| Clin Mol Hepatol > Volume 21(3); 2015 > Article |
ABSTRACT
Background/Aims
Sorafenib is currently the sole molecular targeted agent that improves overall survival in advanced hepatocellular carcinoma (HCC). Despite the efficacy of sorafenib, the response rate varies in patients with advanced HCC. We retrospectively analyzed a series of Korean patients with advanced HCC with complete remission (CR) after sorafenib therapy.
Methods
In total, 523 patients with advanced HCC were treated with sorafenib in 3 large tertiary referral hospitals in Korea. A survey was conducted to collect data on patients who experienced CR after sorafenib monotherapy, and their medical records and follow-up data were analyzed. The tumor response and recurrence rates were assessed by radiologic study, based on modified response evaluation criteria in solid tumors.
Results
Seven patients with advanced HCC experienced CR after sorafenib therapy. The median time to tumor disappearance and the median disease-free survival time were 3 months and 9 months, respectively. HCC recurrence was identified in three cases (42.9%). Of these, two patients discontinued sorafenib before or after achieving CR and the other patient continued sorafenib after achieving CR. HCC recurred at 3, 10, and 42 months after CR in these three patients. Three patients needed dose reduction for toxicity and adverse events.
Hepatocellular carcinoma (HCC), which was the third leading cause of cancer related mortality, is diagnosed in more than 600,000 cases every year, worldwide.1 For early stage HCC, surgical and locoregional procedures, such as radiofrequency ablation, have been found to be curitive.2 However, at the time of diagnosis,the majority of patients present with advanced stage HCC.3 In the past, because of no effective conventional cytotoxic agent for advanced HCC, it was diagnosed at an advanced stage, and prognosis remained dismal.4
In recent years, with developments in targeted agents, the effectiveness of these drugs has been proven for many malignant neoplasms. Sorafenib, which is a multikinase inhibitor, targets Raf, vascular-endothelial growth factor (VEGFR)-2, VEGFR-3, platelet-derived growth factor-β, Flt-3, and c-Kit that inhibit molecular pathogenesis, angiogenesis, and tumor cell proliferation.567 The rationale for the use of sorafenib in patients with advanced HCC is based on two large 3-phase clinical trials, namely, the Sorafenib HCC Assessment Randomized Protocol (SHARP) and the Asia-Pacific (conducted in the Asia-Pacific region) trials.89 Currently, sorafenib is the sole systemic agent that leads to a significant improvement in both overall survival and progression-free survival in patients with advanced HCC. Additionally, the guidelines of the European Association for the Study of the Liver (EASL) and the American Association for the Study of Liver Diseases (AASLD) recommend sorafenib as a first line of treatment in patients with advanced HCC.1011 However, tumor regression was demonstrated only in a small proportion of patients in the SHARP and Asia-Pacific trials. Furthermore, only a relatively small number of complete remission (CR) cases were reported worldwide for sorafenib monotherapy. Of these, two CR cases were reported among 90 patients in a single center.12. Therefore, we evaluated the CR rate and long term outcomes in advanced HCC patients who experienced CR after sorafenib monotherapy at three tertiary medical centers over 4 years.
All patients who were treated with sorafenib monotherapy for advanced HCC at the Kyungpook National University Hospital and Kyungpook National University Medical Center in Daegu, Korea, and the Pusan National University Hospital, Pusan, Korea, between January 1, 2010 and December 31, 2013 were evaluated. A retrospective data review was performed using the three centers as cohorts. Further, a survey was conducted to determine the number of patients who experienced CR according to radiologic or pathologic findings. The study protocol was reviewed and approved by the Kyungpook National University Hospital Institutional Review Board (2009-1027), and the study was conducted in accordance with the principles of the Declaration of Helsinki.
Data on baseline clinical and tumor characteristics of the entire cohort are shown in Table 1. Medical records and follow-up data of all patients who experienced CR were reviewed for this analysis. Follow-up data included radiologic findings, tumor marker levels, time to recurrence, observed disease free survival time, treatment administered after recurrence, dose, and adverse effects of sorafenib.
The diagnosis of HCC was based on histologic or classical imaging features. The clinical diagnosis of HCC was defined as follows: among the patients with chronic hepatitis B or chronic hepatitis C and/or liver cirrhosis with any etiology, a definite diagnosis was established with the presence of a nodule larger than 2cm, showing a typical enhancement pattern (arterial enhancement and early wash-out in the delayed phase) on triphasic computed tomography (CT) and/or magnetic resonance imaging with liver-specific contrast agents.13 Further, advanced HCC was regarded as single or multinodular HCC with portal vein invasion and/or distant metastasis.11
CR was determined by radiologic findings according to the modified response evaluation criteria in solid tumors and pathology.14 CR was defined as follows: (1) No arterial enhancing mass on contrast-enhanced radiologic imaging and (2) pathologic confirmation following surgery.
Sorafenib (Nexavar; Bayer Healthcare Pharmaceuticals, Leverkusen, Germany) was administered orally to patients, initially at a dose of 400 mg, twice daily. All patients received sorafenib until unacceptable toxicity or patient refusal. The dosage of sorafenib could be reduced if grade 2 or 3 toxicity persisted.
Quantitative values are expressed as means with standard deviations or medians with range. Categorical values are expressed as numbers with percentages. As the number of patients who experienced CR was limited, statistical analysis for evaluating the factors associated with tumor response and recurrence could not be assessed. All statistical analyses were performed using the SPSS program (version 20.0; IBM, Somers, NY, USA).
Sorafenib was administered to 523 patients with advanced HCC. Most patients were men with chronic hepatitis B (78.0%) and Child-Pugh Class A (85.5%). At the diagnosis of advanced HCC, radiologic features of the tumors included a relatively large size (mean: 8.5 cm), a single tumor in 48.4% of cases, portal vein invasion in 67.1% of cases, and extrahepatic metastasis in 42.3% of cases. Of these, only 7 patients who were diagnosed with advanced HCC according to the Barcelona Clinic Liver Cancer (BCLC) classification (BCLC Stage C) achieved CR after sorafenib monotherapy (CR rate: 1.3%). Data on baseline clinical characteristics of patients who achieved CR are shown in Table 2. At diagnosis, the age of the patients ranged from 43 to 71 years (median: 53 years) and only 1 patient was female. All patients had chronic hepatitis B (6, 86%) or chronic hepatitis C (1, 14%), which is a high-risk etiology for developing HCC. The reservoir of hepatic function was relatively good (Child-Pugh Class A: 4, Class B: 3). Further, 7 patients' Eastern Cooperative Oncology Group performance status was 0. Four out of these 7 patients were initially diagnosed with advanced HCC, whereas the others were diagnosed after transarterial chemoembolization (TACE) with or without radiofrequency ablation (RFA) or surgery. Only 1 patient was diagnosed with HCC by histology.
Pretreatment radiologic features of the tumors included right lobe predominance, relatively small size (3.5-13 cm, median: 5.3 cm), and portal vein invasion without distant metastasis except in 1 case (Table 3). An elevated serum alpha fetoprotein (AFP) level was observed in 5 patients (315.5-30,376 ng/mL, median 685.6 ng/mL) at the diagnosis of advanced HCC. The level of serum protein induced by vitamin K absence or antagonist-II (PIVKA-II) was elevated (113-2,000 mAU/mL, median: 1,083 mAU/mL) in all except 3 patients, whose data were not available.
The clinical outcomes following CR in patients who received sorafenib have been described in Table 4. The time to tumor disappearance was less than 6 months in all cases (2-6 months, median: 34 months). Before the achievement of CR, the serum AFP level was normalized or maintained at normal in patients with normal serum AFP level, whereas the PIVKA-II level was not normalized. All 7 patients experienced radiologic CR with normalization of the serum AFP level, whereas pathologic CR was not noted in any patient.
The duration of sorafenib use varied across patients (1-44 months, median: 11 months). The dosage of sorafenib was reduced after achieving CR in 3 of the 7 patients because of side effects (Table 4). Before achieving CR, 1 patient (Patient 2) discontinued sorafenib because of the economic burden. After 6 weeks of achieving CR, 1 patient (Patient 1) discontinued sorafenib because of infection (Table 5).
Disease free survival varied across patients (3-43 months, median: 9 months). Three of the 7 patients showed recurrence after sorafenib therapy. Of these, 1 patient (Patient 2) showed recurrence in the liver and lung at 42 months after achieving CR, and then died 3 months later. Two patients experienced distant intrahepatic recurrence at 10 (Patient 1) and 3 months (Patient 5) after achieving CR. After intrahepatic recurrence, 1 patient (Patient 1) was treated with RFA and TACE, while the other patient was treated (Patient 5) with RFA. These patients were followed up for a total of 15 months and for 7 months without recurrence. Mortality, which was not related with disease progression, was noted in 2 out of the 7 patients (Patients 1 and 3).
Based on the SHARP and Asia-Pacific trials, sorafenib is the sole chemotherapeutic agent that has been shown to significantly improve both overall and progression-free survival.89 However, of the 449 patients who received sorafenib, no CR was achieved in patients with advanced HCC. Moreover, only 12 (2.6%) patients achieved partial response in both studies. Theoretically, sorafenib is only able to inhibit tumor progression in HCC patients because its effect is not cytotoxic, but it inhibits tumor proliferation and angiogenesis. However, after the approval of sorafenib in Europe and North America in 2007, the first case in which CR was achieved in a patient with hemochromatosis and metastatic HCC after sorafenib monotherapy was reported in 2008.15 At present, to our knowledge, there have been reports of 13 patients with advanced HCC who experienced CR after sorafenib monotherapy, worldwide, excluding 1 pilot analysis conducted in Japan.1215161718192021222324 A nationwide survey conducted in Japan reported 18 cases of CR after sorafenib in patients with advanced HCC among 3047 patients (CR rate, 0.6%).25 In the entire cohort of 523 patients in the present study, only 7 patients (1.3%) achieved CR after sorafenib therapy. All reported cases of CR achievement after sorafenib monotherapy tend to exhibit a rapid decrease in serum AFP levels before CR.1215161718192021222324 Further, in only 3 cases, it took more than 6 months to achieve CR.161822 In the present study, the time to CR was less than 6 months in all cases. It is more likely to achieve CR with sorafenib because a rapid tumor response has been shown to occur within 6 months. With a rapid tumor response, a rapid decrease in the serum AFP level was also observed after sorafenib therapy (Fig. 1). It is well known that the rapid decrease in serum AFP is a good predictive factor for sorafenib response.26 Moreover, AFP response was an independent surrogate marker that predicted the prognosis of patients with advanced HCC after sorafenib therapy.27 In this study, the radiologic response was well correlated with the serum AFP level. Based on these findings, both a rapid decrease in the serum AFP level and an initial radiologic response within 6 months may be good predictive factors for sorafenib response.
In this study, although patients achieved CR after sorafenib therapy, the recurrence rate was relatively high (3 of 7 patients, 43%). However, recurrence at the target lesion was not noted in any of the patients. Recurrence was in the form of distant metastasis, which could be controlled by locoregional therapy (Patients 1 and 5), except in patients with extrahepatic recurrence. In previous studies, treatment following CR varied from surgery to observation.1215161718192021222324 Although surgery or transplantation is preferred for curative treatment, most HCC patients cannot receive these treatments because of underlying chronic liver disease or limited donors and high costs. Moreover, there are limited data on these curative treatments after achieving CR. Except for 2 cases, followup periods after achieving CR was less than 1 year in most studies.1823 Therefore, sorafenib maintenance therapy can be suggested as a therapeutic option for patients who cannot receive curative treatment. However, there are limited data about the efficacy of sorafenib maintenance therapy after achieving CR. Only 5 cases have reported continuation of sorafenib after achieving CR.12202122 Moreover, recurrence has not been addressed in most studies. In this study, 5 patients underwent sorafenib maintenance therapy. Of these, 1 patient experienced distant intrahepatic recurrence. In contrast, of the 2 patients who discontinued sorafenib before or after achieving CR, 1 patient experienced distant intrahepatic recurrence and the other experienced extrahepatic recurrence after discontinuation of sorafenib. Thus, recurrence was more likely to occur in patients with early discontinuation of sorafenib. However, because of the limited number of cases, the efficacy of sorafenib maintenance therapy is still questionable. Therefore, further investigation is needed on sorafenib maintenance therapy after achieving CR.
In this study, all cases, except 1, were of locally advanced HCC without distant metastasis. It was suggested that VEGF may be closely associated with HCC carcinogenesis, and may also promote portal vein thrombosis through modulating angiogenesis.28 In this regard, sorafenib could play a critical role in both direct regression of tumor cells and recanalization of portal vein thrombosis by inhibition of the VEGF pathway.29 However, there is still no known convincing mechanism of these dramatic responses to sorafenib.
One limitation of our study is that the diagnosis of HCC was made without a biopsy. However, the diagnosis was based on the typical post-contrast pattern of image findings and tumor size of more than 2 cm in all cases. These clinical diagnoses were based on the AASLD and EASL guidelines.1011 Another limitation was that the diagnosis of CR was made based on radiologic findings in all cases. All patients were not candidates for resection because of liver cirrhosis or refusal of liver transplantation. However, although the diagnosis of CR was based on radiologic findings, there was no recurrence of target lesions in any of the patients.
In conclusion, although the number of cases is small, the recurrence rate after achieving CR in advanced HCC after sorafenib therapy is relatively high in our study. Subsequent strategies to reduce the recurrence rate after sorafenib therapy should be investigated. Further studies should identify the molecular features of these tumors to explain the achievement of these dramatic responses.
Acknowledgements
I acknowledge with thanks to corroborate data of cases by Soo Young Park, Won Young Tak, Young Oh Kweon in Kyungpook National University Hospital and Jeong Heo, HyunYoung Woo, Won Im in Pusan National University Hospital.
Abbreviations
AASLD
the American Association for the Study of Liver Diseases
AFP
serum alpha fetoprotein
CR
complete remission
CT
computed tomography
EASL
European Association for the Study of the Liver
HCC
hepatocellular carcinoma
PIVKA-II
protein induced by vitamin K absence or antagonist-II
RFA
radiofrequency ablation
SHARP
Sorafenib HCC Assessment Randomized Protocol trial
TACE
transarterial chemoembolization
VEGFR
vascular-endothelial growth factor
REFERENCES
1. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin 2005;55:74-108. 15761078.


2. Lopez PM, Villanueva A, Llovet JM. Systematic review: evidence-based management of hepatocellular carcinoma-an updated analysis of randomized controlled trials. Aliment Pharmacol Ther 2006;23:1535-1547. 16696801.


4. Nowak AK, Chow PK, Findlay M. Systemic therapy for advanced hepatocellular carcinoma: a review. Eur J Cancer 2004;40:1474-1484. 15196530.


5. Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res 2004;64:7099-7109. 15466206.


6. Chang YS, Adnane J, Trail PA, Levy J, Henderson A, Xue D, et al. Sorafenib (BAY 43-9006) inhibits tumor growth and vascularization and induces tumor apoptosis and hypoxia in RCC xenograft models. Cancer Chemother Pharmacol 2007;59:561-574. 17160391.


7. Wilhelm SM, Adnane L, Newell P, Villanueva A, Llovet JM, Lynch M. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol Cancer Ther 2008;7:3129-3140. 18852116.


8. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378-390. 18650514.


9. Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, doubleblind, placebo-controlled trial. Lancet Oncol 2009;10:25-34. 19095497.


10. Bruix J, Sherman M. American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020-1022. 21374666.



11. European Association For The Study Of The Liver. European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012;56:908-943. 22424438.


12. Kudo M, Ueshima K. Positioning of a molecular-targeted agent, sorafenib, in the treatment algorithm for hepatocellular carcinoma and implication of many complete remission cases in Japan. Oncology 2010;78(Suppl 1):154-166. 20616599.


13. Korean Liver Cancer Study Group and National Cancer Center, Korea. Practice guidelines for management of hepatocellular carcinoma 2009. Korean J Hepatol 2009;15:391-423. 19783891.


14. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 2010;30:52-60. 20175033.


15. So BJ, Bekaii-Saab T, Bloomston MA, Patel T. Complete clinical response of metastatic hepatocellular carcinoma to sorafenib in a patient with hemochromatosis: a case report. J Hematol Oncol 2008;1:18. 18928548.



16. Irtan S, Chopin-Laly X, Ronot M, Faivre S, Paradis V, Belghiti J. Complete regression of locally advanced hepatocellular carcinoma induced by sorafenib allowing curative resection. Liver Int 2011;31:740-743. 21457447.


17. Curtit E, Thiery-Vuillemin A, Nguyen T, Heyd B, Pivot X, Di Martino V, et al. Complete histologic response induced by sorafenib in advanced hepatocellular carcinoma: a case report. J Clin Oncol 2011;29:e330-e332. 21263091.


18. Wang SX, Byrnes A, Verma S, Pancoast JR, Rixe O. Complete remission of unresectable hepatocellular carcinoma treated with reduced dose of sorafenib: a case report. Target Oncol 2010;5:59-63. 20309643.


19. Inuzuka T, Nishikawa H, Sekikawa A, Takeda H, Henmi S, Sakamoto A, et al. Complete response of advanced hepatocellular carcinoma with multiple lung metastases treated with sorafenib: a case report. Oncology 2011;81:152-157. 22212950.


20. Chelis L, Ntinos N, Souftas V, Deftereos S, Xenidis N, Chamalidou E, et al. Complete response after sorafenib therapy for hepatocellular carcinoma in an HIV-HBV co infected patient: Possible synergy with HAART? A case report. Med Oncol 2011;28:S165-S168. 20809183.


21. Sacco R, Bargellini I, Gianluigi G, Bertini M, Bozzi E, Altomare E, et al. Complete response for advanced liver cancer during sorafenib therapy: case report. BMC Gastroenterol 2011;11:4. 21241463.




22. Yeganeh M, Finn RS, Saab S. Apparent remission of a solitary metastatic pulmonary lesion in a liver transplant recipient treated with sorafenib. Am J Transplant 2009;9:2851-2854. 20021481.


23. Ahn SY, Lee HS, Kweon YO, Tak WY, Park SY. Sustained remission over 36 months of advanced hepatocellular carcinoma after short-term sorafenib therapy. Dig Dis Sci 2013;58:1428-1432. 23306847.


24. Kim MS, Jin YJ, Lee JW, Lee JI, Kim YS, Lee SY, et al. Complete remission of advanced hepatocellular carcinoma by sorafenib: A case report. World J Gastrointest Oncol 2013;15:38-42. 23556056.



25. Shiba S, Okusaka T, Ikeda M, Saito H, Ichida T. Characteristics of 18 patients with hepatocellular carcinoma who obtained a complete response after treatment with sorafenib. Hepatol Res 2014;44:1268-1276. 24405694.


26. Yau T, Yao TJ, Chan P, Wong H, Pang R, Fan ST, et al. The significance of early alpha-fetoprotein level changes in predicting clinical and survival benefits in advanced hepatocellular carcinoma patients receiving sorafenib. Oncologist 2011;16:1270-1279. 21885876.



27. Personeni N, Bozzarelli S, Pressiani T, Rimassa L, Tronconi MC, Sclafani F, et al. Usefulness of alpha-fetoprotein response in patients treated with sorafenib for advanced hepatocellular carcinoma. J Hepatol 2012;57:101-107. 22414760.


Figure 1
Serial changes of serum alpha fetoprotein level after sorafenib therapy during follow-up period.
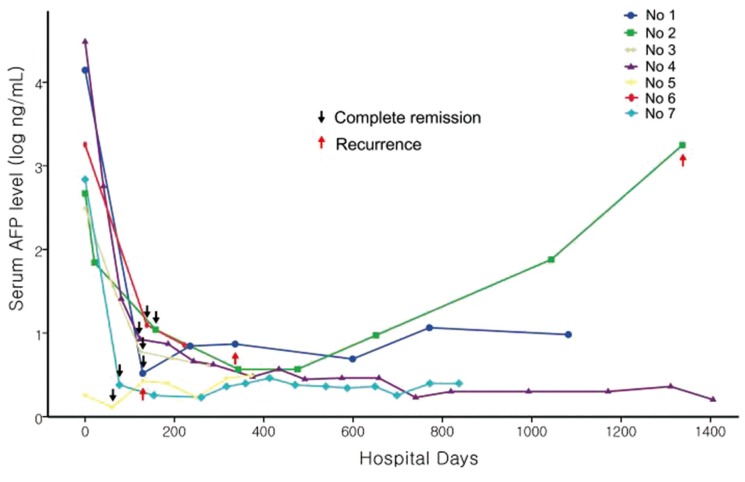
Table 1.
Baseline characteristics of all patients
Table 2.
Baseline characteristics of patients achieving complete remission after sorafenib monotherapy
Table 3.
Pretreatment tumor characteristics and tumor markers of patients
Table 4.
Clinical status and tumor marker of patients at the time of complete response
| Patient No | AFP (ng/mL) | PIVKA-II (mAU/mL) | Side effect of sorafenib | Dosage of sorafenib (mg) | Time to disappearance (months) | Duration of sorafenib use (months) |
|---|---|---|---|---|---|---|
| 1 | 3.33 | - | No | 800 | 2 | 5 |
| 2 | 10.97 | - | No | 800* | 6 | 1 |
| 3 | 36.5 | 1043 | No | 800 | 2 | 9 |
| 4 | 8.42 | - | Yes | 800† | 3 | 47 |
| 5 | 2.5 | 383 | Yes | 800‡ | 2 | 12 |
| 6 | 12.5 | - | No | 800 | 5 | 11 |
| 7 | 2.4 | - | No | 800§ | 3 | 28 |
Table 5.
Clinical status of patients after achieving complete response
| Patient No | Sorafenib maintenance | Recurrence, intrahepatic | Recurrence, extrahepatic | Disease Free survival | Next treatment after recurrence |
|---|---|---|---|---|---|
| 1* | No† | Yes | No | 10 | TACE, RFA |
| 2‡ | No§ | Yes | Yes | 42 | - |
| 3* | Yes | No | No | 9 | - |
| 4 | Yes | No | No | 43 | - |
| 5 | Yes | Yes | No | 3 | RFA |
| 6 | Yes | No | No | 7 | - |
| 7 | Yes | No | No | 25 | - |



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print




