YW, Li HY, Chang HY, et al. 2022 Taiwan lipid guidelines for primary prevention. J Formos Med Assoc 2022;121:2393-2407.


98. Wang TD, Chiang CE, Chao TH, Cheng HM, Wu YW, Wu YJ, et al. 2022 Guidelines of the Taiwan Society of Cardiology and the Taiwan Hypertension Society for the management of hypertension. Acta Cardiol Sin 2022;38:225-325.


99. Ueng KC, Chiang CE, Chao TH, Wu YW, Lee WL, Li YH, et al. 2023 Guidelines of the Taiwan Society of Cardiology on the diagnosis and management of chronic coronary syndrome. Acta Cardiol Sin 2023;39:4-96.


100. Chiang CE, Hung CL, Wu YW, Lin TH, Ueng KC, Sung SH, et al. 2023 Consensus of Taiwan Society of Cardiology on the pharmacological treatment of chronic heart failure. Acta Cardiol Sin 2023;39:361-390.


101. Thoma C, Day CP, Trenell MI. Lifestyle interventions for the treatment of non-alcoholic fatty liver disease in adults: a systematic review. J Hepatol 2012;56:255-266.


102. Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, Torres-Gonzalez A, Gra-Oramas B, Gonzalez-Fabian L, et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology 2015;149:367-378.e5 quiz e14-15.


103. Wong VW, Chan RS, Wong GL, Cheung BH, Chu WC, Yeung DK, et al. Community-based lifestyle modification programme for non-alcoholic fatty liver disease: a randomized controlled trial. J Hepatol 2013;59:536-542.


104. Malespin MH, Barritt AS 4th, Watkins SE, Schoen C, Tincopa MA, Corbin KD, et al. Weight loss and weight regain in usual clinical practice: Results from the TARGET-NASH observational cohort. Clin Gastroenterol Hepatol 2022;20:2393-2395.e4.


105. Christensen QH, Brecht RM, Dudekula D, Greenberg EP, Nagarajan R. Evolution of acyl-substrate recognition by a family of acyl-homoserine lactone synthases. PLoS One 2014;9:e112464.



106. Mazzotti A, Caletti MT, Brodosi L, Di Domizio S, Forchielli ML, Petta S, et al. An internet-based approach for lifestyle changes in patients with NAFLD: Two-year effects on weight loss and surrogate markers. J Hepatol 2018;69:1155-1163.


107. Mouzaki M, Allard JP. The role of nutrients in the development, progression, and treatment of nonalcoholic fatty liver disease. J Clin Gastroenterol 2012;46:457-467.


108. Vilar-Gomez E, Nephew LD, Vuppalanchi R, Gawrieh S, Mladenovic A, Pike F, et al. High-quality diet, physical activity, and college education are associated with low risk of NAFLD among the US population. Hepatology 2022;75:1491-1506.



109. Yasutake K, Nakamuta M, Shima Y, Ohyama A, Masuda K, Haruta N, et al. Nutritional investigation of non-obese patients with non-alcoholic fatty liver disease: the significance of dietary cholesterol. Scand J Gastroenterol 2009;44:471-477.


110. Meng G, Zhang B, Yu F, Li C, Zhang Q, Liu L, et al. Soft drinks consumption is associated with nonalcoholic fatty liver disease independent of metabolic syndrome in Chinese population. Eur J Nutr 2018;57:2113-2121.



111. Hall KD, Guo J. Obesity energetics: Body weight regulation and the effects of diet composition. Gastroenterology 2017;152:1718-1727.e3.



112. Antraco VJ, Hirata BKS, de Jesus Sim├Żo J, Cruz MM, da Silva VS, da Cunha de S├Ī RDC, et al. Omega-3 polyunsaturated fatty acids prevent nonalcoholic steatohepatitis (NASH) and stimulate adipogenesis. Nutrients 2021;13:622.



113. European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of nonalcoholic fatty liver disease. J Hepatol 2016;64:1388-1402.


114. Eslam M, Sarin SK, Wong VW, Fan JG, Kawaguchi T, Ahn SH, et al. The Asian Pacific Association for the Study of the Liver clinical practice guidelines for the diagnosis and management of metabolic associated fatty liver disease. Hepatol Int 2020;14:889-919.



115. Properzi C, OŌĆÖSullivan TA, Sherriff JL, Ching HL, Jeffrey GP, Buckley RF, et al. Ad libitum mediterranean and low-fat diets both significantly reduce hepatic steatosis: A randomized controlled trial. Hepatology 2018;68:1741-1754.



116. Pugliese N, Plaz Torres MC, Petta S, Valenti L, Giannini EG, et al. Is there an ŌĆśidealŌĆÖ diet for patients with NAFLD? Eur J Clin Invest 2022;52:e13659.



117. Yaskolka Meir A, Rinott E, Tsaban G, Zelicha H, Kaplan A, Rosen P, et al. Effect of green-Mediterranean diet on intrahepatic fat: the DIRECT PLUS randomised controlled trial. Gut 2021;70:2085-2095.



118. Chen YP, Lu FB, Hu YB, Xu LM, Zheng MH, Hu ED. A systematic review and a dose-response meta-analysis of coffee dose and nonalcoholic fatty liver disease. Clin Nutr 2019;38:2552-2557.


119. Wijarnpreecha K, Thongprayoon C, Ungprasert P. Coffee consumption and risk of nonalcoholic fatty liver disease: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol 2017;29:e8-e12.


120. Diniz TA, de Lima Junior EA, Teixeira AA, Biondo LA, da Rocha LAF, Valad├Żo IC, et al. Aerobic training improves NAFLD markers and insulin resistance through AMPK-PPAR-╬▒ signaling in obese mice. Life Sci 2021;266:118868.


121. Zheng F, Cai Y. Concurrent exercise improves insulin resistance and nonalcoholic fatty liver disease by upregulating PPAR-╬│ and genes involved in the beta-oxidation of fatty acids in ApoE-KO mice fed a high-fat diet. Lipids Health Dis 2019;18:6.




123. Ennequin G, Sirvent P, Whitham M. Role of exercise-induced hepatokines in metabolic disorders. Am J Physiol Endocrinol Metab 2019;317:E11-E24.


124. Pugh CJ, Spring VS, Kemp GJ, Richardson P, Shojaee-Moradie F, Umpleby AM, et al. Exercise training reverses endothelial dysfunction in nonalcoholic fatty liver disease. Am J Physiol Heart Circ Physiol 2014;307:H1298-1306.


125. Kistler KD, Brunt EM, Clark JM, Diehl AM, Sallis JF, Schwimmer JB, et al. Physical activity recommendations, exercise intensity, and histological severity of nonalcoholic fatty liver disease. Am J Gastroenterol 2011;106:460-468 quiz 469.



126. Rinella ME, Neuschwander-Tetri BA, Siddiqui MS, Abdelmalek MF, Caldwell S, Barb D, et al. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology 2023;77:1797-1835.



127. Villareal DT, Aguirre L, Gurney AB, Waters DL, Sinacore DR, Colombo E, et al. Aerobic or resistance exercise, or both, in dieting obese older adults. N Engl J Med 2017;376:1943-1955.



128. Garc├Ła-Hermoso A, Ram├Łrez-V├®lez R, Ram├Łrez-Campillo R, Peterson MD, Mart├Łnez-Vizca├Łno V. Concurrent aerobic plus resistance exercise versus aerobic exercise alone to improve health outcomes in paediatric obesity: a systematic review and meta-analysis. Br J Sports Med 2018;52:161-166.


129. Liu X, Miller YD, Burton NW, Brown WJ. A preliminary study of the effects of Tai Chi and Qigong medical exercise on indicators of metabolic syndrome, glycaemic control, health-related quality of life, and psychological health in adults with elevated blood glucose. Br J Sports Med 2010;44:704-709.


130. Singh AK, Kaur N, Kaushal S, Tyagi R, Mathur D, Sivapuram MS, et al. Partitioning of radiological, stress and biochemical changes in pre-diabetic women subjected to Diabetic Yoga Protocol. Diabetes Metab Syndr 2019;13:2705-2713.


131. Thorp A, Stine JG. Exercise as medicine: The IMPACT of exercise training on nonalcoholic fatty liver disease. Curr Hepatol Rep 2020;19:402-411.




132. Fakhry TK, Mhaskar R, Schwitalla T, Muradova E, Gonzalvo JP, Murr MM. Bariatric surgery improves nonalcoholic fatty liver disease: a contemporary systematic review and meta-analysis. Surg Obes Relat Dis 2019;15:502-511.


133. Wiggins T, Guidozzi N, Welbourn R, Ahmed AR, Markar SR. Association of bariatric surgery with all-cause mortality and incidence of obesity-related disease at a population level: A systematic review and meta-analysis. PLoS Med 2020;17:e1003206.



134. Bazerbachi F, Vargas EJ, Rizk M, Maselli DB, Mounajjed T, Venkatesh SK, et al. Intragastric balloon placement induces significant metabolic and histologic improvement in patients with nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol 2021;19:146-154.e4.



135. Elsaid MI, Li Y, Bridges JFP, Brock G, Minacapelli CD, Rustgi VK. Association of bariatric surgery with cardiovascular outcomes in adults with severe obesity and nonalcoholic fatty liver disease. JAMA Netw Open 2022;5:e2235003.



136. Krishnan A, Hadi Y, Alqahtani SA, Woreta TA, Fang W, Abunnaja S, et al. Cardiovascular outcomes and mortality after bariatric surgery in patients with nonalcoholic fatty liver disease and obesity. JAMA Netw Open 2023;6:e237188.



137. Gollisch KS, Lindhorst A, Raddatz D. EndoBarrier gastrointestinal liner in type 2 diabetic patients improves liver fibrosis as assessed by liver elastography. Exp Clin Endocrinol Diabetes 2017;125:116-121.


138. Lee YM, Low HC, Lim LG, Dan YY, Aung MO, Cheng CL, et al. Intragastric balloon significantly improves nonalcoholic fatty liver disease activity score in obese patients with nonalcoholic steatohepatitis: a pilot study. Gastrointest Endosc 2012;76:756-760.


139. Jirapinyo P, McCarty TR, Dolan RD, Shah R, Thompson CC. Effect of endoscopic bariatric and metabolic therapies on nonalcoholic fatty liver disease: A systematic review and meta-analysis. Clin Gastroenterol Hepatol 2022;20:511-524.e1.


140. Stefan N, H├żring HU, Cusi K. Non-alcoholic fatty liver disease: causes, diagnosis, cardiometabolic consequences, and treatment strategies. Lancet Diabetes Endocrinol 2019;7:313-324.


141. Vuppalanchi R, Noureddin M, Alkhouri N, Sanyal AJ. Therapeutic pipeline in nonalcoholic steatohepatitis. Nat Rev Gastroenterol Hepatol 2021;18:373-392.



142. Alkhouri N, Feldstein AE. The TONIC trial: a step forward in treating pediatric nonalcoholic fatty liver disease. Hepatology 2012;55:1292-1295.


143. Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med 2010;362:1675-1685.



144. Usman M, Bakhtawar N. Vitamin E as an adjuvant treatment for non-alcoholic fatty liver disease in adults: A systematic review of randomized controlled trials. Cureus 2020;12:e9018.



145. Sato K, Gosho M, Yamamoto T, Kobayashi Y, Ishii N, Ohashi T, et al. Vitamin E has a beneficial effect on nonalcoholic fatty liver disease: a meta-analysis of randomized controlled trials. Nutrition 2015;31:923-930.


146. Xu R, Tao A, Zhang S, Deng Y, Chen G. Association between vitamin E and non-alcoholic steatohepatitis: a meta-analysis. Int J Clin Exp Med 2015;8:3924-3934.


147. Bril F, Biernacki DM, Kalavalapalli S, Lomonaco R, Subbarayan SK, Lai J, et al. Role of vitamin E for nonalcoholic steatohepatitis in patients with type 2 diabetes: A randomized controlled trial. Diabetes Care 2019;42:1481-1488.



148. Miller ER 3rd, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med 2005;142:37-46.


149. Lonn E, Bosch J, Yusuf S, Sheridan P, Pogue J, Arnold JM, et al. Effects of long-term vitamin E supplementation on cardiovascular events and cancer: a randomized controlled trial. JAMA 2005;293:1338-1347.


150. Klein EA, Thompson IM Jr, Tangen CM, Crowley JJ, Lucia MS, Goodman PJ, et al. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2011;306:1549-1556.


151. Cheng P, Wang L, Ning S, Liu Z, Lin H, Chen S, et al. Vitamin E intake and risk of stroke: a meta-analysis. Br J Nutr 2018;120:1181-1188.


152. Sch├╝rks M, Glynn RJ, Rist PM, Tzourio C, Kurth T. Effects of vitamin E on stroke subtypes: meta-analysis of randomised controlled trials. BMJ 2010;341:c5702.



153. Neuschwander-Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet 2015;385:956-965.


154. Younossi ZM, Ratziu V, Loomba R, Rinella M, Anstee QM, Goodman Z, et al. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet 2019;394:2184-2196.

155. Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018;67:328-357.



156. Chitturi S, Wong VW, Chan WK, Wong GL, Wong SK, Sollano J, et al. The Asia-Pacific Working Party on Non-alcoholic Fatty Liver Disease guidelines 2017-Part 2: Management and special groups. J Gastroenterol Hepatol 2018;33:86-98.



157. Marchesini G, Roden M, Vettor R. Response to: Comment to ŌĆ£EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver diseaseŌĆØ. J Hepatol 2017;66:466-467.


158. Momtazi-Borojeni AA, Banach M, Ruscica M, Sahebkar A. The role of PCSK9 in NAFLD/NASH and therapeutic implications of PCSK9 inhibition. Expert Rev Clin Pharmacol 2022;15:1199-1208.


159. Duseja A, Das A, Dhiman RK, Chawla YK, Thumburu KT, Bhadada S, et al. Metformin is effective in achieving biochemical response in patients with nonalcoholic fatty liver disease (NAFLD) not responding to lifestyle interventions. Ann Hepatol 2007;6:222-226.


160. Loomba R, Lutchman G, Kleiner DE, Ricks M, Feld JJ, Borg BB, et al. Clinical trial: pilot study of metformin for the treatment of non-alcoholic steatohepatitis. Aliment Pharmacol Ther 2009;29:172-182.



161. Torres DM, Jones FJ, Shaw JC, Williams CD, Ward JA, Harrison SA. Rosiglitazone versus rosiglitazone and metformin versus rosiglitazone and losartan in the treatment of nonalcoholic steatohepatitis in humans: a 12-month randomized, prospective, open-label trial. Hepatology 2011;54:1631-1639.


162. Li Y, Liu L, Wang B, Wang J, Chen D. Metformin in non-alcoholic fatty liver disease: A systematic review and meta-analysis. Biomed Rep 2013;1:57-64.



163. Musso G, Cassader M, Rosina F, Gambino R. Impact of current treatments on liver disease, glucose metabolism and cardiovascular risk in non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of randomised trials. Diabetologia 2012;55:885-904.



164. Sawangjit R, Chongmelaxme B, Phisalprapa P, Saokaew S, Thakkinstian A, Kowdley KV, et al. Comparative efficacy of interventions on nonalcoholic fatty liver disease (NAFLD): A PRISMA-compliant systematic review and network meta-analysis. Medicine (Baltimore) 2016;95:e4529.


165. Belfort R, Harrison SA, Brown K, Darland C, Finch J, Hardies J, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med 2006;355:2297-2307.


166. Blazina I, Selph S. Diabetes drugs for nonalcoholic fatty liver disease: a systematic review. Syst Rev 2019;8:295.




167. Bril F, Kalavalapalli S, Clark VC, Lomonaco R, Soldevila-Pico C, Liu IC, et al. Response to pioglitazone in patients with nonalcoholic steatohepatitis with vs without type 2 diabetes. Clin Gastroenterol Hepatol 2018;16:558-566.e2.


168. Bugianesi E, Moscatiello S, Ciaravella MF, Marchesini G. Insulin resistance in nonalcoholic fatty liver disease. Curr Pharm Des 2010;16:1941-1951.


169. Gastaldelli A, Cusi K. From NASH to diabetes and from diabetes to NASH: Mechanisms and treatment options. JHEP Rep 2019;1:312-328.



170. Dougherty JA, Guirguis E, Thornby KA. A systematic review of newer antidiabetic agents in the treatment of nonalcoholic fatty liver disease. Ann Pharmacother 2021;55:65-79.



171. Mann JFE, ├śrsted DD, Brown-Frandsen K, Marso SP, Poulter NR, Rasmussen S, et al. Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med 2017;377:839-848.


172. Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016;375:311-322.



173. Armstrong MJ, Gaunt P, Aithal GP, Barton D, Hull D, Parker R, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet 2016;387:679-690.


174. Newsome PN, Buchholtz K, Cusi K, Linder M, Okanoue T, Ratziu V, et al. A placebo-controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. N Engl J Med 2021;384:1113-1124.


175. Zhu Y, Xu J, Zhang D, Mu X, Shi Y, Chen S, et al. Efficacy and safety of GLP-1 receptor agonists in patients with type 2 diabetes mellitus and non-alcoholic fatty liver disease: A systematic review and meta-analysis. Front Endocrinol (Lausanne) 2021;12:769069.



176. Nespoux J, Vallon V. SGLT2 inhibition and kidney protection. Clin Sci (Lond) 2018;132:1329-1339.




177. Palmiero G, Cesaro A, Vetrano E, Pafundi PC, Galiero R, Caturano A, et al. Impact of SGLT2 inhibitors on heart failure: From pathophysiology to clinical effects. Int J Mol Sci 2021;22:5863.



178. Ohki T, Isogawa A, Toda N, Tagawa K. Effectiveness of ipragliflozin, a sodium-glucose co-transporter 2 inhibitor, as a second-line treatment for non-alcoholic fatty liver disease patients with type 2 diabetes mellitus who do not respond to incretin-based therapies including glucagon-like peptide-1 analogs and dipeptidyl peptidase-4 inhibitors. Clin Drug Investig 2016;36:313-319.



179. Seko Y, Sumida Y, Tanaka S, Mori K, Taketani H, Ishiba H, et al. Effect of sodium glucose cotransporter 2 inhibitor on liver function tests in Japanese patients with non-alcoholic fatty liver disease and type 2 diabetes mellitus. Hepatol Res 2017;47:1072-1078.



180. Ito D, Shimizu S, Inoue K, Saito D, Yanagisawa M, Inukai K, et al. Comparison of ipragliflozin and pioglitazone effects on non-alcoholic fatty liver disease in patients with type 2 diabetes: A randomized, 24-week, open-label, active-controlled trial. Diabetes Care 2017;40:1364-1372.



181. Akuta N, Kawamura Y, Watanabe C, Nishimura A, Okubo M, Mori Y, et al. Impact of sodium glucose cotransporter 2 inhibitor on histological features and glucose metabolism of nonalcoholic fatty liver disease complicated by diabetes mellitus. Hepatol Res 2019;49:531-539.



182. Shimizu M, Suzuki K, Kato K, Jojima T, Iijima T, Murohisa T, et al. Evaluation of the effects of dapagliflozin, a sodium-glucose co-transporter-2 inhibitor, on hepatic steatosis and fibrosis using transient elastography in patients with type 2 diabetes and non-alcoholic fatty liver disease. Diabetes Obes Metab 2019;21:285-292.



183. Chehrehgosha H, Sohrabi MR, Ismail-Beigi F, Malek M, Reza Babaei M, Zamani F, et al. Empagliflozin improves liver steatosis and fibrosis in patients with non-alcoholic fatty liver disease and type 2 diabetes: A randomized, double-blind, placebo-controlled clinical trial. Diabetes Ther 2021;12:843-861.




184. Wong C, Yaow CYL, Ng CH, Chin YH, Low YF, Lim AYL, et al. Sodium-glucose co-transporter 2 inhibitors for non-alcoholic fatty liver disease in Asian patients with type 2 diabetes: A meta-analysis. Front Endocrinol (Lausanne) 2021;11:609135.



185. Xing B, Zhao Y, Dong B, Zhou Y, Lv W, Zhao W. Effects of sodium-glucose cotransporter 2 inhibitors on non-alcoholic fatty liver disease in patients with type 2 diabetes: A meta-analysis of randomized controlled trials. J Diabetes Investig 2020;11:1238-1247.




186. Dwinata M, Putera DD, Hasan I, Raharjo M. SGLT2 inhibitors for improving hepatic fibrosis and steatosis in non-alcoholic fatty liver disease complicated with type 2 diabetes mellitus: a systematic review. Clin Exp Hepatol 2020;6:339-346.



187. Langley AK, Suffoletta TJ, Jennings HR. Dipeptidyl peptidase IV inhibitors and the incretin system in type 2 diabetes mellitus. Pharmacotherapy 2007;27:1163-1180.


188. Avogaro A, Fadini GP. The effects of dipeptidyl peptidase-4 inhibition on microvascular diabetes complications. Diabetes Care 2014;37:2884-2894.



189. Hattori S. Ten-year follow-up of sitagliptin treatment in patients with type 2 diabetes mellitus. Diabetol Metab Syndr 2021;13:117.




190. Clifton P. Do dipeptidyl peptidase IV (DPP-IV) inhibitors cause heart failure? Clin Ther 2014;36:2072-2079.


191. Fu AZ, Johnston SS, Ghannam A, Tsai K, Cappell K, Fowler R, et al. Association between hospitalization for heart failure and dipeptidyl peptidase 4 inhibitors in patients with type 2 diabetes: An observational study. Diabetes Care 2016;39:726-734.



192. Yang TY, Liaw YP, Huang JY, Chang HR, Chang KW, Ueng KC. Association of Sitagliptin with cardiovascular outcome in diabetic patients: a nationwide cohort study. Acta Diabetol 2016;53:461-468.



193. Smits MM, Tonneijck L, Muskiet MH, Kramer MH, Pouwels PJ, Pieters-van den Bos IC, et al. Twelve week liraglutide or sitagliptin does not affect hepatic fat in type 2 diabetes: a randomised placebo-controlled trial. Diabetologia 2016;59:2588-2593.




194. Olaywi M, Bhatia T, Anand S, Singhal S. Novel anti-diabetic agents in non-alcoholic fatty liver disease: a mini-review. Hepatobiliary Pancreat Dis Int 2013;12:584-588.


195. Ha KH, Kim B, Shin HS, Lee J, Choi H, Kim HC, et al. Comparative cardiovascular risks of dipeptidyl peptidase-4 inhibitors: Analyses of real-world data in Korea. Korean Circ J 2018;48:395-405.




196. Tani S, Nagao K, Hirayama A. Association between urinary albumin excretion and low-density lipoprotein heterogeneity following treatment of type 2 diabetes patients with the dipeptidyl peptidase-4 inhibitor, vildagliptin: a pilot study. Am J Cardiovasc Drugs 2013;13:443-450.



197. Tsochatzis E, Papatheodoridis GV, Manesis EK, Chrysanthos N, Kafiri G, Archimandritis AJ. Hepatic steatosis in chronic hepatitis B develops due to host metabolic factors: a comparative approach with genotype 1 chronic hepatitis C. Dig Liver Dis 2007;39:936-942.


198. Peleg N, Issachar A, Sneh Arbib O, Cohen-Naftaly M, Braun M, Leshno M, et al. Liver steatosis is a strong predictor of mortality and cancer in chronic hepatitis B regardless of viral load. JHEP Rep 2019;1:9-16.



199. Wang X, Xie Q. Metabolic dysfunction-associated fatty liver disease (MAFLD) and viral hepatitis. J Clin Transl Hepatol 2022;10:128-133.

200. Hu D, Wang H, Wang H, Wang Y, Wan X, Yan W, et al. Non-alcoholic hepatic steatosis attenuates hepatitis B virus replication in an HBV-immunocompetent mouse model. Hepatol Int 2018;12:438-446.



201. Choi HSJ, Brouwer WP, Zanjir WMR, de Man RA, Feld JJ, Hansen BE, et al. Nonalcoholic steatohepatitis is associated with liver-related outcomes and all-cause mortality in chronic hepatitis B. Hepatology 2020;71:539-548.



202. Koike K, Moriya K. Metabolic aspects of hepatitis C viral infection: steatohepatitis resembling but distinct from NASH. J Gastroenterol 2005;40:329-336.



203. Chen CH, Huang JF, Huang CF, Yeh ML, Yang JF, Hsieh MY, et al. Interferon-associated hepatic steatosis is related to discrepancies in biochemical and virological responses of chronic hepatitis C to IFN-based therapy. Hepatol Int 2013;7:162-170.



204. Lonardo A, Adinolfi LE, Loria P, Carulli N, Ruggiero G, Day CP. Steatosis and hepatitis C virus: mechanisms and significance for hepatic and extrahepatic disease. Gastroenterology 2004;126:586-597.


-
METRICS

- ORCID iDs
-
Yen-Wen Wu

https://orcid.org/0000-0003-1520-1166Chun-Jen Liu

https://orcid.org/0000-0002-6202-0993 - Related articles



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



