Sinusoidal obstruction syndrome after oxaliplatin-based chemotherapy
Article information
INTRODUCTION
Drug-induced liver injury presents with extremely diverse histologic patterns, including necroinflammatory, cholestatic, steatotic and vascular patterns. Sinusoidal obstruction syndrome (SOS), also referred to as toxic sinusoidal injury, veno-occlusive disease or "blue liver syndrome", is a commonly recognized vascular pattern of drug-induced liver injury,1 and has been frequently associated with oxaliplatin-based chemotherapy.2,3,4,5,6
In this issue, we present a case of SOS after 6 cycles of oxaliplatin-based preoperative chemotherapy for colorectal cancer liver metastasis and discuss the morphologic findings of SOS.
CASE SUMMARY
A 58-year-old Korean woman presented with hematochezia during 2 months in June, 2011. She had no significant past medical history and also denied any previous intake of alcohol, smoking and herbal agents. The initial computed tomography (CT) scan demonstrated two masses in the rectum and ascending colon, multiple enlarged regional lymph nodes, two metastatic nodules in segments 6 and 8 of the liver and also pulmonary metastasis. She was treated with 6 cycles of XELOX (oral capecitabine [1,000 mg/m2 twice daily on days 1 to 14] plus oxaliplatin [130 mg/m2 on day 1]) over a 5-month period. A follow-up CT scan at 5 months after chemotherapy revealed a partial tumor response, and after one month, she underwent low anterior resection of the rectum, right hemihepatectomy of the liver, and left lobectomy of the lung. Preoperative liver function tests were within normal limits: aspartate aminotransferase (AST) 24 IU/L, alanine aminotransferase (ALT) 12 IU/L, alkaline phosphatase (ALP) 60 IU/L, total bilirubin 0.8 mg/dL, and prothrombin time (PT) 1.07 INR. Serologic tests for hepatitis B and hepatitis C virus were negative.
PATHOLOGIC FINDINGS
On gross examination, the resected liver showed 2 yellowish-white nodules corresponding to the metastatic lesions, and the background liver showed a distinct mottled appearance, with areas of congestion alternating with relatively normal-appearing hepatic parenchyme (Fig. 1). Microscopically, the nodules were consistent with metastatic adenocarcinomas from the colorectum. The non-tumorous liver parenchyme showed diffuse sinusoidal dilatation and congestion with extravasation of red blood cells, which predominantly affected the centrilobular zones. Hepatocytic plate disruption was seen in areas of severe sinusoidal congestion, and parenchymal extinction lesions (PELs) - defined as the approximation of hepatic vein remnants and portal tracts with loss of intervening hepatocytes - were noted. Masson's trichrome stain high-lighted centrilobular venular fibrosis and perisinusoidal fibrosis, and fibrous occlusion of small terminal hepatic venules was seen (Fig. 2). Macrovesicular and microvesicular steatosis was seen in less than 33% of hepatocytes. Hepatocellular ballooning was not prominent, and there was no significant lobular or portal inflammation. There was no significant cholestasis or ductular reaction.
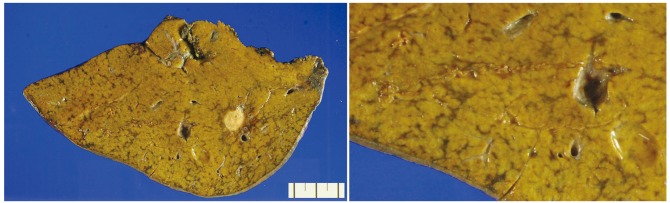
Macroscopic finding of sinusoidal obstruction syndrome. The non-neoplastic hepatic parenchyme is diffusely mottled, with geographic areas of congestion alternating with relatively normal-appearing liver.
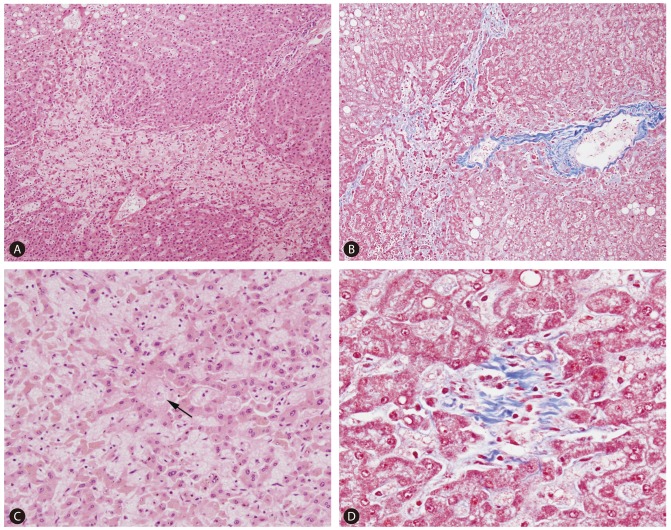
Representative microscopic findings of sinusoidal obstruction syndrome (A-D). Patchy perivenular sinusoidal dilatation and congestion with hepatocyte plate disruption (A ×100 magnification). Approximation of portal structures and hepatic vein with loss of intervening hepatocytes, and perivenular and perisinusoidal fibrosis (B ×100 magnification, Masson's trichrome stain). Sinusoidal dilatation and congestion with atrophy and disruption of hepatocyte plates (C ×200 magnification). An obliterated terminal hepatic venule is seen in the center (arrow). Masson's trichrome stain demonstrating fibrous obliteration of a terminal hepatic venule (D ×400 magnification).
DISCUSSION
Oxaliplatin-based chemotherapeutic regimens such as FOLFOX (5-fluorouracil (5-FU), leucovorin and oxaliplatin) and XELOX (capecitabine and oxaliplatin) are commonly used for patients with colorectal cancer liver metastases - preoperative chemotherapy has been shown to reduce the size of the hepatic metastases, subsequently rendering the tumor more amenable to curative resection.3,4 However, while these drugs have demonstrated impressive results in the treatment of colorectal cancers, they have also been associated with various degrees of hepatotoxicity. 5-FU has been associated with hepatic steatosis, possibly mediated by excessive production of reactive oxygen species resulting in the accumulation of lipid vesicles in hepatocytes,3,7,8 and an association between irinotecan and steatohepatitis has been reported.8,9 Oxaliplatin has been frequently associated with SOS. SOS has been described in association with drugs including oxaliplatin, azathioprine, cysteamine, dacarbazine, dactinomycin, carmustine (BCNU), 6-mercaptopurine, 6-thioguanine, busulfan, dimethyl busulfan, cytosine arabinoside, cyclophosphamide, indicine-N-oxide, mustine-HCl, doxorubicin, urethane, vincristine, mitomycin-C, etoposide, arsenic, thorium dioxide (Thorotrast), and intra-arterial flurodeoxyuridine.10 SOS related to oxaliplatin administration was first described by Rubbia-Brandt et al,5 and is characterized by the following histologic findings: sinusoidal dilatation and congestion, centrilobular vein fibrosis and obstruction, perisinusoidal fibrosis, necrosis of pericentral hepatocytes, PELs, hepatocyte plate disruption, and nodular regenerative hyperplasia.2,3,4,6,10,11 These changes are generally irregularly distributed within the hepatic parenchyme; however, if the changes are more extensive it is possible to recognize SOS on gross examination, where the liver appears diffusely "nodular" due to alternating areas of congestion/hemorrhage with relatively normal-looking areas.
Although the pathogenesis of chemotherapy-induced SOS is still unclear, it has been suggested that initial toxic damage to sinusoidal endothelial cells results in the disruption of the sinusoidal wall, resulting in marked sinusoidal dilatation and extravasation of erythrocytes into the Disse's spaces through the discontinuities in the endothelial lining. In addition, activation of hepatic stellate cells results in the deposition of collagen matrix in the perisinusoidal spaces and centrilobular vein.3,5 The degree of SOS has been suggested to be time-and dose-dependent- the extent of sinusoidal dilatation has been shown to be significantly higher in livers exposed to higher numbers of chemotherapy cycles.3,11 Interestingly, Overman et al.12 demonstrated that oxaliplatin-increased SOS was associated with portal hypertension, splenomegaly and subsequent thrombocytopenia, and suggested that increased spleen size could correlate with increasing grade of hepatic sinusoidal injury.12
The relationship between preoperative chemotherapy-related liver pathology and postoperative outcome is still controversial. Some studies have demonstrated a lack of significant association between chemotherapy-associated hepatopathy and postoperative outcome.6,9,13 On the other hand, preoperative chemotherapy was significantly correlated with increased postoperative morbidity and abnormal liver function tests such as AST, ALT, and total bilirubin in another recent study, suggesting that preoperative chemotherapy may lead to some derangement of liver function.3 There is limited data regarding the postoperative outcome in relation to SOS following oxaliplatin-based treatment, but an association between the presence of SOS and increased postoperative morbidity has been suggested: sinusoidal injury has been associated with a significantly higher complication rate and poorer liver functional reserve among patients undergoing a major hepatectomy, suggesting the importance of careful surgical candidate selection in patients previously exposed to oxaliplatin-based treatment.11
Notes
The authors have no conflicts to disclose.
Abbreviations
PEL
Parenchymal extinction lesion
SOS
sinusoidal obstruction syndrome