| Clin Mol Hepatol > Volume 30(2); 2024 > Article |
|
See the commentary-article "Clinical impact of five cardiometabolic risk factors in metabolic dysfunction-associated steatotic liver disease (MASLD): Insights into regional and ethnic differences" on page 168.
See the letter "Correspondence on Letter regarding “Prognosis of biopsy-confirmed MASLD: A sub-analysis of the CLIONE study”" on page 281.
See the letter "Correspondence on Letter regarding “Prognosis of biopsy-confirmed MASLD: A sub-analysis of the CLIONE study”" on page 281.
ABSTRACT
Background/Aims
Metabolic dysfunction-associated steatotic liver disease (MASLD) was recently proposed as an alternative disease concept to nonalcoholic fatty liver disease (NAFLD). We aimed to investigate the prognosis of patients with biopsy-confirmed MASLD using data from a multicenter study.
Methods
This was a sub-analysis of the Clinical Outcome Nonalcoholic Fatty Liver Disease (CLIONE) study that included 1,398 patients with NAFLD. Liver biopsy specimens were pathologically diagnosed and histologically scored using the NASH Clinical Research Network system, the FLIP algorithm, and the SAF score. Patients who met at least one cardiometabolic criterion were diagnosed with MASLD.
Results
Approximately 99% of cases (n=1,381) were classified as MASLD. Patients with no cardiometabolic risk (n=17) had a significantly lower BMI than patients with MASLD (20.9 kg/m2 vs. 28.0 kg/m2, P<0.001), in addition to significantly lower levels of inflammation, ballooning, NAFLD activity score, and fibrosis stage based on liver histology. These 17 patients had a median follow-up of 5.9 years, equivalent to 115 person-years, with no deaths, liver-related events, cardiovascular events, or extrahepatic cancers. The results showed that the prognosis for pure MASLD was similar to that for the original CLIONE cohort, with 47 deaths and one patient who underwent orthotopic liver transplantation. The leading cause of death was extrahepatic cancer (n=10), while the leading causes of liver-related death were liver failure (n=9), hepatocellular carcinoma (n=8), and cholangiocarcinoma (n=4).
Conclusions
Approximately 99% of NAFLD cases were considered MASLD based on the 2023 liver disease nomenclature. The NAFLD-only group, which is not encompassed by MASLD, had a relatively mild histopathologic severity and a favorable prognosis. Consequently, the prognosis of MASLD is similar to that previously reported for NAFLD.
Graphical Abstract
The main shortcomings of the terms nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH) are their dependence on exclusionary confounding factors and the potential use of stigmatizing language. Three large pan-national liver associations (AASLD, EASL, and ALEH) announced the official development and finalization of a new liver disease nomenclature in June 2023 [1]. The term “steatotic liver disease (SLD)” was selected as a comprehensive label to encompass the different causes of steatosis [2], and NAFLD has been renamed metabolic dysfunction-associated steatotic liver disease (MASLD) [3]. MASLD includes patients with hepatic steatosis and at least one of five cardiometabolic risk factors. Furthermore, metabolic dysfunction-associated steatohepatitis (MASH) is an alternative term for NASH [1,4].
Song et al. recently found little difference in the prevalence of NAFLD (25.7%) and MASLD (26.7%) in a random subset of 1,016 community subjects from Hong Kong who were examined using proton-magnetic resonance spectroscopy [5]. Hagström et al. reported that 99% of patients with biopsy-confirmed NAFLD (n=1,333) had MASLD (n=1,329) based on cardiometabolic criteria. The 10-year mortality rates for patients with NAFLD and MASLD were 10.4% and 10.3%, respectively [6]. Nonetheless, this well-documented liver biopsy case report originates from Sweden, one of the Nordic countries. The Asian-based study Clinical Outcomes in Nonalcoholic Fatty Liver Disease (CLIONE) found that Westerners and Asians have significantly different NAFLD prognoses [7]. In this study, we examined the prognosis of pure MASLD as a subanalysis of the CLIONE study.
We utilized a dataset from the CLIONE study carried out in Asia. Detailed information on how this cohort was formed and the primary findings of the CLIONE study can be found in a separate publication [7]. This study was approved by the Institutional Review Board of Saga University Hospital (approval no. 2020-04-R-02; June 30, 2020), which waived the requirement for informed consent due to the use of preexisting data. This study adhered to the reporting guidelines provided by Strengthening the Reporting of Observational Studies in Epidemiology (STROBE).
We identified all patients who received a diagnosis of biopsy-confirmed NAFLD from December 1, 1994, to December 31, 2020. We tracked this cohort’s progress until March 31, 2021, to determine clinical outcomes related to hepatocellular carcinoma (HCC). Patients with excessive alcohol consumption (>30 g/day for men; >20 g/day for women) and those with liver disease caused by viral hepatitis, autoimmune hepatitis, drug-induced liver disease, primary biliary cholangitis, or biliary obstruction were excluded from the study.
We collected data on several factors including body mass index (BMI), blood pressure, daily alcohol consumption, smoking habits, past medical history, and current medication usage. Blood samples were collected after an overnight fast of at least 8 hours to measure plasma glucose, lipids, and liver-related biochemistry values. Diagnoses of type 2 diabetes mellitus (DM), hypertension, and dyslipidemia were based on established diagnostic criteria [10-12]. Cardiometabolic (CM) criteria in the MASLD definition are as follows: i) BMI ≥23 kg/m² (for Asian populations); ii) insulin resistance, defined as fasting blood glucose ≥100 mg/dL, HbA1C ≥ 5.7%, diagnosis of DM, or treatment for DM; iii) high blood pressure indicated by blood pressure ≥130/85 mmHg or the use of antihypertensive medication; iv) elevated triglycerides, with plasma triglyceride levels ≥150 mg/dL or the use of lipid-lowering medication; and v) dyslipidemia, characterized by plasma HDL-cholesterol levels ≤40 mg/dL (for males) and ≤50 mg/dL (for females) or the use of lipid-lowering treatment. Cardiometabolic criteria are considered met when at least one of the above five criteria is met [1].
Liver biopsy samples were acquired percutaneously with the guidance of ultrasound. Formalin-fixed and paraffin-embedded liver sections were stained using hematoxylin and eosin or azan before being sent for centralized evaluation. NAFLD was defined as the presence of ≥5% hepatic steatosis, following the criteria established by Kleiner and colleagues [13]. Grading and staging were based on the NAFLD activity score (NAS) of the NASH Clinical Research Network system (NASHCRN) according to Brunt et al. [14] and Kleiner et al. [13] by an experienced pathologist (S.A.) at Saga University, who was blinded to the patients’ clinical and laboratory data. MASH was diagnosed according to steatosis activity fibrosis (SAF) and the fatty liver inhibition of progression (FLIP) algorithms [15]. In this study, active MASH was defined as NAS of 4 or higher and a fibrosis stage of 2 or higher.
The follow-up period commenced on the date of biopsy and extended until the date of the last visit, patient death, or receipt of an orthotopic liver transplantation. Patients were monitored at intervals ranging from 3 to 12 months following NAFLD diagnosis, with repeated measurements of anthropometric parameters and metabolic assessments during each follow-up visit. For liver-related events, which encompassed the composite endpoint of gastroesophageal varices/bleeding, HCC, or decompensated cirrhosis, only the initial occurrence of these events following liver biopsy was considered. Recurring cases were not included in the analysis. In addition, we continued to record other cancer events, cardiovascular diseases (coronary artery disease, heart failure, arrhythmia), and stroke. The definition of cardiovascular disease was taken from our previous report [7]. In cases of hospitalizations, we recorded the diagnosis upon admission. Additionally, we recorded the date and cause of death for all individuals. The duration of follow-up was determined as the time from the date of the biopsy to the date of the most recent follow-up.
Continuous and ordinal variables are expressed as the mean (standard deviation [SD]) and were compared using the unpaired t-test. Some data are expressed as number (%). Categorical variables were compared using Fisher’s exact test. Clinical outcomes (overall mortality and liver-related events) are presented as Kaplan–Meier curves. Presence or absence of cardiometabolic criteria, histologic features such as fibrosis stage, presence of MASH based on the FLIP algorithm, and high or low cumulative number of cardiometabolic risk factors were compared using the log-rank test. Two-sided P-values <0.05 were considered significant. All statistical tests, except those for spline curve creation, were conducted using JMP® 17.2.0 software (SAS Institute Inc., Cary, NC, USA).
Cardiometabolic criteria were met by 1,381 of the 1,398 patients in the CLIONE cohort, which accounts for 98.8% of the total cohort (Table 1). Table 2 describes the differences in clinical characteristics according to the presence or absence of cardiometabolic criteria. Patients with cardiometabolic criteria (n=1,381) had a significantly higher BMI (28.0 vs. 20.9 kg/m2, P<0.001), TG level (159 vs. 107 mg/dL, P<0.029), and FBS (114 vs. 86.6 mg/dL, P=0.002) than those without and had significantly lower HDL-C levels (49.6 vs. 59.9 mg/dL, P=0.009). Table 3 shows the differences in pathological features according to the presence or absence of cardiometabolic criteria. Inflammation, ballooning, NAFLD activity score, fibrosis stage, and the proportion of MASH and active MASH were significantly higher in patients with cardiometabolic criteria than in those without.
Median follow-up duration of patients with pure MASLD (n=1,381) was 4.6 years (range: 0.3–21.6 years), for a total of 8,759 person-years. The number of patients lost to follow-up was 717 (51.3%) at 5 years, 284 (20.3%) at 5–10 years, and 257 (18.4%) at 10–15 years. Kaplan-Meier curve analysis of allcause mortality and liver-related events, stratified by the presence or absence of cardiometabolic criteria in NAFLD patients, is presented in Figure 1. The Kaplan–Meier curves of overall mortality and liver-related events stratified by fibrosis stage and presence of MASH are presented in Figure 2. When patients were divided into four groups by fibrosis stage, overall survival and liver-related event-free rates were significantly stratified (log-rank P=0.009 and P<0.001, respectively). Furthermore, when patients were divided into two groups by the presence of MASH, overall survival and liver-related event-free rates were significantly stratified (log-rank P=0.007 and P<0.001, respectively).
In Supplementary Figure 1, CM risk factors had no effect on long-term prognosis, including mortality or the incidence of liver-related events, when the cumulative number of risk factors was divided into low (0–2) and high (3–5) groups.
In contrast, the 17 patients who did not meet CM criteria had a median follow-up duration of 5.9 years (range, 0.2–16.7 years), equivalent to 115 person-years, without death, liver-related events, cardiovascular events, or extrahepatic cancer. Therefore, the number of deaths and causes of death in patients with pure MASLD (n=1,381) were the same as those reported in the original CLIONE study. During the observation period, 47 patients with pure MASLD died and one patient underwent orthotopic liver transplantation. The primary cause of death was extrahepatic cancer (n=10). The leading liver-related causes of death were liver failure (n=9), HCC (n=8), and cholangiocellular carcinoma (n=4) (Supplementary Table 1). Five-year and 10-year mortality rates were 1.9% and 4.9%, respectively. Finally, during the observation period, 77 new cases of liver-related events, 51 new cases of cardiovascular disease, and 21 new cases of stroke occurred (Supplementary Table 2). Finally, the types of new cancers observed during follow-up are were hepatocellular carcinoma (n=37), breast cancer (n=16), and stomach cancer (n=10) (Supplementary Table 3).
Analysis of a subset of data from the CLIONE study revealed that approximately 99% of NAFLD cases could be classified as MASLD cases. The NAFLD-only group that did not meet the MASLD criteria exhibited milder histopathologic severity than the MASLD cohort and had a favorable prognosis. The prognosis of MASLD was similar to that of NAFLD.
Several studies have reported that MASLD is largely synonymous with NAFLD [5,6]. Indeed, as shown in Table 1, about 99% of SLD patients met the CM criteria for MASLD. SLD has a bidirectional association with components of metabolic syndrome (MetS), and type 2 diabetes increases the risk of cirrhosis and related complications [16]. Furthermore, the definition of MAFLD also includes MetS factors [17]. Cardiometabolic criteria do not include high-sensitivity CRP or Homeostatic Model Assessment for Insulin Resistance, which is more realistic for clinical practice. In MAFLD, the group without metabolic factors showed less frequent fibrosis development than the group with metabolic factors [18]. The prevalence of significant fibrosis also increased with the number of metabolic abnormalities [19]. Furthermore, it has been reported that patients with steatotic liver but no metabolic risk factors have a better prognosis than those with metabolic risk factors [20]. In this study, we demonstrated that the NAFLD-only group, which comprised NAFLD patients without CM risk factors that would have resulted in the classification of MASLD, had a milder histologic severity and better prognosis than the MASLD group. Our findings are consistent with those of previous studies. Interestingly, the cumulative number of CM risk factors did not affect long-term outcomes such as mortality or occurrence of liver-related events. Compared to CM risk factors, we believe that the presence or absence of MASH and liver fibrosis have more influence on the long-term prognosis of MASLD.
The cohort we studied had a lower mortality rate than those reported for Western cohorts. One possible reason for the lower mortality in Asian studies is that there are fewer CVD-related deaths in Asia than in the West. In a previous Western cohort of NAFLD, the CVD-related mortality rate in patients with biopsy-proven NAFLD was in the 20% range [21-23], whereas that in our cohort was only 4.2%. The prognosis of MASLD needs to be discussed in the Asian population. Previous reports on the prognosis of MASLD (formerly called NAFLD) indicated that the leading cause of death was cardiovascular disease (CVD) [21-23]. Leung and colleagues followed 307 patients in Hong Kong with biopsy-confirmed MASLD for an average of 49 months [24]. Six patients died during the observation period, but only one was due to cardiovascular disease (ruptured abdominal aortic aneurysm). In Japan, the unadjusted mortality rates for heart diseases per 100,000 people in 2000, 2010, and 2019 were 117, 144, and 163 for men and 116, 155, and 172 for women, respectively. In contrast, the age-adjusted mortality rates for all heart diseases showed a consistent decline from 1995 to 2019 [25]. A well-developed universal health insurance system, high statin adherence rates among high-risk patients, and the use of superior therapeutic devices may contribute to the lower CVD mortality rate in Japan than in Western countries. It should also be noted that the impact on clinical outcomes differs between population-based big data studies and hospital-based biopsy cohort studies. In the Korean health examination big data, the cumulative CVD incidence rate for MASLD was 8.5 per 1,000 person-years [26].
Our study’s key strengths include the large size of the patient cohort; verification of all NAFLD cases via liver biopsy; assessment of biopsy features by a single, experienced liver pathologist to eliminate inter-observer differences; and adoption of well-established scoring systems, such as the FLIP algorithm, for grading and staging liver biopsy features [15].
This study also had some limitations. Many of these limitations are inherent to retrospective studies, including the lack of specific treatment protocols, the lack of follow-up endoscopic evaluations, the lack of tracking of alcohol consumption, the lack of imaging in non-cirrhotic patients, interval censoring, and residual confounding effects. Therefore, the number of liver-related events may have been underestimated in our cohort. Waist diameter could also not be assessed because it was not included in routine clinical observations. Second, we did not assess patatin-like phospholipase domain-containing protein 3 (PNPLA3) polymorphisms, which are more common among Asian than Western populations [27]. PNPLA3 polymorphisms are detected in homozygous forms in approximately 20% of the general Japanese population [28]. Recently, Seko et al. [29] reported the impact of PNPLA3 polymorphisms on liver-related events. In that study, 1,550 Japanese patients with MASLD were followed for a mean of 7.1 years. Multivariate analysis identified the presence of PNPLA3 CG/GG (hazard ratio 16.04, P=0.006) as a predictor of liver-related events. Evaluation of single nucleotide polymorphisms, including PNPLA3, will aid in our understanding of the pathogenesis of SLD. Third, only 17 patients did not meet the CM criteria for MASLD, and although Table 3 shows significant differences in some histopathologic factors, the data should be interpreted with caution since the significance could change if the histologic data changed in even one case. Finally, selection bias may have been present because all participants in this study were diagnosed by liver biopsy.
In conclusion, approximately 99% of NAFLD cases could be classified as MASLD cases, whereas those with NAFLD only who did not meet the MASLD criteria had milder histopathological traits and a more positive outlook. Therefore, the prognosis of MASLD closely resembles that of what was previously referred to as NAFLD. Further studies with a larger number of patients will help clarify the pathogenesis of MASLD and patients with NAFLD-only who do not meet the MASLD criteria.
ACKNOWLEDGMENTS
This research was financially supported by the Japan Strategic Medical Administration Research Center (J-SMARC). The authors are grateful to Ms. Keiko Ota and Ms. Masayo Kitano (Osaka Metropolitan University) for their technical assistance in conducting this REDCap project. The authors would also like to thank Ms. Kozue Tashiro and Ms. Maki Miyahara (Saga University Hospital) and Ms. Miki Noguchi (Kawasaki Medical School) for their technical assistance in creating virtual slides. The authors thank eWorldediting (https://www.eworldediting.com/) for English language editing.
FOOTNOTES
SUPPLEMENTAL MATERIAL
Supplementary material is available at Clinical and Molecular Hepatology website (http://www.e-cmh.org).
Supplementary Table 1.
Causes of death and mortality in the patients with pure MASLD (n=1,381)
Supplementary Table 2.
Major complications in the patients with pure MASLD (n=1,381)
Supplementary Table 3.
Cancer incidence rates in patients with pure MASLD (n=1,381)
Supplementary Figure 1.
Overall mortality and liver-related events stratified according to cumulative number of cardiometabolic risk factors in patients with NAFLD (n=1,398). (A) Overall mortality, and (B) liver-related events according to cumulative number of cardiometabolic risk factors in patients with NAFLD. NAFLD, nonalcoholic fatty liver disease.
Figure 1.
Overall mortality and liver-related events stratified according to cardiometabolic criteria in patients with NAFLD (n=1,398). (A) Overall mortality and (B) liver-related events according to the presence of cardiometabolic criteria. NAFLD, nonalcoholic fatty liver disease.
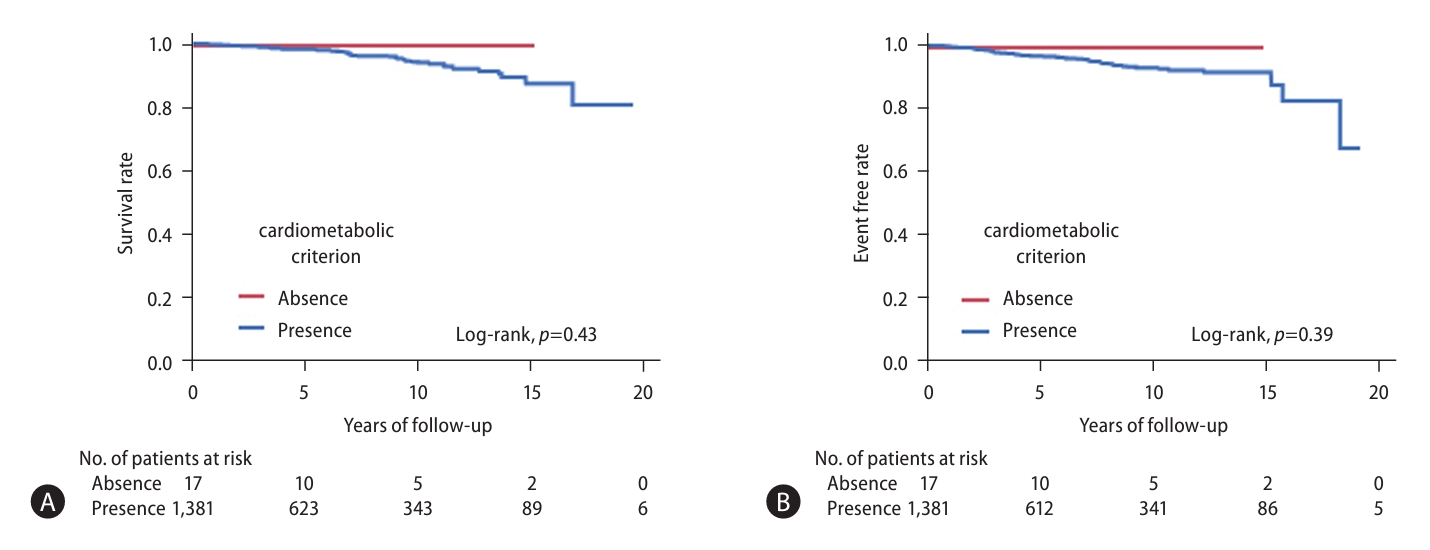
Figure 2.
Overall mortality and liver-related events stratified according to histological characteristics in patients with pure MASLD (n=1,381). (A) Overall mortality and (B) liver-related events according to fibrosis stage. (C) Overall mortality and d, liver-related events according to the presence or absence of MASH. MASH, metabolic dysfunction-associated steatohepatitis; MASLD, metabolic dysfunction-associated steatotic liver disease.
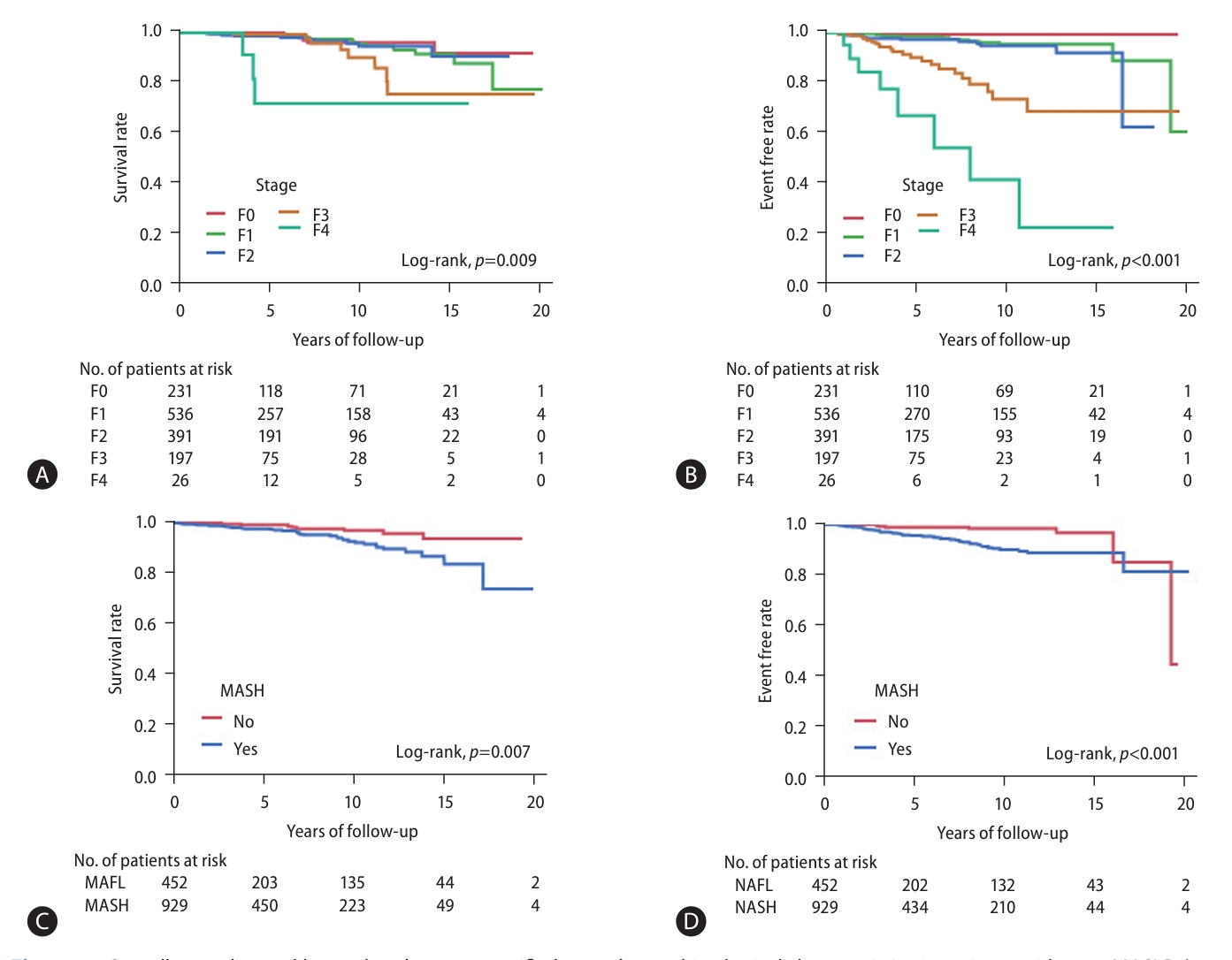
Table 1.
MASLD diagnosis criteria
| Criteria | Number | Percentage |
|---|---|---|
| 1. BMI | 1,224/1,395 | 87.7% |
| 2. Insulin resistance | 1,065/1,294 | 82.3% |
| 3. Blood pressure | 770/1,089 | 70.7% |
| 4. Elevated triglycerides | 577/1,303 | 44.3% |
| 5. Dyslipidemia | 722/1,204 | 51.6% |
| 6. Cardiometabolic criteria* | 1,381/1,398 | 98.8% |
Table 2.
Differences in clinical characteristics in the presence and absence of cardiometabolic criteria (n=1,398)
Values are presented as number (percent).
ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; DM, diabetes mellitus; GGT, gamma-glutamyltransferase; HDL, high-density lipoprotein; Hg, hemoglobin; SD, standard Deviation; TC, total cholesterol; TG, triglycerides.
Table 3.
Differences in pathological features in the presence and absence of cardiometabolic criteria (n=1,398)
| Variables | Cardiometabolic criteria (+) (n=1,381) | Cardiometabolic criteria (–) (n=17) | P-value |
|---|---|---|---|
| Steatosis | |||
| 0* | 8 (0.6) | 0 (0) | 0.20 |
| 1 | 967 (70.0) | 16 (94.1) | |
| 2 | 270 (19.6) | 1 (5.9) | |
| 3 | 136 (9.8) | 0 (0) | |
| Inflammation | |||
| 0 | 65 (4.7) | 4 (23.5) | 0.007 |
| 1 | 870 (63.0) | 12 (70.6) | |
| 2 | 365 (26.4) | 1 (5.9) | |
| 3 | 81 (5.9) | 0 (0) | |
| Ballooning | |||
| 0 | 453 (32.1) | 11 (64.7) | 0.019 |
| 1 | 609 (44.1) | 5 (29.4) | |
| 2 | 329 (23.8) | 1 (5.9) | |
| NAFLD activity score (NAS) | |||
| 1–2 | 272 (19.7) | 11 (64.7) | <0.001 |
| 3–4 | 744 (53.9) | 5 (29.4) | |
| 5–8 | 365 (26.4) | 1 (5.9) | |
| Fibrosis stage (F) | |||
| 0 | 231 (16.7) | 10 (58.8) | <0.001 |
| 1 | 536 (38.8) | 3 (17.7) | |
| 2 | 391 (28.3) | 3 (17.7) | |
| 3 | 197 (14.3) | 1 (5.9) | |
| 4 | 26 (1.9) | 0 (0) | |
| MASH | |||
| No | 452 (32.7) | 11 (64.7) | 0.008 |
| Yes | 929 (67.3) | 6 (35.3) | |
| Active MASH | |||
| NAS≥4+F≥2 | 462 (33.5) | 1 (5.9) | 0.017 |
| Others | 919 (66.5) | 16 (94.1) |
Abbreviations
CI
confidence interval
DM
diabetes mellitus
HCC
hepatocellular carcinoma
HR
hazard ratio
MASH
metabolic dysfunction-associated steatohepatitis
MASLD
metabolic dysfunction-associated steatotic liver disease
NAFLD
nonalcoholic fatty liver disease
NAS
NAFLD activity score
NASH
nonalcoholic steatohepatitis
REFERENCES
1. Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, et al. A multi-society Delphi consensus statement on new fatty liver disease nomenclature. Ann Hepatol 2024;29:101133.

2. Kim GA, Moon JH, Kim W. Critical appraisal of metabolic dysfunction-associated steatotic liver disease: Implication of Janus-faced modernity. Clin Mol Hepatol 2023;29:831-843.




3. Yoon EL, Jun DW. Waiting for the changes after the adoption of steatotic liver disease. Clin Mol Hepatol 2023;29:844-850.




4. AASLD. New NAFLD nomenclature. AASLD web site, <https://www.aasld.org/new-masld-nomenclature>. Accessed 19 Oct 2023.
5. Song SJ, Lai JC, Wong GL, Wong VW, Yip TC. Can we use old NAFLD data under the new MASLD definition? J Hepatol 2024;80:e54-e56.


6. Hagström H, Vessby J, Ekstedt M, Shang Y. 99% of patients with NAFLD meet MASLD criteria and natural history is therefore identical. J Hepatol 2024;80:e76-e77.


7. Fujii H, Iwaki M, Hayashi H, Toyoda H, Oeda S, Hyogo H, et al. Clinical outcomes in biopsy-proven nonalcoholic fatty liver disease patients: A multicenter registry-based cohort study. Clin Gastroenterol Hepatol 2023;21:370-379.


8. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377-381.



9. Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform 2019;95:103208.



10. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2010;33 Suppl 1:S62-S69.




11. Umemura S, Arima H, Arima S, Asayama K, Dohi Y, Hirooka Y, et al. The Japanese society of hypertension guidelines for the management of hypertension (JSH 2019). Hypertens Res 2019;42:1235-1481.

12. Teramoto T, Sasaki J, Ueshima H, Egusa G, Kinoshita M, Shimamoto K, et al. Diagnostic criteria for dyslipidemia. Executive summary of Japan Atherosclerosis Society (JAS) guideline for diagnosis and prevention of atherosclerotic cardiovascular diseases for Japanese. J Atheroscler Thromb 2007;14:155-158.


13. Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005;41:1313-1321.


14. Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol 1999;94:2467-2474.


15. Bedossa P; FLIP Pathology Consortium. Utility and appropriateness of the fatty liver inhibition of progression (FLIP) algorithm and steatosis, activity, and fibrosis (SAF) score in the evaluation of biopsies of nonalcoholic fatty liver disease. Hepatology 2014;60:565-575.


17. Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, et al. A new definition for metabolic dysfunctionassociated fatty liver disease: An international expert consensus statement. J Hepatol 2020;73:202-209.

18. Tsutsumi T, Eslam M, Kawaguchi T, Yamamura S, Kawaguchi A, Nakano D, et al. MAFLD better predicts the progression of atherosclerotic cardiovascular risk than NAFLD: Generalized estimating equation approach. Hepatol Res 2021;51:1115-1128.



19. Yamamura S, Eslam M, Kawaguchi T, Tsutsumi T, Nakano D, Yoshinaga S, et al. MAFLD identifies patients with significant hepatic fibrosis better than NAFLD. Liver Int 2020;40:3018-3030.



20. Nguyen VH, Le MH, Cheung RC, Nguyen MH. Differential clinical characteristics and mortality outcomes in persons with NAFLD and/or MAFLD. Clin Gastroenterol Hepatol 2021;19:2172-2181.e6.


21. Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology 2005;129:113-121.


22. Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 2015;149:389-397.e10.



23. Hagström H, Nasr P, Ekstedt M, Hammar U, Stål P, Hultcrantz R, et al. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J Hepatol 2017;67:1265-1273.


24. Leung JC, Loong TC, Wei JL, Wong GL, Chan AW, Choi PC, et al. Histological severity and clinical outcomes of nonalcoholic fatty liver disease in nonobese patients. Hepatology 2017;65:54-64.



25. Ohira T, Eguchi E, Hayashi F, Kinuta M, Imano H. Epidemiology of cardiovascular disease in Japan: An overview study. J Cardiol 2024;83:191-200.


26. Moon JH, Jeong S, Jang H, Koo BK, Kim W. Metabolic dysfunction-associated steatotic liver disease increases the risk of incident cardiovascular disease: a nationwide cohort study. EClinicalMedicine 2023;65:102292.



27. Carlsson B, Lindén D, Brolén G, Liljeblad M, Bjursell M, Romeo S, et al. Review article: the emerging role of genetics in precision medicine for patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther 2020;51:1305-1320.




- TOOLS
-
METRICS

- ORCID iDs
-
Hideki Fujii

https://orcid.org/0000-0003-1267-9623 - Related articles
-
Reply to correspondence: “Prognosis of biopsy-confirmed MASLD: A sub-analysis of the CLIONE study”




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement1
Supplement1 Print
Print



