The rise of non-invasive tools in the diagnosis of portal hypertension: Validation of the Baveno VII consensus
Article information
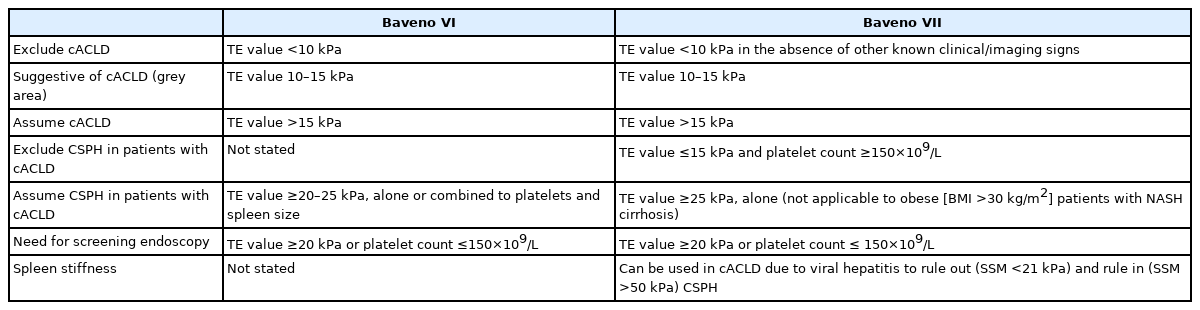
Since its inception in 1986 by professor Robert deFranchis, the Baveno consensus is published every 5 years and has become the world’s most-cited diagnostic guideline for portal hypertension [1]. Significant changes were made in the Baveno VII consensus released in 2021 compared to the 2015 Baveno VI consensus [2,3]. Table 1 is the comparison of the Baveno VI and VII criteria showing the unique features of the Baveno VII. Conditions like compensated advanced chronic liver disease (cACLD) and clinically significant portal hypertension (CSPH) are emphasized in both Baveno VI and VII. However, in Baveno VII, i) hepatic venous pressure gradient (HVPG) measurement and clinical importance were further underscored, ii) usefulness of transient elastography (TE) as a noninvasive test was highlighted, and iii) a cut-off for spleen stiffness to estimate CSPH was presented [2].
The Baveno consensus is based on the results of recent research such as ANTICIPATE study and PREDESCI study [4,5]. However, the criteria of cACLD or CSPH based on a non-invasive test need to be validated in a large cohort to verify the clinical utility of the cut-offs for real-word applications [6,7]. Recently, a study was conducted to validate the recompensation criteria of the Baveno VII [8], but few studies thus far have validated the CSPH criteria based on a non-invasive assessment. At an opportune time, Wong et al. [9] conducted a large-scale multinational study to validate the CSPH criteria based on TE and platelet count in cACLD patients (TE value ≥10 kPa). In this study, CSPH was classified into four groups. First two groups were: i) definite CSPH, TE >25 kPa; ii) excluded CSPH, TE <15 kPa and platelet count ≥150×109/L. If patients do not meet either of the first two criteria, they were classified as grey zone while patients in the grey zone were categorized into two groups; iii) high probability of CSPH, TE value between 20–25 kPa and platelet count <150×109/L, or TE value between 15–20 kPa and platelet count <110×109/L; and iv) low probability of CSPH (remainder of the patients within the grey zone who do not meet the high probability of CSPH condition).
Wong et al. [9] showed that the definite CSPH (TE >25 kPa) criterion can effectively predict liver decompensation and liver-related events. In particular, the fact that any patient with the excluded CSPH condition did not experience decompensation augments the reliability of the exclusion criteria. It is worth noting that the condition of high probability of CSPH was associated with multiple patterns of CSPH-related symptoms depending on etiology. In the grey zone, patients with viral etiology rarely experienced hepatic decompensation, whereas patients with non-viral etiology had a relatively increased risk of hepatic decompensation. This interpretation is based on the fact that all of the viral etiology patients enrolled in the study of Wong et al. [9] were in the state of viral suppression by antiviral treatment. On the other hand, the increased risk of non-viral etiology despite the exclusion of all patients with significant alcohol consumption in the study suggests that the risk of hepatic decompensation in the grey zone may be markedly higher in real clinical practice dealing with cases of active alcoholic cirrhosis. Regarding the grey zone, more empirical research is needed to examine the feasibility of a fine-tuned scheme of CSPH cut-off for each etiology [10]. For a more accurate prognosis of the CSPH among the patients within the grey zone, the spleen stiffness (SS) can be jointly considered in line with the proposition of the recent studies [11,12]. The use of SS was recommended for the first time in the Baveno VII, and SS can be used in viral hepatitis cACLD patients to rule out (SS <21 kPa) and rule in (SS >50 kPa) CSPH. Therefore, if SS values are used in combination with TE and platelet counts, the gray zone condition can be further subdivided. In particular, the results of SS would be promising because the current criteria for CSPH (TE >5 kPa) cannot be used for obese (body mass index >30 kg/m2) patients with nonalcoholic steatohepatitis (NASH)-associated cirrhosis. The costly process of measuring SS in a large number of cACLD patients remains a challenge, though.
The criteria for recommending the non-selective beta-blockers (NSBBs) in the Baveno VII were not clearly defined. In this study, decision curve analysis demonstrated that an overall net benefit of using NSBB in cACLD patients is largest when treating only CSPH (HVPG ≥10 mmHg) rather than treating all varix or the probable CSPH group [9]. Similarly, NSBB was used only in the compensated cirrhosis with CSPH (HVPG ≥10 mmHg) patients in the PREDESCI study [4]. To identify the patient group in which the NSBB is clinically helpful, more studies are needed to further refine the cut-offs [13].
The most notable limitation of this study is that four cohorts with high heterogenicity were pooled [9]. Specifically, the distribution of etiology markedly different across the four countries (i.e., Italy, hepatitis C only; Singapore, hepatitis C dominant; India and China, hepatitis B dominant) is likely to be a confounding factor. Also, patients with active viral hepatitis and active alcohol drinking were excluded and few obese NASH patients were included in the study, potentially limiting the generalizability of the cut-offs for CSPH in a broad range of patients.
The Baveno VII consensus and this study demonstrate the expanding application of TE to the prediction of cACLD, CSPH, and determination of NSBB appropriateness. In medical fields, there are trends of gradually decreasing use of invasive methods such as HVPG and endoscopy, and increasing use of non-invasive approaches [13]. In the future, it is expected that various cut-offs for portal hypertension would be developed in consideration of shear wave elastography and spleen stiffness measurement as well as TE. Although endoscopy is a useful tool for variceal surveillance, it is recommended to patients only when it is inevitable. Compared to other Western countries, endoscopy is easier to access and relatively inexpensive in South Korea due to its unique health care system. That being said, some potential adverse effects of endoscopy such as emission of greenhouse gases [14] can be mitigated by the use of endoscopy for sensibly targeted patients only.
Notes
Authors’ contribution
Jeong-Ju Yoo contributed to write the manuscript. Sang Gyune Kim contributed to study concept and writing.
Conflicts of Interest
The authors have no conflicts to disclose.
Acknowledgements
This study was supported by the Soonchunhyang University Research Fund.
Abbreviations
cACLD
compensated advanced chronic liver disease
CSPH
clinically significant portal hypertension
HVPG
hepatic venous pressure gradient
NASH
nonalcoholic steatohepatitis
NSBB
non-selective beta-blocker
SS
spleen stiffness
TE
transient elastography