Hepatitis B core-related antigen dynamics and risk of subsequent clinical relapses after nucleos(t)ide analog cessation
Article information
See the commentary-article "Enhancing off-nucleos(t)ide analogue outcome predictions in chronic hepatitis B with time-varying hepatitis B core-related antigen" on page 154.
Abstract
Background/Aims
Finite nucleos(t)ide analog (NA) therapy has been proposed as an alternative treatment strategy for chronic hepatitis B (CHB), but biomarkers for post-treatment monitoring are limited. We investigated whether measuring hepatitis B core-related antigen (HBcrAg) after NA cessation may stratify the risk of subsequent clinical relapse (CR).
Methods
This retrospective multicenter analysis enrolled adults with CHB who were prospectively monitored after discontinuing entecavir or tenofovir with negative HBeAg and undetectable HBV DNA at the end of treatment (EOT). Patients with cirrhosis or malignancy were excluded. CR was defined as serum alanine aminotransferase > two times the upper limit of normal with recurrent viremia. We applied time-dependent Cox proportional hazard models to clarify the association between HBcrAg levels and subsequent CR.
Results
The cohort included 203 patients (median age, 49.8 years; 76.8% male; 60.6% entecavir) who had been treated for a median of 36.9 months (interquartile range [IQR], 36.5–40.1). During a median post-treatment follow-up of 31.7 months (IQR, 16.7–67.1), CR occurred in 104 patients with a 5-year cumulative incidence of 54.8% (95% confidence interval [CI], 47.1–62.4%). Time-varying HBcrAg level was a significant risk factor for subsequent CR (adjusted hazard ratio [aHR], 1.53 per log U/mL; 95% CI, 1.12–2.08) with adjustment for EOT HBsAg, EOT anti-HBe, EOT HBcrAg and time-varying HBsAg. During follow-up, HBcrAg <1,000 U/mL predicted a lower risk of CR (aHR, 0.41; 95% CI, 0.21–0.81).
Conclusions
Dynamic measurement of HBcrAg after NA cessation is predictive of subsequent CR and may be useful to guide post-treatment monitoring.
Graphical Abstract
INTRODUCTION
Nucleos(t)ide analogs (NA) suppress the replication of hepatitis B virus (HBV) by inhibiting the viral reverse transcriptase, thereby reducing hepatic inflammation, other liver-related comorbidities, and hepatocellular carcinoma (HCC) [1-3]. However, the ideal treatment endpoint, such as hepatitis B surface antigen (HBsAg) seroclearance, is rarely achieved during NA treatment [4,5]. Most patients who remain HBsAg-positive experienced viral relapse after stopping NA. Hepatitis flares can occur following viral relapse, and severe acute exacerbations can lead to fatal consequences in some patients [6,7]. Nevertheless, a limited number of studies have reported high HBsAg seroclearance rates after cessation of NA [8,9] and ex vivo studies also indicate that HBV-specific T cell responses could be enhanced by discontinuing treatment with viral suppressants [10,11]. Thus, the identification of groups of patients with chronic hepatitis B (CHB) who need to maintain NA or who can safely discontinue NA is an important yet unresolved issue.
HBcrAg is a potentially novel and useful biomarker that includes the hepatitis B core antigen (HBcAg), hepatitis B e antigen (HBeAg), and a truncated precore protein (p22Cr) [12]. Serum HBcrAg level correlates with nuclear cccDNA activity, and could be used to predict HBeAg seroconversion, sustained treatment response to NA, and the risk of development or recurrence of HCC [13-16]. Prior studies have demonstrated that serum HBcrAg level at the time of NA cessation is useful for predicting off-therapy relapse and identifying patients for whom NA discontinuation may be potentially suitable [17-19]. However, the levels of HBcrAg can change after cessation of treatment, and it is unclear whether dynamic measurements of HBcrAg off-therapy may better predict subsequent CR than the fixed HBcrAg level measured at end of treatment (EOT). Furthermore, no studies have yet compared the kinetics of HBV viral markers post-treatment to their association with clinical relapse risk following NA discontinuation. To address this knowledge gap, our study retrospectively analyzed data and biospecimens from a multicenter cohort of prospectively enrolled patients, serially measuring HBcrAg, HBsAg, and HBV DNA levels after therapy cessation. Our aim was to elucidate the relationship between the dynamic changes in these markers off-therapy and the subsequent CR after stopping NA.
MATERIALS AND METHODS
Study design and setting
We retrospectively analyzed data and assayed serum collected from a multicenter cohort of patients with CHB who were prospectively followed up after discontinuation of NA treatment. The protocol was previously reported in detail [19,20]. Patients were prospectively enrolled from the E-Da Hospital (Kaohsiung, Taiwan), the Lotung Poh-Ai Hospital (Yilan, Taiwan), and the National Taiwan University Hospital Yun-Lin Branch (Yunlin, Taiwan) between July 1, 2011 and January 31, 2022. All participants provided signed informed consent before enrollment and agreed to the storage of their blood samples for subsequent studies. The present study was approved by the institutional review boards of the E-Da Healthcare System (EMRP-110-100).
Study participants
Patients older than twenty years who had been diagnosed with CHB for longer than six months before treatment, who had continuously received either entecavir or tenofovir for at least two years, and who had undetectable HBV DNA and negative HBeAg at EOT were eligible for this analysis. Patients with detectable HBV DNA or positive HBeAg at discontinuation of NA, liver cirrhosis diagnosed either by histology or clinical features, co‐infection with hepatitis C virus or human immunodeficiency virus, ascites, hepatic encephalopathy, variceal bleeding, malignancy, those who were organ transplant recipients, or who had received cytotoxic or immunosuppressive agents were excluded.
Patients enrolled in this study had to discontinue NA in accordance with the national health insurance policy of Taiwan. Details of the reimbursement rules were previously reported [2]. Briefly, patients who were HBeAg‐negative before treatment had to discontinue antiviral therapy after a maximum treatment duration of three years, but patients who were HBeAg‐positive before treatment could maintain antiviral therapy until one year after HBeAg seroconversion. Patients could opt to pay for NA therapy themselves when their treatment period ended.
Laboratory examinations, including quantification of serum HBcrAg
The study baseline measurements at cessation of NA included the patients’ demographic, biochemical, serological, and virological data. Quantification of HBV DNA was performed using polymerase chain reaction (COBAS TaqMan HBV Test, version 2.0; Roche Molecular Systems, Inc., Alameda, CA, USA); the range of detection was 20 to 1.7×108 IU/mL. Serum levels of HBsAg were quantified using a micro‐particle immunoassay (Abbott Architect i2000; Abbott Laboratories, Abbott Park, IL, USA). The upper limit of automated quantification was 250 IU/mL. Serum samples obtained at EOT, one and two years after treatment cessation that had been stored at –80°C, were used to analyze the dynamic changes in HBcrAg. Serum HBcrAg was quantified using the Lumipulse G HBcrAg assay on a Lumipulse G1200 Analyzer (Fujirebio Inc., Sagamihara Facility, Japan). The detection range varied from 1,000 to 107 U/mL. Samples with either HBsAg or HBcrAg levels higher than the upper limits of automatic detection were manually diluted to enable precise quantification.
Follow‐up and definitions of outcomes after cessation of NA
After discontinuation of NA, patients were followed up every three months. Follow-up included a physical health check and blood examinations, such as serum biochemistry, HBeAg, antibody to hepatitis B e antigen (anti-HBe), and HBV DNA, depending on the patient’s clinical situation at the time of the visit. Patients also underwent an abdominal ultrasound and serum alpha-fetoprotein tests every six months for HCC surveillance. Occurrence of CR was the primary study outcome and was defined as elevation of serum alanine transaminase (ALT) two times above the upper limit of the normal range (ULN) plus serum HBV DNA higher than 2,000 IU/mL. The ULN of serum was set at 40 U/L in accordance with the Asian-Pacific guidelines [21]. The clinical outcomes of all patients were monitored until they restarted antiviral treatment, were lost to follow‐up, or after January 31, 2022. The annual measurement of serum HBcrAg and HBsAg continued until either the occurrence of a CR or the end of the study. The temporal relationship between repeated measurement of serum HBcrAg levels and CR are shown in Supplementary Figure 1. According to the national health insurance policy in Taiwan, patients did not restart antiviral therapy for either virological relapses or transient hepatitis flares. NA was resumed for persistent hepatitis, defined as an elevation of serum ALT two times above ULN for at least three months and concerns of hepatic decompensation, with serum total bilirubin >2 mg/dL or prolonged prothrombin time >3 seconds.
Statistical analyses
Descriptive results for categorical and continuous variables are expressed as exact numbers with percentages and medians with interquartile ranges (IQR), respectively.
HBcrAg was imputed as 500 U/mL for statistical analysis if serum HBcrAg level was lower than 1,000 U/mL. Serum levels of HBsAg (IU/mL) and HBcrAg (U/mL) were transformed logarithmically for analysis. HBcrAg levels were also analyzed as a categorical variable above or below 1,000 U/mL. The cumulative incidences of CR were estimated by the Kaplan–Meier method and comparisons among different patient subgroups were performed using the log‐rank test. Cox proportional hazard regression was used to identify potential risk factors. We analyzed the dynamic changes in HBcrAg, HBsAg, and HBV DNA levels at the EOT, and one and two years posttreatment cessation, as time-varying variables. Time-dependent models were employed to assess the association of these viral markers’ changes over time with the risk of CR. We applied the stepwise approach to eliminate factors that were not statistically significant during variable selection. For comparative purposes, however, we deliberately included the EOT levels of HBcrAg and HBsAg, as well as their time-varying levels, in the multivariable model, irrespective of their statistical significance. Hazard ratios (HR) were reported along with a 95% confidence interval (CI). All statistical examinations were two sided and P-values less than 0.05 were defined as statistically significant. All statistical analyses were performed using IBM SPSS Statistics for Windows, Version 25.0 (IBM Co., Armonk, NY, USA), except for time-dependent Cox regression model which was generated using R (version 4.1.3; R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Baseline characteristics of the study participants
This study included 156 male (76.8%) and 47 female (23.2%) patients with CHB who discontinued NA, with the median age of 49.8 years (IQR, 41.9–59.0). Most patients (n=123, 60.6%) received entecavir and the median duration of treatment was 36.9 months (IQR, 36.5–40.1). The EOT serum HBsAg was 2.7 (IQR, 2.0–3.0) log IU/mL and the EOT serum HBcrAg was 3.0 (IQR, 2.0–3.9) log U/mL, respectively. The median follow‐up time was 31.7 months (IQR, 16.7–67.1) (Table 1).
CR after discontinuation of NA and association with EOT HBcrAg level
During the follow‐up period, CR occurred in 104 patients with a cumulative incidence of 49.5% (95% CI, 42.2–56.7%), 54.8% (95% CI, 47.1–62.4%) and 58.2% (95% CI, 50.1–66.0%) at 3, 5, and 7 years, respectively (Fig. 1). Eight patients experienced hyperbilirubinemia with serum total bilirubin over 2 mg/dL after CR, but recovered fully after resumption of NA therapy.

The cumulative incidence of clinical relapse following discontinuation of nucleos(t)ide analogues in the study population.
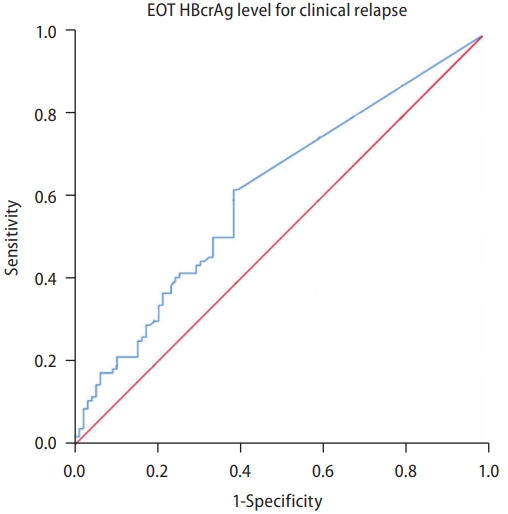
The lower limit of HBcrAg detection in the current study was 1,000 U/mL. This value has been used as the cutoff as suggested by the Japan Society of Hepatology (JSH) [22]. The incidence of CR was significantly higher with the EOT HBcrAg ≥1,000 U/mL than <1,000 U/mL (P=0.002) (Fig. 2). In the univariable analysis, the HR for CR was 1.30 (95% CI, 1.10–1.53) per log U/mL (P=0.005) for the EOT HBcrAg (Table 2). In the receiver operating characteristic curve designed to evaluate the performance of EOT HBcrAg, however, the EOT HBcrAg level alone could not sufficiently predict the risk of CR, with an AUC of 0.61 (95% CI, 0.53–0.69) (Fig. 3).
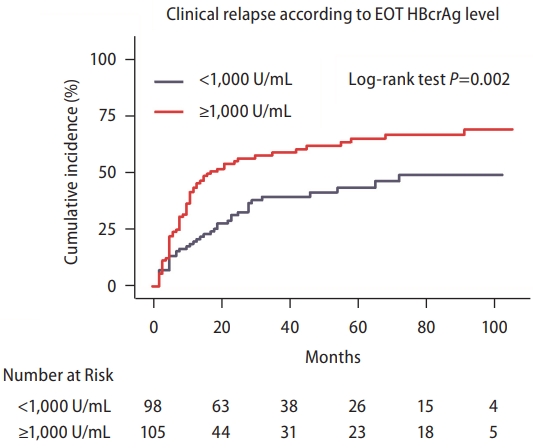
The cumulative incidence of clinical relapse according to serum level of hepatitis B core-related antigen measured at the end of treatment. EOT, end-of-treatment; HBcrAg, hepatitis B core‐related antigen.

Univariable and multivariable time-dependent Cox proportional hazard models for the risk of clinical relapse
Changes in serum HBcrAg in post-treatment monitoring and the associations with CR
After stopping treatment, 114 and 71 patients remained off NA after the first and second years. Their posttreatment fluctuations in HBcrAg levels were illustrated in relation to the occurrence of CR (Fig. 4). At treatment cessation (Fig. 4A), 59.6% (n=59) of patients without CR (n=99) had HBcrAg levels below 3 log U/mL, compared to 37.5% (n=39) of patients with CR (n=104). At one year post NA cessation, these figures were 67.1% (n=53 out of 79) and 54.3% (n=19 of 35), respectively (Fig. 4B), and the corresponding figures at two years were 68.4% (n=39 of 57) and 35.7% (n=5 of 14), respectively (Fig. 4C). On the other hand, a higher proportion with HBcrAg levels above 4 log U/mL was consistently observed in patients with CR at all time points: 29.8% (n=31 of 104) at cessation, 11.4% (n=4 of 35) at one year, and 14.3% (n=2 of 14) at two years, compared to 18.2% (n=18 of 99), 6.3% (n=5 of 79), and 5.3% (n=3 of 57), respectively for those without CR.
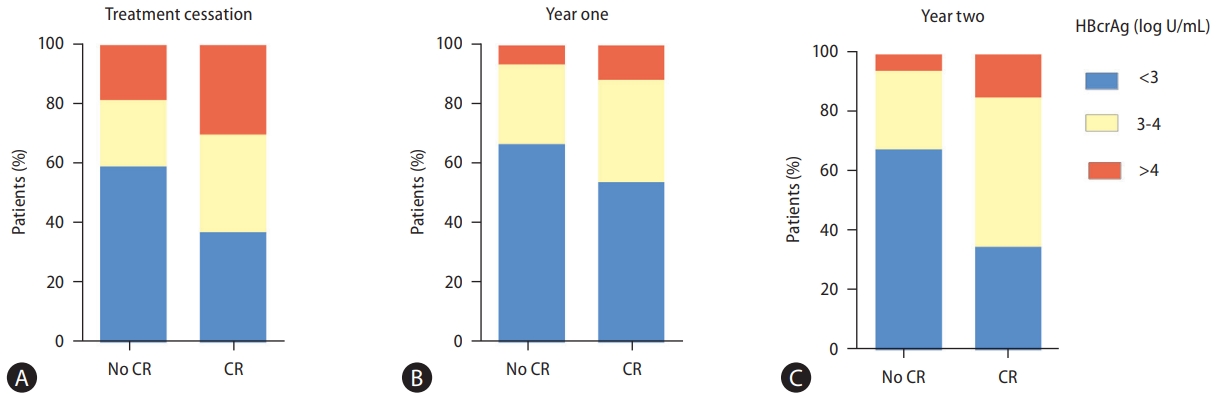
The bar charts illustrates the proportion of serum HBcrAg levels <3 log U/mL, 3–4 log U/mL, >4 log U/mL measured at treatment cessation (A), one year afterwards (B), and two years afterwards (C) in all patients, categorized by the occurrence or absence of clinical relapse. HBcrAg, hepatitis B core‐related antigen; CR, clinical relapse.
The proportions of patients with different HBV DNA and HBsAg levels in relation to subsequent CR at various time points were illustrated in Supplementary Figures 2 and 3, respectively.
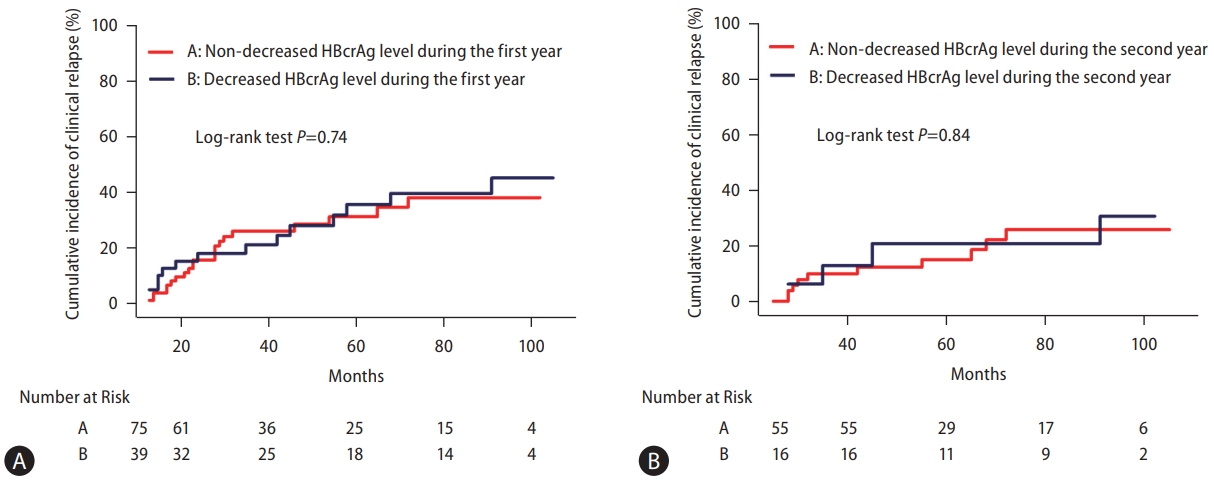
We further clarified the pattern of posttreatment changes in HBcrAg and explored the association with CR. Among the 114 patients who did not resume antiviral therapy at year one, serum HBcrAg decreased in 39 patients (34.2%), did not change in 62 patients (54.4%) and increased in 13 patients (11.4%). The incidence of subsequent CR did not differ between patients with and without decrease in HBcrAg in the first year (P=0.74; Fig. 5A). During the second year, serum HBcrAg decreased in 16 (22.5%), did not change in 41 (57.8%), and increased in 14 patients (19.7%). Similarly, there were no difference in the risk of CR between patients with and without HBcrAg decreases in year two (P=0.84; Fig. 5B).
Time-dependent Cox models to examine posttreatment HBcrAg level as an independent risk factor for CR
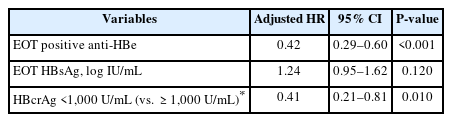
In the univariable analysis, male sex, EOT HBsAg, timevarying HBsAg, EOT HBcrAg, time-varying HBcrAg, timevarying HBV DNA, and EOT ALT level were linked to a higher risk of CR, while longer therapy duration was associated with a lower risk (Table 2). In the multivariable model, the posttreatment time-varying HBcrAg level remained a significant predictor of CR, with an adjusted hazard ratio (aHR) of 1.53 per log U/mL (95% CI: 1.12–2.08), after adjusting for EOT HBsAg, time-varying HBsAg, and EOT HBcrAg, alongside factors that were selected according to statistical significance. In addition to time-varying HBcrAg, independent predictors of CR in the fully adjusted model included EOT anti-HBe positivity (aHR: 0.19; 95% CI: 0.10–0.37) and EOT HBsAg level (aHR: 2.47 per log IU/mL; 95% CI: 1.28–4.77).
For practical clinical use, the association between posttreatment HBcrAg levels and CR risk was further evaluated using a dichotomous approach, with 1,000 U/mL as the cutoff. In the time-dependent multivariable Cox model adjusted for anti-HBe positivity and EOT HBsAg (Table 3), posttreatment HBcrAg levels below 1,000 U/mL (as compared to 1,000 U/mL or above) were associated with a significantly lower risk of CR, with an aHR of 0.41 (95% CI: 0.21–0.81).
DISCUSSION
In this multicenter cohort study, we found that dynamic HBcrAg levels were more predictive of CR than static EOT HBcrAg and dynamic HBsAg levels in CHB patients discontinuing entecavir or tenofovir, although the posttreatment change in HBcrAg was generally mild and the pattern (i.e., with or without decrease during the preceding year) was not independently associated with CR. We further validated the results were consistent with posttreatment levels of HBcrAg set < or ≥1,000 U/mL, a convenient cutoff that can be easily applied in clinical practice. These findings implicate that dynamic measurement of serum HBcrAg may inform posttreatment monitoring in CHB patients who stop NA therapy.
Previous studies have shown that lower HBsAg or HBcrAg level at EOT were associated with lower rates of relapse after discontinuation of NA [17,20]. Sonneveld et al. [23] reported in a multicenter study with a median follow-up of 295 weeks that a lower EOT HBcrAg level was related to better outcomes after cessation of NA, including sustained virologic response, HBsAg loss, and a lower ALT flare rate. Other studies suggested that a combination of the EOT HBcrAg level with either HBV RNA or HBsAg level at EOT may be an acceptable predictor of off-therapy relapse [19,24,25]. In the present study, we also found the incidence of CR was significantly lower among patients with HBcrAg <1,000 U/mL at EOT. Despite the significant association with CR, EOT HBcrAg level alone is not accurate enough, as indicated by its AUC of 0.61 (95% CI, 0.53–0.69) in our study.
The serum levels of HBsAg and HBcrAg may change over time following NA cessation, and it has been unclear whether dynamic measurements may add to the accuracy of prediction for subsequent CR. In our prior study with 140 patients, we reported that the time-varying gradient of serum HBsAg was associated with both clinical and virological relapse [26]. In the current study, we established for the first time that dynamic HBcrAg levels are superior predictors of CR compared to static EOT HBcrAg levels and dynamic HBsAg levels (Table 2).
Currently, only serum HBV DNA and ALT levels are recommended in guidelines for post-treatment monitoring [27]. However, elevation of serum ALT is actually a marker of liver injury that already occurs and does not serve to forecast. With regards to serum HBV DNA, viremia typically relapses within months and can rapidly fluctuate. Therefore, it is recommended that serum HBV DNA should be frequently measured, such as monthly measurement for the first three months and then bi-monthly or tri-monthly measurements in the first year, barring relapses [27]. In contrast, serum HBsAg and HBcrAg levels generally do not undergo sudden changes in most patients and can stratify patients at different risks of relapses that may not be imminent. Thus, serum HBcrAg may serve as a complementary biomarker to serum HBV DNA in the posttreatment monitoring of patients stopping NA therapy. Further research is warranted to elucidate how HBcrAg and HBV DNA may be combined together in clinical practice.
While serum HBcrAg levels may change over time following NA cessation, we found the fluctuation was generally mild during the first and second years off-NA therapy. In fact, the serum level of HBcrAg was stable without marked increase or decrease in most patients. Accordingly, the lack of association between the pattern of changes in serum HBcrAg and CR might not be surprising given that the interval difference was mild for most patients. This finding suggested that cessation of NA therapy did not significantly affect the transcriptional activity of HBV cccDNA but more studies, preferably with quantitative analysis of viral expression in the hepatocytes, are needed to fully understand the biological underpinning.
The strengths of our study are as follows: First, HBcrAg kinetics during the off-therapy period were assessed using serum HBcrAg measurements regularly obtained three times according to a prespecified protocol. Second, the participants were prospectively enrolled from multiple sites and the concern of selection bias commonly observed in retrospective analyses and/or single-center experience could be mitigated. Third, patients who took either entecavir or tenofovir were included, which reflects the current standard of care. Although the patterns of relapse for patients on these two regimens differ [28], the design of this study more closely reflects daily clinical practice. Fourth, the follow-up period was sufficiently long enough to observe the outcomes after cessation of NA. Even though the incidence of hepatitis flares was high, and many patients resumed NA and discontinued observation, more than one-third of the patients (n=74, 36.5%) in this cohort remained at risk of CR after three years of follow‐up. Finally, our multivariable Cox proportional hazard analysis was developed with the adjustment for EOT HBsAg level and EOT HBcrAg level that were important factors associated with subsequent CR after NA cessation.
There are several limitations to this study. First, due to the health insurance policy in Taiwan, the present study enrolled patients with a median treatment duration of 36.9 months (IQR, 36.5–40.1) [2]. The small range of the treatment duration may lead to selection bias, and we could not examine the association between the duration and outcome of treatment. Second, we could not precisely quantify serum HBcrAg levels when HBcrAg levels were lower than 1,000 U/mL. However, the same commercial assay was employed in several prior studies, and is widely used in the clinic [29]. Third, this cohort only included Asian patients and the most common HBV genotypes were type B or C [30]. Finally, we could not exactly investigate the association between the relapse risks and the different patterns of patients with non-decreased HBcrAg levels because of the limited number of patients who remained untreated. Therefore, future studies of more participants with different ethnicities, HBV genotypes, and NA treatment durations are required to validate our findings.
In conclusion, this study revealed that the dynamic measurements of serum HBcrAg after cessation of NA outperformed the EOT HBcrAg level and was more accurate than the dynamic HBsAg level, as an independent predictor for subsequent CR. HBcrAg level <1,000 U/mL could be a useful cutoff value to forecast a low risk of subsequent CR during the off-therapy follow-up. These finding may help to design a safer monitoring strategy for patients who discontinue NA and may inspire further research to optimize the finite strategy of NA therapy.
Notes
Authors’ contribution
Guarantor of the article: Ying-Nan Tsai, Yao-Chun Hsu. Concept: Ying-Nan Tsai, Yao-Chun Hsu. Design: Ying-Nan Tsai, Yao-Chun Hsu. Data collection: Cheng-Hao Tseng, Tzu-Haw Chen, Chieh-Chang Chen, Yu-Jen Fang, Tzeng-Huey Yang, Jaw-Town Lin, Yao-Chun Hsu. Data analysis: Ying-Nan Tsai, Jia-Ling Wu, Yao-Chun Hsu. Data interpretation: Ying-Nan Tsai, Jia-Ling Wu, Cheng-Hao Tseng, Yi-Ling Wu, Mindie H. Nguyen, Yao-Chun Hsu. Manuscript drafting: Ying-Nan Tsai. Manuscript edition and final approval: all authors.
Conflicts of Interest
The authors have no conflicts to disclose.
Acknowledgements
The authors are grateful to our colleagues who treated the study participants. This work was funded by E-Da Hospital (EDCHP111007), the National Science and Technology Council in Taiwan (110-2314-B-214 -006 -MY3), and the Tomorrow Medical Foundation (112-1). The authors declare no conflict of interest relevant to the study.
Abbreviations
ALT
alanine transaminase
anti-HBe
hepatitis B e antibody
CHB
chronic hepatitis B
CI
confidence interval
CR
clinical relapse
EOT
end of treatment
HBcrAg
hepatitis B core-related antigen
HBeAg
hepatitis B e antigen
HBsAg
hepatitis B surface antigen
HBV
hepatitis B virus
HCC
hepatocellular carcinoma
HR
hazard ratio
IQR
interquartile range
NA
nucleos(t)ide analogs
SUPPLEMENTAL MATERIAL
Supplementary material is available at Clinical and Molecular Hepatology website (http://www.e-cmh.org).
The different patterns of the temporal relationship between repeated measurement of serum hepatitis B corerelated antigen (HBcrAg) and clinical relapse (CR). (A) End of treatment HBcrAg level predicted CR within one year. (B) HBcrAg level at one year predicted CR between one and two years. (C) HBcrAg level at two year predicted CR after two years. (D) CR did not happen during the period of study.
The proportions of patients with serum HBV DNA levels <3 log IU/mL, 3–4 log IU/mL, and >4 log IU/mL by the absence or presence of clinical relapses were respectively 100% vs. 100%, 0 vs. 0, and 0 vs. 0 at treatment cessation (A), 68.4% (n=54) vs. 45.7% (n=16), 17.7% (n=14) vs. 20.0% (n=7), and 13.9% (n=11) vs. 34.3% (n=12) at the first year (B), and 63.2% (n=36) vs. 42.9% (n=6), 24.5% (n=14) vs. 42.9% (n=6), and 12.3% (n=7) vs. 14.2% (n=2), respectively, at the second year (C). HBV, hepatitis B virus; CR, clinical relapse.
The proportions of patients with serum HBsAg <2 log IU/mL, 2–3 log IU/mL, >3 log IU/mL as categorized by absence or presence of subsequent clinical relapse were respectively 42.4% (n=42) vs. 11.5% (n=12), 36.4% (n=36) vs. 54.8% (n=57), and 21.2% (n=21) vs. 33.7% (n=35) at treatment cessation (A), 44.3% (n=35) vs. 11.4% (n=4), 34.2% (n=27) vs. 51.4% (n=18), and 21.5% (n=17) vs. 37.2% (n=13) at one year (B), and 47.4% (n=27) vs. 14.3% (n=2), 36.8% (n=21) vs. 57.1% (n=8), and 15.8% (n=9) vs. 28.6% (n=4), respectively at the two years (C). HBsAg, hepatitis B surface antigen; CR, clinical relapse.
References
Article information Continued
Notes
Study Highlights
• Posttreatment HBcrAg levels more accurately predict clinical relapse following discontinuation of entecavir or tenofovir than end-of-treatment HBcrAg and dynamic HBsAg levels do
• An HBcrAg level below 1,000 U/mL during the posttreatment follow-up indicates a low risk of subsequent clinical relapse
• Changes in HBcrAg levels after treatment in CHB patients are typically minor and the patterns of changes are not independently linked to clinical relapse risk.