| Clin Mol Hepatol > Volume 26(3); 2020 > Article |
|
ABSTRACT
Background/Aims
Methods
Results
ACKNOWLEDGMENTS
FOOTNOTES
SUPPLEMENTAL MATERIAL
Supplementary Table 1.
Supplementary Table 2.
Supplementary Table 3.
Supplementary Table 4.
Supplementary Figure 1.
Supplementary Figure 2.
Supplementary Figure 3.
Supplementary Figure 4.
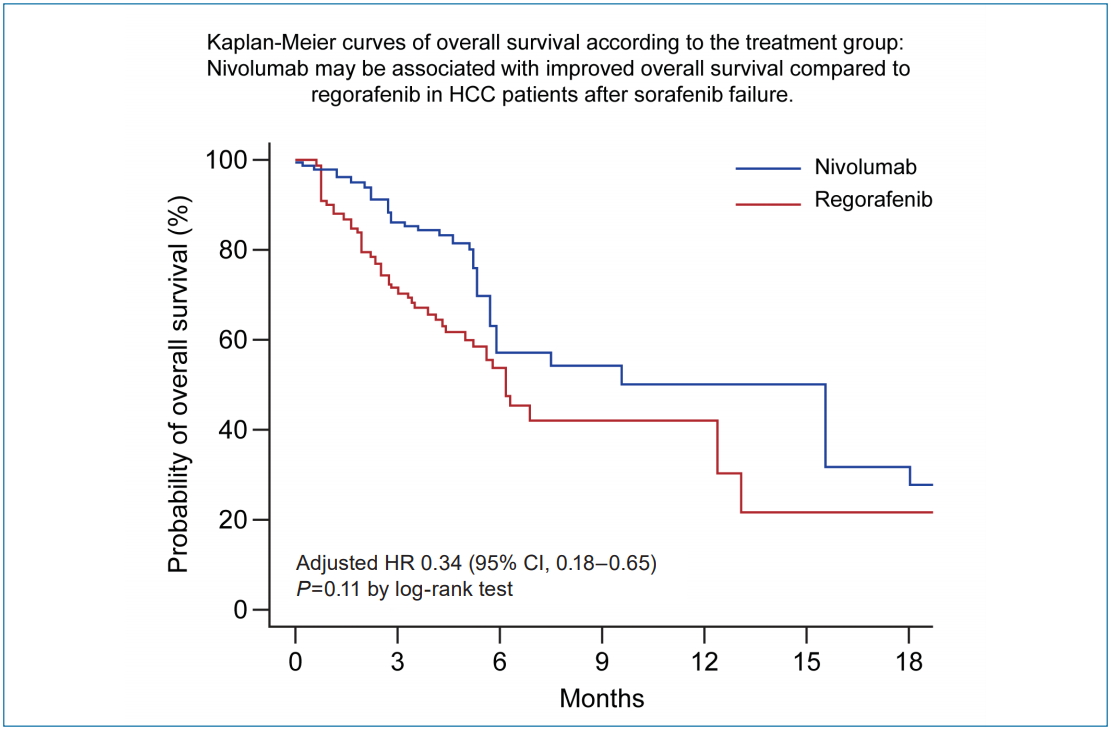
Figure 1.

Figure 2.

Figure 3.
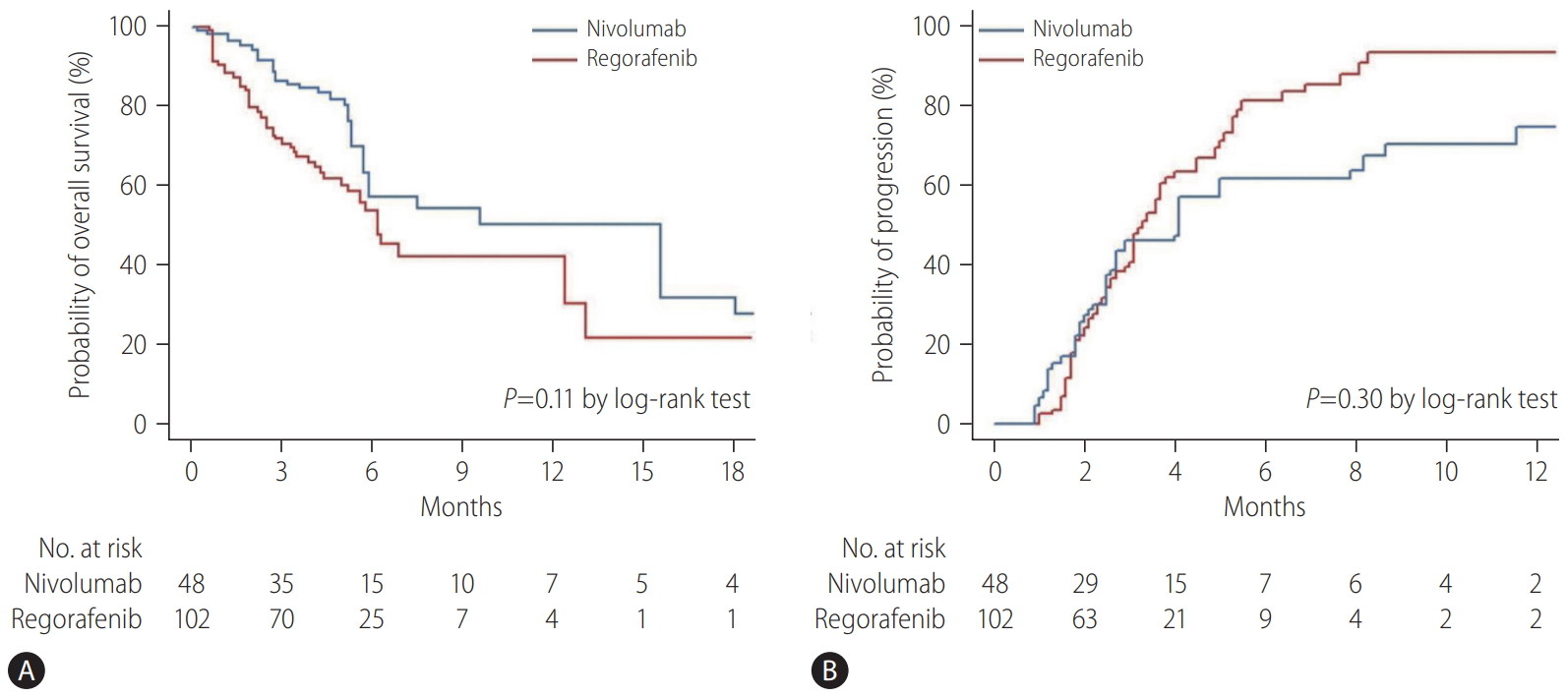
Table 1.
| Characteristic | Overall (n=150) |
Group |
P-value | |
|---|---|---|---|---|
| Regorafenib (n=102) | Nivolumab (n=48) | |||
| Age (years) | 62 (55–70) | 62 (56–71) | 61 (54–67) | 0.14 |
| Male | 122 (81.3) | 83 (81.4) | 39 (81.2) | 0.99 |
| HCC etiology | 0.44 | |||
| HBV | 124 (82.7) | 86 (84.3) | 38 (79.2) | |
| Others | 26 (17.3) | 16 (15.7) | 10 (20.8) | |
| Child-Pugh score | 0.003 | |||
| 5 | 86 (57.3) | 66 (64.7) | 20 (41.7) | |
| 6 | 51 (34.0) | 32 (31.4) | 19 (39.6) | |
| 7–9 | 13 (8.7) | 4 (3.9) | 9 (18.8) | |
| Vascular invasion | 66 (44.0) | 42 (41.2) | 24 (50.0) | 0.31 |
| Biliary invasion | 7 (4.7) | 5 (4.9) | 2 (4.2) | 0.84 |
| Intrahepatic tumor burden | 0.40 | |||
| None | 25 (16.7) | 19 (18.6) | 6 (12.5) | |
| <50% | 93 (62.0) | 64 (62.7) | 29 (60.4) | |
| ≥50% | 32 (21.3) | 19 (18.6) | 13 (27.1) | |
| Extrahepatic metastases | 120 (80.0) | 79 (77.5) | 41 (85.4) | 0.26 |
| Lymph node | 54 (36.0) | 37 (36.3) | 17 (35.4) | |
| Lung | 77 (51.3) | 46 (45.1) | 31 (64.6) | |
| Bone | 32 (21.3) | 21 (20.6) | 11 (22.9) | |
| Peritoneum | 24 (16.0) | 16 (15.7) | 8 (16.7) | |
| Others | 3 (2.0) | 1 (1.0) | 2 (4.2) | |
| Portal hypertension | 64 (42.7) | 42 (41.2) | 22 (45.8) | 0.59 |
| BCLC stage | 0.56 | |||
| B | 5 (3.3) | 4 (3.9) | 1 (2.1) | |
| C | 145 (96.7) | 98 (96.1) | 47 (97.9) | |
| Laboratory data | ||||
| Albumin (g/dL) | 3.7 (3.3–4.0) | 3.8 (3.4–4.1) | 3.7 (3.2–3.9) | 0.08 |
| Total bilirubin (mg/dL) | 1.0 (0.7–1.4) | 1.0 (0.7–1.3) | 1.1 (0.7–1.7) | 0.29 |
| AST (IU/L) | 49 (33–80) | 45 (32–77) | 60 (39–90) | 0.16 |
| ALT (IU/L) | 37 (22–59) | 37 (22–57) | 38 (23–60) | 0.42 |
| ALP (IU/L) | 138 (88–220) | 133 (87–220) | 144 (92–212) | 0.88 |
| Creatinine (mg/dL) | 0.81 (0.68–0.93) | 0.82 (0.68–0.93) | 0.80 (0.69–0.93) | 0.44 |
| Platelet count (×109/L) | 154 (105–218) | 164 (107–228) | 149 (101–204) | 0.28 |
| INR | 1.08 (1.02–1.15) | 1.06 (1.00–1.12) | 1.10 (1.04–1.21) | 0.002 |
| AFP (ng/mL) | 431 (12.5–4,185.0) | 338.0 (11.9–3,799.3) | 760.0 (18.4–4,665.0) | 0.90 |
| PIVKA (mAU/mL) | 1,453 (135–8,898) | 1,365 (149–8,699) | 1,803 (107–10,545) | 0.48 |
| MoRAL | 570.6 (205.1–1,254.3) | 570.6 (240.1–1,224.0) | 559.5 (234.2–1,276.5) | 0.62 |
| Intolerant to sorafenib | 13 (8.7) | 6 (5.9) | 7 (14.6) | 0.08 |
| Duration of sorafenib* (months) | 2.8 (2.0–4.7) | 3.0 (2.3–6.2) | 2.5 (1.4–3.1) | <0.001 |
| Time interval between sorafenib and treatment (months) | 1.2 (0.0–4.7) | 0.9 (0.0–4.6) | 1.8 (0.3–5.8) | 0.89 |
Values are presented as median (interquartile range) or number (%) of patients.
HCC, hepatocellular carcinoma; HBV, hepatitis B virus; BCLC, Barcelona Clinic Liver Cancer; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; INR, international normalized ratio; AFP, alpha-fetoprotein; PIVKA, protein induced by vitamin K absence or antagonist; MoRAL, model to predict tumor recurrence after living donor liver transplantation.
Table 2.
P values were determined using Cox proportional hazards regression models. P<0.05 indicated a significant difference.
HR, hazards ratio; CI, confidence interval; HCC, hepatocellular carcinoma; HBV, hepatitis B virus; BCLC, Barcelona Clinic Liver Cancer; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; AFP, alpha-fetoprotein; PIVKA, protein induced by vitamin K absence or antagonist; MoRAL, model to predict tumor recurrence after living donor liver transplantation.
Table 3.
P values were determined using Cox proportional hazards regression models. P<0.05 indicated a significant difference.
HR, hazards ratio; CI, confidence interval; HCC, hepatocellular carcinoma; HBV, hepatitis B virus; BCLC, Barcelona Clinic Liver Cancer; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; AFP, alpha-fetoprotein; PIVKA, protein induced by vitamin K absence or antagonist; MoRAL, model to predict tumor recurrence after living donor liver transplantation.
Table 4.
| Regorafenib (n=102) | Nivolumab (n=48) | |
|---|---|---|
| Best overall response* | ||
| Complete response | 0 | 0 |
| Partial response | 6 (5.9) | 8 (16.7) |
| Stable disease | 42 (41.2) | 16 (33.3) |
| Progressive disease | 37 (36.3) | 17 (35.4) |
| Not assessed | 17 (16.7) | 7 (14.6) |
| Objective response† | 6 (5.9) | 8 (16.7) |
| Disease control‡ | 48 (47.1) | 24 (50.0) |
Abbreviations
REFERENCES
- TOOLS



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Supplement1
Supplement1 Print
Print





